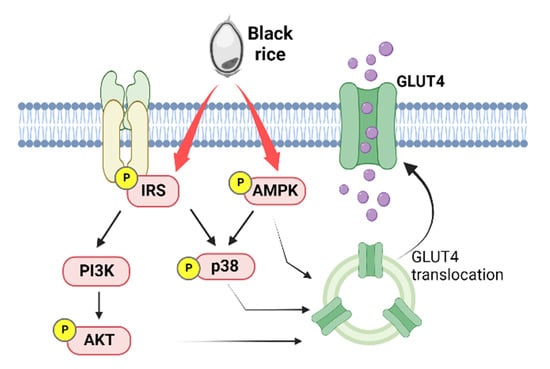
Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Black Rice Extracts
2.2. Cell Culture and Differentiation
2.3. Cell Viability Assay
2.4. Total Anthocyanin Content Analysis
2.5. Glucose Uptake Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of Black Rice Extracts on Cell Viability in C2C12 Myotubes
3.2. Effects of HBRE and CBRE on Glucose Uptake in C2C12 Myotubes
3.3. Effects of HBRE and CBRE on IRS/PI3K/Akt Signaling Pathways
3.4. Effects of HBRE and CBRE on AMPK/p38 MAPK/ERK Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 30 May 2022).
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., 3rd; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tunduguru, R.; Thurmond, D.C. Promoting Glucose Transporter-4 Vesicle Trafficking along Cytoskeletal Tracks: PAK-Ing Them Out. Front. Endocrinol. 2017, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.M.; Stock, J.L.; McCoid, S.C.; Stukenbrok, H.A.; Pessin, J.E.; Stevenson, R.W.; Milici, A.J.; McNeish, J.D. Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J. Clin. Investig. 1995, 95, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Kanai, F.; Ito, K.; Todaka, M.; Hayashi, H.; Kamohara, S.; Ishii, K.; Okada, T.; Hazeki, O.; Ui, M.; Ebina, Y. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem. Biophys. Res. Commun. 1993, 195, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Shrestha, M.M.; Lim, C.Y.; Bi, X.; Robinson, R.C.; Han, W. Tmod3 Phosphorylation Mediates AMPK-Dependent GLUT4 Plasma Membrane Insertion in Myoblasts. Front. Endocrinol. 2021, 12, 653557. [Google Scholar] [CrossRef]
- Jessen, N.; Pold, R.; Buhl, E.S.; Jensen, L.S.; Schmitz, O.; Lund, S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J. Appl. Physiol. 2003, 94, 1373–1379. [Google Scholar] [CrossRef]
- Nayeem, S.; Venkidasamy, B.; Sundararajan, S.; Kuppuraj, S.P.; Ramalingam, S. Differential expression of flavonoid biosynthesis genes and biochemical composition in different tissues of pigmented and non-pigmented rice. J. Food Sci. Technol. 2021, 58, 884–893. [Google Scholar] [CrossRef]
- Dias, A.L.S.; Pachikian, B.; Larondelle, Y.; Quetin-Leclercq, J. Recent advances on bioactivities of black rice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 470–476. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Tantipaiboonwong, P.; Pintha, K.; Chaiwangyen, W.; Chewonarin, T.; Pangjit, K.; Chumphukam, O.; Kangwan, N.; Suttajit, M. Anti-hyperglycaemic and anti-hyperlipidaemic effects of black and red rice in streptozotocin-induced diabetic rats. Scienceasia 2017, 43, 281. [Google Scholar] [CrossRef]
- Xu, P.T.; Song, Z.; Zhang, W.C.; Jiao, B.; Yu, Z.B. Impaired translocation of GLUT4 results in insulin resistance of atrophic soleus muscle. BioMed. Res. Int. 2015, 2015, 291987. [Google Scholar] [CrossRef] [PubMed]
- Ganasegeran, K.; Hor, C.P.; Jamil, M.F.A.; Loh, H.C.; Noor, J.M.; Hamid, N.A.; Suppiah, P.D.; Abdul Manaf, M.R.; Ch’ng, A.S.H.; Looi, I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5723. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Helmyati, S.; Kiasaty, S.; Amalia, A.W.; Sholihah, H.; Kurnia, M.; Wigati, M.; Rohana, A.J.; Ishak, W.R.W.; Hamid, N.A.; Malik, V.; et al. Substituting white rice with brown and black rice as an alternative to prevent diabetes mellitus type 2: A case-study among young adults in Yogyakarta, Indonesia. J. Diabetes Metab. Disord. 2020, 19, 749–757. [Google Scholar] [CrossRef]
