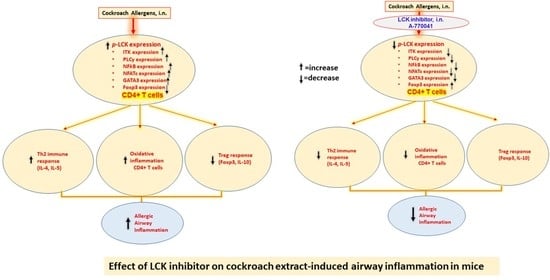
Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Development of Cockroach Extract (CE)-Induced Allergic Inflammation in Mice
2.3. Experimental Groups
2.4. BAL Procedure
2.5. Immunofluorescent Analysis of LCK Signaling and Different Th2 Related Markers in Pulmonary Cell Suspension Using Flow Cytometry
2.6. Evaluation of Allergic Cytokine in the Lung by ELISA
2.7. Evaluation of mRNA Expression by Real-Time PCR
2.8. Histopathology of the Lung Tissue Using H&E and PAS Staining
2.9. Chemicals and Reagents
2.10. Statistical Analysis
3. Results
3.1. LCK Is Activated during Allergic Airway Inflammation and Its Inhibition Causes Reduction in Downstream Pathways in CD4+ T Cells of CE-Exposed Mice
3.2. Inhibition of LCK Signaling Causes Reduction in Airway Inflammation in CE-Induced Allergic Model of Asthma
3.3. Blockade of LCK Signaling Leads to Attenuation of Th2 Related Transcription Factor Signaling in CE-Exposed Mice
3.4. Blockade of LCK Signaling Leads to Attenuation of Th2 Related Cytokines in CE-Exposed Mice
3.5. Blockade of LCK Signaling Leads to Augmentation of Treg Cells in CE-Exposed Mice
3.6. Blockade of LCK Signaling Leads to Attenuation Oxidative Stress in CD4+ T Cells in CE-Exposed Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The Immunology of Asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018, 15, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; Liu, X. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010; NCHS Data Brief; US Department of Health and Human Services, Centers for Disease Control and Prevention: Washington, DC, USA, 2012; pp. 1–8.
- Al Ghobain, M.O.; Algazlan, S.S.; Oreibi, T.M. Asthma Prevalence among Adults in Saudi Arabia. Saudi Med. J. 2018, 39, 179–184. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Nadeem, A.; Siddiqui, N.; Alharbi, N.O.; Alharbi, M.M. Airway and systemic oxidant-antioxidant dysregulation in asthma: A possible scenario of oxidants spill over from lung into blood. Pulm. Pharmacol. Ther. 2014, 29, 31–40. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Hodes, R.J. Regulation of T cell development by c-Cbl: Essential role of Lck. Int. Immunol. 2015, 27, 245–251. [Google Scholar] [CrossRef]
- Kumar, S.P.; Kashyap, A.; Silakari, O. Exploration of the therapeutic aspects of Lck: A kinase target in inflammatory mediated pathological conditions. Biomed. Pharm. 2018, 108, 1565–1571. [Google Scholar] [CrossRef]
- Nika, K.; Soldani, C.; Salek, M.; Paster, W.; Gray, A.; Etzensperger, R. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 2010, 32, 766–777. [Google Scholar] [CrossRef] [Green Version]
- Kemp, K.L.; Levin, S.D.; Bryce, P.J.; Stein, P.L. LCK mediates Th2 differentiation through effects on T-bet and GATA-3. J. Immunol. 2010, 184, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Fowell, D.J.; Shinkai, K.; Liao, X.C.; Beebe, A.M.; Coffman, R.L.; Littman, D.R.; Locksley, R.M. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity 1999, 11, 399–409. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Ahmad, S.F.; Almutairi, M.; Alanazi, A.Z.; Ibrahim, K.E.; Alqarni, S.A.; Alqahtani, F.; Alhazzani, K.; Alharbi, M.; Alasmari, F.; et al. Lck signaling inhibition causes improvement in clinical features of psoriatic inflammation through reduction in inflammatory cytokines in CD4+ T cells in imiquimod mouse model. Cell Immunol. 2022, 376, 104531. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, P.; Murali, A.K.; Al-Khami, A.A.; Mehrotra, S. Redox regulation of T-cell function: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2013, 18, 1497–1534. [Google Scholar] [CrossRef]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 12, 635021. [Google Scholar] [CrossRef]
