Profiling Phospholipids within Atlantic Salmon Salmo salar with Regards to a Novel Terrestrial Omega-3 Oil Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fish Growth Conditions and Sampling
2.3. Lipid Extraction
2.4. Untargeted Supercritical Fluid Chromatography (SFC)-MS/MS
2.5. Semi-targeted Reverse Phase LC-MS/MS
2.6. Data Analysis
3. Results
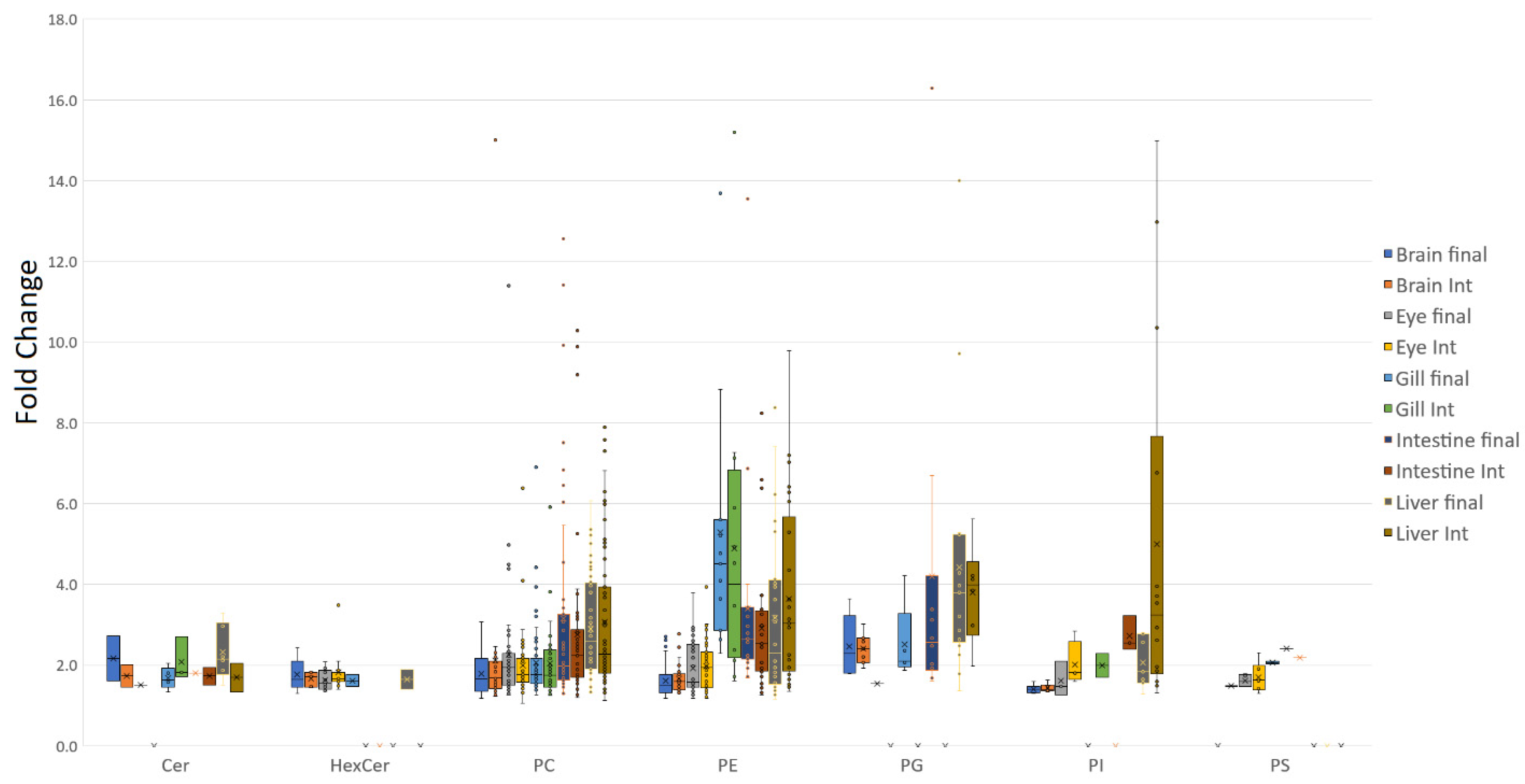
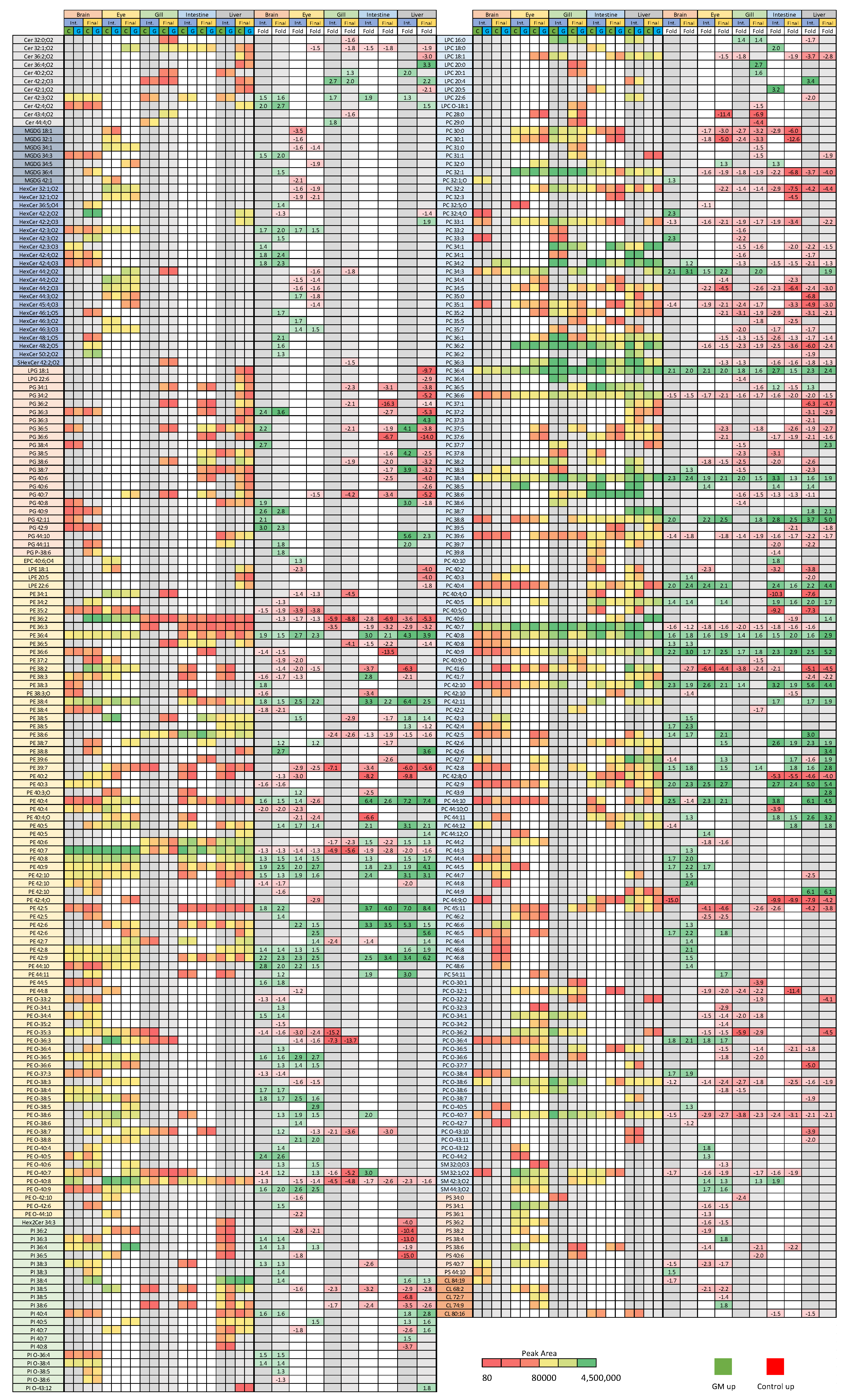
3.1. Tissue Responses to GM and Control Diets
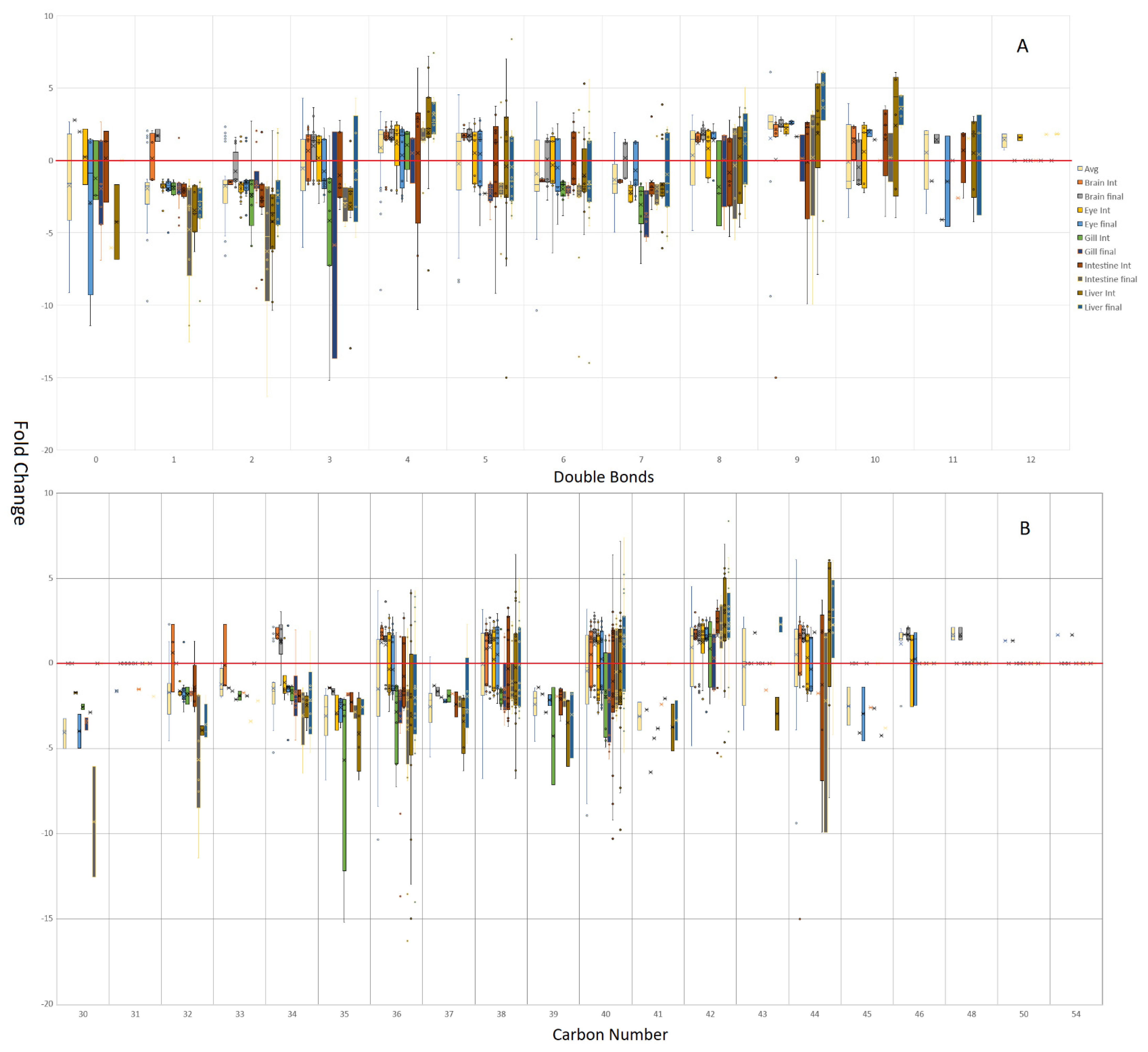
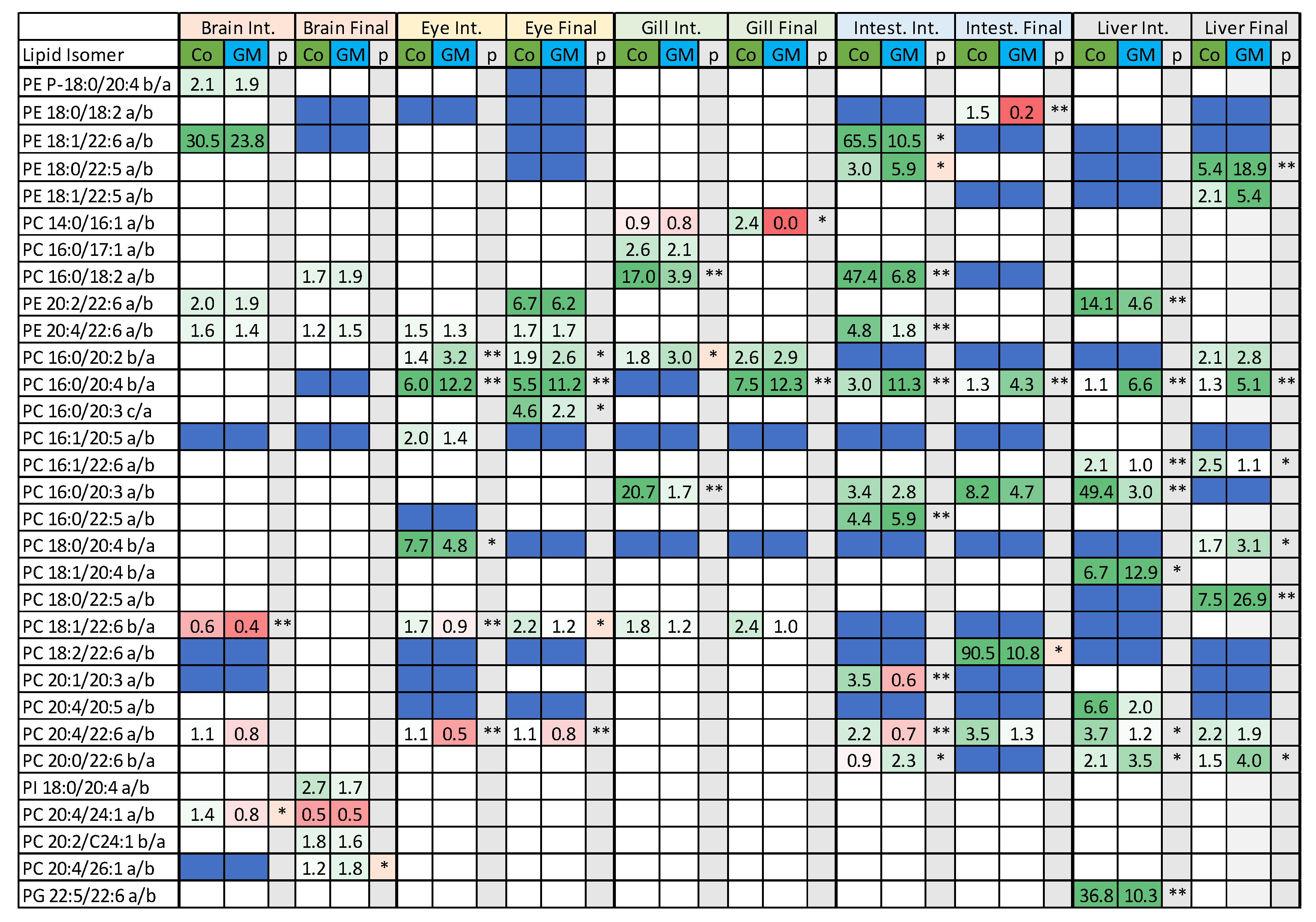
3.2. Isomeric Analysis
4. Discussion
4.1. Lipid Class Trends
4.2. Tissue Plasticity
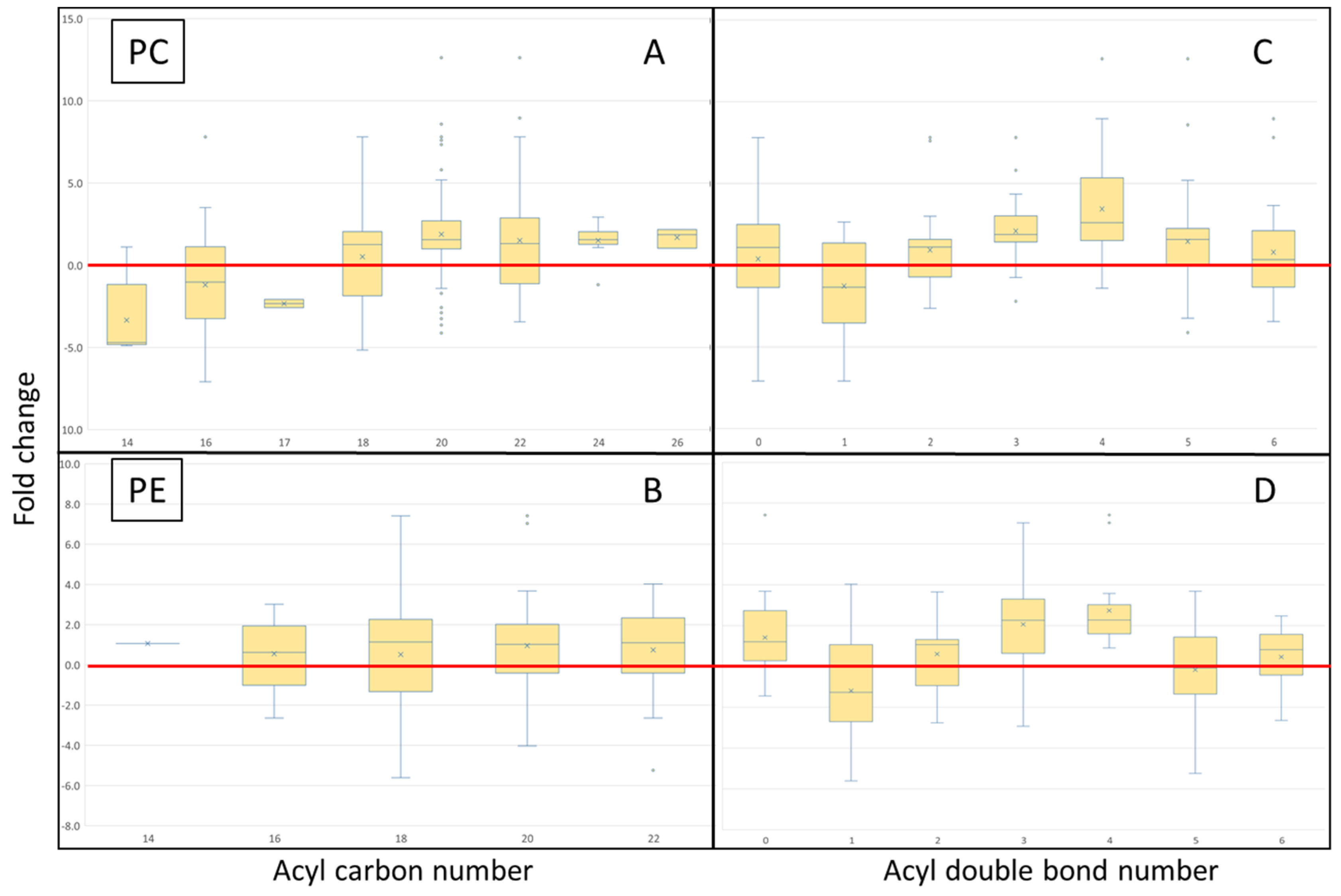
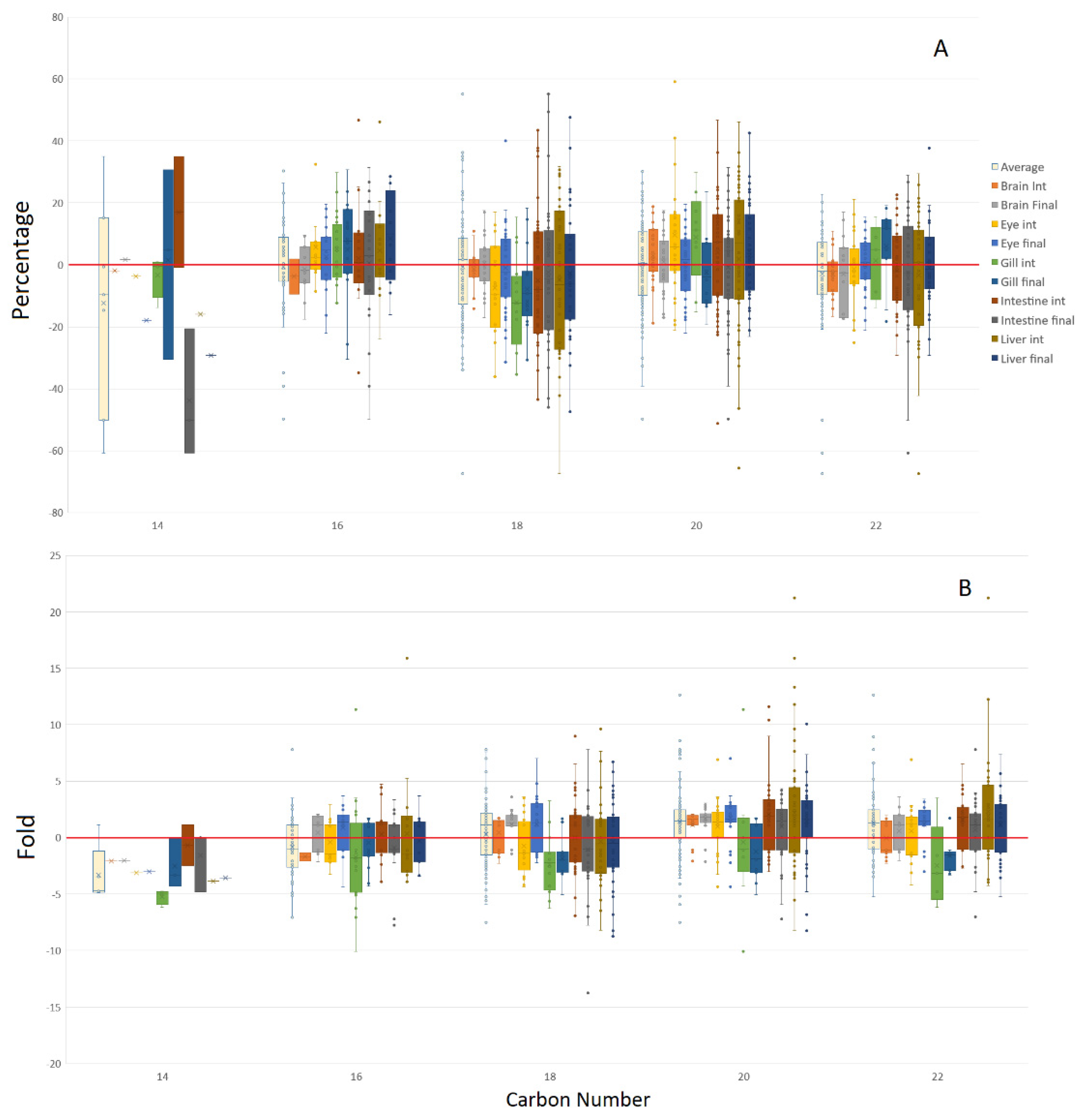
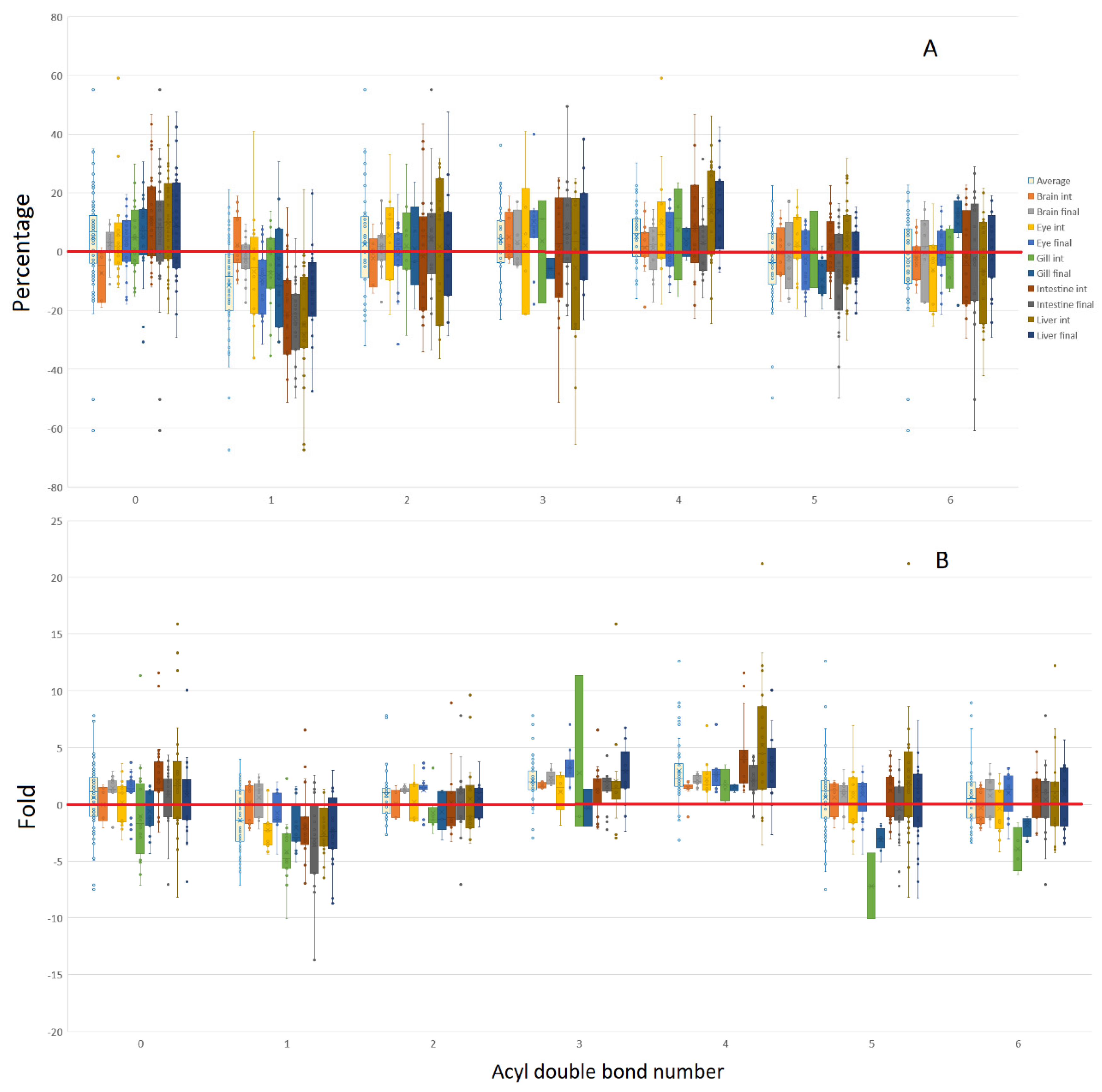
4.3. Acyl Complexity and Counter Trends
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020; Volume 32, p. 244. [Google Scholar] [CrossRef]
- Barange, M.; Bahri, T.; Beveridge, M.; Cochrane, K.; Funge-Smith, S.; Poulain, F. Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; FAO: Rome, Italy, 2018; pp. 1–628. [Google Scholar]
- Sprague, M.; Chau, T.C.; Givens, D.I. Iodine Content of Wild and Farmed Seafood and Its Estimated Contribution to UK Dietary Iodine Intake. Nutrients 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Fawcett, S.; Betancor, M.; Struthers, W.; Tocher, D. Variation in the nutritional composition of farmed Atlantic salmon (Salmo salar L.) fillets with emphasis on EPA and DHA contents. J. Food Compos. Anal. 2020, 94, 103618. [Google Scholar] [CrossRef]