- Ding, W.; Liu, H.; Qin, Z.; Liu, M.; Zheng, M.; Cai, D.; Liu, J. Dietary Antioxidant Anthocyanins Mitigate Type II Diabetes through Improving the Disorder of Glycometabolism and Insulin Resistance. J. Agric. Food Chem. 2021, 69, 13350–13363. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.; Santander, F.; Paz, R. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Inv. Agr. 2014, 41, 49–60. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Guo, Q.; Xu, L.; Santhanam, R.K.; Gao, X.; Wang, C.; Panichayupakaranant, P.; Chen, H. Anthocyanins from dietary black soybean potentiate glucose uptake in L6 rat skeletal muscle cells via up-regulating phosphorylated Akt and GLUT4. J. Funct. Foods 2019, 52, 663–669. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Cyanidin-3-rutinoside increases glucose uptake by activating the PI3K/Akt pathway in 3T3-L1 adipocytes. Environ. Toxicol. Pharmacol. 2017, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baldini, G.; Hohman, R.; Charron, M.J.; Lodish, H.F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J. Biol. Chem. 1991, 266, 4037–4040. [Google Scholar] [CrossRef]
- de Vries-Smits, A.M.; Burgering, B.M.; Leevers, S.J.; Marshall, C.J.; Bos, J.L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature 1992, 357, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Zhou, J.; Luo, L.P.; Han, B.; Li, F.; Chen, J.Y.; Zhu, Y.F.; Chen, W.; Yu, X.P. Black Rice Anthocyanins Suppress Metastasis of Breast Cancer Cells by Targeting RAS/RAF/MAPK Pathway. BioMed. Res. Int. 2015, 2015, 414250. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, K.; Konrad, D.; Klip, A.; Marette, A. The AMP-activated protein kinase activator AICAR does not induce GLUT4 translocation to transverse tubules but stimulates glucose uptake and p38 mitogen-activated protein kinases alpha and beta in skeletal muscle. FASEB J. 2003, 17, 1658–1665. [Google Scholar] [CrossRef]
- Musi, N.; Goodyear, L.J. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol. Scand. 2003, 178, 337–345. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Holman, G.D.; Marley, A.; James, D.E.; Stockli, J.; Coster, A.C. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J. Biol. Chem. 2010, 285, 1653–1660. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Sozen, B.; Ozturk, S.; Yaba, A.; Demir, N. The p38 MAPK signalling pathway is required for glucose metabolism, lineage specification and embryo survival during mouse preimplantation development. Mech. Dev. 2015, 138 Pt 3, 375–398. [Google Scholar] [CrossRef]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.-Y.; Wu, S.-J.; Chang, Y.-C.; Ng, L.-T.; Chang, S.-J. Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites 2022, 12, 856. https://doi.org/10.3390/metabo12090856
Feng S-Y, Wu S-J, Chang Y-C, Ng L-T, Chang S-J. Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites. 2022; 12(9):856. https://doi.org/10.3390/metabo12090856
Chicago/Turabian StyleFeng, Shui-Yuan, Shu-Jing Wu, Yun-Ching Chang, Lean-Teik Ng, and Sue-Joan Chang. 2022. "Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells" Metabolites 12, no. 9: 856. https://doi.org/10.3390/metabo12090856
APA StyleFeng, S.-Y., Wu, S.-J., Chang, Y.-C., Ng, L.-T., & Chang, S.-J. (2022). Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites, 12(9), 856. https://doi.org/10.3390/metabo12090856