- Muehling, L.M.; Lawrence, M.G.; Woodfolk, J.A. Pathogenic CD4+ T cells in patients with asthma. J. Allergy Clin. Immunol. 2017, 140, 1523–1540. [Google Scholar] [CrossRef]
- Corren, J. New Targeted Therapies for Uncontrolled Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1394–1403. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, L.K.; Choi, J.M. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front. Immunol. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Hermann-Kleiter, N.; Baier, G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 2010, 115, 2989–2997. [Google Scholar] [CrossRef]
- Alfardan, A.S.; Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Harbi, M.M.; AlSharari, S.D. Plasticizer, di(2-ethylhexyl)phthalate (DEHP) enhances cockroach allergen extract-driven airway inflammation by enhancing pulmonary Th2 as well as Th17 immune responses in mice. Environ. Res. 2018, 164, 327–339. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Ibrahim, K.E.; Siddiqui, N.; Al-Harbi, M.M.; Attia, S.M.; Bakheet, S.A. Inhibition of Bruton’s tyrosine kinase and IL-2 inducible T-cell kinase suppresses both neutrophilic and eosinophilic airway inflammation in a cockroach allergen extract-induced mixed granulocytic mouse model of asthma using preventative and therapeutic strategy. Pharmacol. Res. 2019, 148, 104441. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta, Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Alharbi, N.O.; Vliagoftis, H.; Tyagi, M.; Ahmad, S.F.; Sayed-Ahmed, M.M. Proteinase activated receptor-2-mediated dual oxidase-2 up-regulation is involved in enhanced airway reactivity and inflammation in a mouse model of allergic asthma. Immunology 2015, 145, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Al-Harbi, N.O.; Ahmad, S.F.; Ibrahim, K.E.; Alotaibi, M.R.; Siddiqui, N.; Alsharari, S.D.; Attia, S.M.; Al-Harbi, M.M. Protease activated receptor-2 mediated upregulation of IL-17 receptor signaling on airway epithelial cells is responsible for neutrophilic infiltration during acute exposure of house dust mite allergens in mice. Chem. Biol. Interact. 2019, 304, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.S. Kinase inhbitors for the treatment of inflammatory and autoimmune disorders. Purinergic Signal. 2009, 5, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, A.A.; Bao, K.; Lupardus, P.; Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug Discov. 2020, 20, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Gadina, M. Advances in kinase inhibition: Treating rheumatic diseases and beyond. Curr. Opin. Rheumatol. 2014, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, R.; Zheng, Y. The effect of siRNA-mediated lymphocyte-specific protein tyrosine kinase (Lck) inhibition on pulmonary inflammation in a mouse model of asthma. Int. J. Clin. Exp. Med. 2015, 8, 15146–15154. [Google Scholar]
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int. J. Mol. Sci. 2019, 20, 3500. [Google Scholar] [CrossRef] [PubMed]
- Do, D.C.; Zhao, Y.; Gao, P. Cockroach allergen exposure and risk of asthma. Allergy 2016, 71, 463–474. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Hodes, J.R. T cell development is regulated by the coordinated function of proximal and distal Lck promoters active at different developmental stages. Eur. J. Immunol. 2016, 46, 2401–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozso, S.J.; Kang, J.J.H.; Nagendran, J. The role of competing mechanisms on Lck regulation. Immunol. Res. 2020, 68, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y. GATA3: A master of many trades in immune regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef]