- de Roos, B.; Sneddon, A.A.; Sprague, M.; Horgan, G.W.; Brouwer, I.A. The potential impact of compositional changes in farmed fish on its health-giving properties: Is it time to reconsider current dietary recommendations? Public Health Nutr. 2017, 20, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Jónasdóttir, S.H.; Visser, A.W.; Richardson, K.; Heath, M.R. Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proc. Natl. Acad. Sci. USA 2015, 112, 12122–12126. [Google Scholar] [CrossRef] [PubMed]
- Cavan, E.L.; Belcher, A.; Atkinson, A.; Hill, S.L.; Kawaguchi, S.; McCormack, S.; Meyer, B.; Nicol, S.; Ratnarajah, L.; Schmidt, K.; et al. The importance of Antarctic krill in biogeochemical cycles. Nat. Commun. 2019, 10, 4742. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.A.; Bevan, S.A.; Gachotte, D.J.; Larsen, C.M.; Moskal, W.A.; Merlo, P.A.O.; Sidorenko, L.V.; Hampton, R.E.; Stoltz, V.; Pareddy, D.; et al. Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat. Biotechnol. 2016, 34, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Zhou, X.-R.; Leonforte, A.; McAllister, J.; Shrestha, P.; Kennedy, Y.; Belide, S.; Buzza, G.; Gororo, N.; Gao, W.; et al. Development of a Brassica napus (Canola) Crop Containing Fish Oil-Like Levels of DHA in the Seed Oil. Front. Plant Sci. 2020, 11, 727. [Google Scholar] [CrossRef]
- Napier, J.A.; Olsen, R.; Tocher, D. Update on GM canola crops as novel sources of omega-3 fish oils. Plant Biotechnol. J. 2018, 17, 703–705. [Google Scholar] [CrossRef]
- Han, L.; Usher, S.; Sandgrind, S.; Hassall, K.L.; Sayanova, O.; Michaelson, L.V.; Haslam, R.; Napier, J.A. High level accumulation of EPA and DHA in field-grown transgenic Camelina—A multi-territory evaluation of TAG accumulation and heterogeneity. Plant Biotechnol. J. 2020, 18, 2280–2291. [Google Scholar] [CrossRef]
- Usher, S.; Han, L.; Haslam, R.; Michaelson, L.V.; Sturtevant, D.; Aziz, M.; Chapman, K.D.; Sayanova, O.; Napier, J.A. Tailoring seed oil composition in the real world: Optimising omega-3 long chain polyunsaturated fatty acid accumulation in transgenic Camelina sativa. Sci. Rep. 2017, 7, 6570. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Stubhaug, I.; Igapharraguerre, I.; Rimbach, G.; Menoyo, D. Positional Distribution of Fatty Acids in Triacylglycerols and Phospholipids from Fillets of Atlantic Salmon (Salmo Salar) Fed Vegetable and Fish Oil Blends. Mar. Drugs 2015, 13, 4255–4269. [Google Scholar] [CrossRef] [PubMed]
- Moharana, T.R.; Byreddy, A.R.; Puri, M.; Barrow, C.; Rao, N.M. Selective Enrichment of Omega-3 Fatty Acids in Oils by Phospholipase A1. PLoS ONE 2016, 11, e0151370. [Google Scholar] [CrossRef]