- Athari, S.S. Targeting cell signaling in allergic asthma. Sig. Transduct. Target Ther. 2019, 4, 45. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Justice, J.P.; Crosby, J.; Borchers, M.T.; Tomkinson, A.; Lee, J.J.; Lee, N.A. CD4(+) T cell-dependent airway mucus production occurs in response to IL-5 expression in lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L1066–L1074. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, S.; Dasgupta, P.; Dorsey, N.J.; Keegan, A.D. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J. Leukoc. Biol. 2010, 87, 1011–1018. [Google Scholar] [CrossRef]
- Ray, A.; Khare, A.; Krishnamoorthy, N.; Qi, Z.; Ray, P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010, 3, 216–229. [Google Scholar] [CrossRef]
- Robinson, D.S. Regulatory T cells and asthma. Clin. Exp. Allergy 2009, 39, 1314–1323. [Google Scholar] [CrossRef]
- Wei, J.; Duramad, O.; Perng, O.A.; Reiner, S.L.; Liu, Y.J.; Qin, F.X. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2007, 104, 18169–18174. [Google Scholar] [CrossRef]
- Martín-Orozco, E.; Norte-Muñoz, M.; Martínez-García, J. Regulatory T Cells in Allergy and Asthma. Front. Pediatr. 2017, 5, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Yu, S.F.; Peng, M.; Lai, D.H.; Hide, G.; Wu, Z.D.; Lun, Z.R. iNOS is essential to maintain a protective Th1/Th2 response and the production of cytokines/chemokines against Schistosoma japonicum infection in rats. PLoS Negl. Trop. Dis. 2022, 16, e0010403. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, L.; Bogeski, I. Redox regulation of T-cell receptor signaling. Biol. Chem. 2015, 396, 555–568. [Google Scholar] [CrossRef]
- Previte, D.M.; Piganelli, J.D. Reactive Oxygen Species and Their Implications on CD4+ T Cells in Type 1 Diabetes. Antioxid. Redox Signal. 2018, 29, 1399–1414. [Google Scholar] [CrossRef] [PubMed]
- Shatynski, K.E.; Chen, H.; Kwon, J.; Williams, M.S. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: Role in T helper development. Eur. J. Immunol. 2012, 42, 3202–3211. [Google Scholar] [CrossRef]
- Medeiros, M.L.; de Oliveira, M.G.; Tavares, E.G.; Mello, G.C.; Anhê, G.F.; Mónica, F.Z.; Antunes, E. Long-term methylglyoxal intake aggravates murine Th2-mediated airway eosinophil infiltration. Int. Immunopharmacol. 2020, 81, 106254. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, Z.; Zhang, Y.; Wu, T.; Ma, Y.; Lai, W.; Guo, Z. LCK inhibitor attenuates atherosclerosis in ApoE-/- mice via regulating T cell differentiation and reverse cholesterol transport. J. Mol. Cell. Cardiol. 2020, 139, 87–97. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhang, M.; Wu, X.; Zhu, J. Silencing LCK inactivates the NF-κB pathway to attenuate cartilage injury in osteoarthritis: An in vivo and in vitro study. J. Gene Med. 2021, 10, e3387. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.W.; Jeong, H.O.; Lee, B.; Chung, K.W.; Lee, Y.; Jung, H.J.; Hyun, M.K.; Lee, A.K.; Kim, B.M.; et al. Novel Role of Lck in Leptin-Induced Inflammation and Implications for Renal Aging. Aging Dis. 2019, 10, 1174–1186. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, S.A.; Bineid, A.; Ahmad, S.F.; Al-Harbi, N.O.; Alqahtani, F.; Ibrahim, K.E.; Ali, N.; Nadeem, A. Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites 2022, 12, 793. https://doi.org/10.3390/metabo12090793
Alqarni SA, Bineid A, Ahmad SF, Al-Harbi NO, Alqahtani F, Ibrahim KE, Ali N, Nadeem A. Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites. 2022; 12(9):793. https://doi.org/10.3390/metabo12090793
Chicago/Turabian StyleAlqarni, Saleh A., Abdulwahab Bineid, Sheikh F. Ahmad, Naif O. Al-Harbi, Faleh Alqahtani, Khalid E. Ibrahim, Nemat Ali, and Ahmed Nadeem. 2022. "Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma" Metabolites 12, no. 9: 793. https://doi.org/10.3390/metabo12090793
APA StyleAlqarni, S. A., Bineid, A., Ahmad, S. F., Al-Harbi, N. O., Alqahtani, F., Ibrahim, K. E., Ali, N., & Nadeem, A. (2022). Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma. Metabolites, 12(9), 793. https://doi.org/10.3390/metabo12090793