- Ando, Y.; Satake, M.; Takahashi, Y. Reinvestigation of positional distribution of fatty acids in docosahexaenoic acid-rich fish oil triacyl-sn-glycerols. Lipids 2000, 35, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Adcock, J.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic Engineering Camelina sativa with Fish Oil-Like Levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. High Sn-2 Docosahexaenoic Acid Lipids for Brain Benefits, and Their Enzymatic Syntheses: A Review. Engineering 2020, 6, 424–431. [Google Scholar] [CrossRef]
- Lankhorst, P.P.; Chang, A.-N. The Application of NMR in Compositional and Quantitative Analysis of Oils and Lipids. In Modern Magnetic Resonance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1743–1764. [Google Scholar] [CrossRef]
- van Erp, H.; Bryant, F.M.; Martin-Moreno, J.; Michaelson, L.V.; Bhutada, G.; Eastmond, P.J. Engineering the stereoisomeric structure of seed oil to mimic human milk fat. Proc. Natl. Acad. Sci. USA 2019, 116, 20947–20952. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Broughton, R.; Tocher, D.R.; Betancor, M.B. Development of a C18 Supercritical Fluid Chromatography-Tandem Mass Spectrometry Methodology for the Analysis of Very-Long-Chain Polyunsaturated Fatty Acid Lipid Matrices and Its Application to Fish Oil Substitutes Derived from Genetically Modified Oilseeds in the Aquaculture Sector. ACS Omega 2020, 5, 22289–22298. [Google Scholar] [CrossRef]
- Nakanishi, H.; Iida, Y.; Shimizu, T.; Taguchi, R. Separation and quantification of sn-1 and sn-2 fatty acid positional isomers in phosphatidylcholine by RPLC-ESIMS/MS. J. Biochem. 2010, 147, 245–256. [Google Scholar] [CrossRef]
- Betancor, M.; Sprague, M.; Sayanova, O.; Usher, S.; Campbell, P.; Napier, J.; Caballero, M.; Tocher, D. Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): Effects on tissue fatty acid composition, histology and gene expression. Aquaculture 2015, 444, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Howarth, F.J.; Glencross, B.D.; Tocher, D.R. Influence of dietary docosahexaenoic acid in combination with other long-chain polyunsaturated fatty acids on expression of biosynthesis genes and phospholipid fatty acid compositions in tissues of post-smolt Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 172-173, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, C.; You, C.; Chen, B.; Wang, S.; Li, Y. Effects of different dietary ratios of docosahexaenoic to eicosapentaenoic acid (DHA/EPA) on the growth, non-specific immune indices, tissue fatty acid compositions and expression of genes related to LC-PUFA biosynthesis in juvenile golden pompano Trachinotus ovatus. Aquaculture 2019, 505, 488–495. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Amlund, H.; Torstensen, B.E. Dietary lipids modulate methylmercury toxicity in Atlantic salmon. Food Chem. Toxicol. 2011, 49, 3258–3271. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ueno, M.; Kubo, K.; Yamaguchi, M. Dose-Response Effect of Dietary Docosahexaenoic Acid on Fatty Acid Profiles of Serum and Tissue Lipids in Rats. J. Agric. Food Chem. 1998, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kitajka, K.; Puskás, L.G.; Zvara, Á.; Hackler, L.; Barceló-Coblijn, G.; Yeo, Y.K.; Farkas, T. The role of n-3 polyunsaturated fatty acids in brain: Modulation of rat brain gene expression by dietary n-3 fatty acids. Proc. Natl. Acad. Sci. USA 2002, 99, 2619–2624. [Google Scholar] [CrossRef]
- Borchman, D.; Yappert, M.C. Lipids and the ocular lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Brush, R.S.; Tran, J.-T.A.; Henry, K.R.; McClellan, M.E.; Elliott, M.H.; Mandal, N.A. Retinal Sphingolipids and Their Very-Long-Chain Fatty Acid–Containing Species. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4422–4431. [Google Scholar] [CrossRef] [Green Version]
- Schlame, M.; Xu, Y.; Erdjument-Bromage, H.; Neubert, T.A.; Ren, M. Lipidome-wide 13C flux analysis: A novel tool to estimate the turnover of lipids in organisms and cultures. J. Lipid Res. 2020, 61, 95–104. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 1183–1194. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Haslam, R.P.; Usher, S.; Napier, J.A.; Sayanova, O. An alternative pathway for the effective production of the omega-3 long-chain polyunsaturates EPA and ETA in transgenic oilseeds. Plant Biotechnol. J. 2015, 13, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Sasaki, K.; Watanabe, H.; Nagao, K.; Inoue, N.; Shirouchi, B.; Yanagita, T.; Nagai, T.; Mizobe, H.; Kojima, K.; et al. Differential effects of triacylglycerol positional isomers containing n-3 series highly unsaturated fatty acids on lipid metabolism in C57BL/6J mice. J. Nutr. Biochem. 2015, 26, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Damen, C.W.N.; Isaac, G.; Langridge, J.; Hankemeier, T.; Vreeken, R.J. Enhanced lipid isomer separation in human plasma using reversed-phase UPLC with ion-mobility/high-resolution MS detection. J. Lipid Res. 2014, 55, 1772–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Control | GM | |
|---|---|---|
| Feed ingredients (%) | ||
| Fishmeal a | 7.5 | 7.5 |
| Vegetable protein a | 36.1 | 36.1 |
| Land animal protein a | 10.0 | 10.0 |
| Wheat a | 11.2 | 11.2 |
| Fish oil a | 10.8 | - |
| Rapeseed oil a | 20.8 | - |
| Camelina oil (transgenic) b | - | 31.6 |
| Premix c | 3.6 | 3.6 |
| Yttrium oxide c | 0.005 | 0.005 |
| Fatty acid profile (%) | ||
| Total saturated1 | 18.1 | 14.7 |
| Total monoenes2 | 49.1 | 22.2 |
| 18:2n-6 | 15.2 | 19.6 |
| 18:3n-6 | 0.1 | 1.5 |
| 20:4n-6 | 0.4 | 1.7 |
| Total n-6 PUFA | 16.0 | 25.2 |
| 18:3n-3 | 5.8 | 19.1 |
| 20:5n-3 | 4.8 | 5.7 |
| 22:5n-3 | 0.7 | 3.8 |
| 22:6n-3 | 2.4 | 5.3 |
| Total n-3 PUFA | 15.1 | 37.7 |
| MOPA | ||
| Moisture % | 6 | 6 |
| Energy–crude (MJ/Kg) | 26 | 26 |
| Protein–crude (%) | 36 | 36 |
| Fat–crude (%) | 36 | 36 |
| Ash (%) | 4.2 | 4.2 |
| Solvent | ||||
|---|---|---|---|---|
| Time | A | B | C | D |
| 0 | 100 | 0 | 0 | 0 |
| 5 | 100 | 0 | 0 | 0 |
| 5.1 | 0 | 100 | 0 | 0 |
| 18.5 | 0 | 100 | 0 | 0 |
| 35 | 0 | 50.2 | 49.8 | 0 |
| 42 | 0 | 20 | 80 | 0 |
| 42.1 | 35 | 0 | 0 | 65 |
| 52 | 35 | 0 | 0 | 65 |
| 56 | 100 | 0 | 0 | 0 |
| 61 | 100 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broughton, R.; Tocher, D.R.; Napier, J.A.; Betancor, M.B. Profiling Phospholipids within Atlantic Salmon Salmo salar with Regards to a Novel Terrestrial Omega-3 Oil Source. Metabolites 2022, 12, 851. https://doi.org/10.3390/metabo12090851
Broughton R, Tocher DR, Napier JA, Betancor MB. Profiling Phospholipids within Atlantic Salmon Salmo salar with Regards to a Novel Terrestrial Omega-3 Oil Source. Metabolites. 2022; 12(9):851. https://doi.org/10.3390/metabo12090851
Chicago/Turabian StyleBroughton, Richard, Douglas R. Tocher, Johnathan A. Napier, and Mónica B. Betancor. 2022. "Profiling Phospholipids within Atlantic Salmon Salmo salar with Regards to a Novel Terrestrial Omega-3 Oil Source" Metabolites 12, no. 9: 851. https://doi.org/10.3390/metabo12090851
APA StyleBroughton, R., Tocher, D. R., Napier, J. A., & Betancor, M. B. (2022). Profiling Phospholipids within Atlantic Salmon Salmo salar with Regards to a Novel Terrestrial Omega-3 Oil Source. Metabolites, 12(9), 851. https://doi.org/10.3390/metabo12090851








