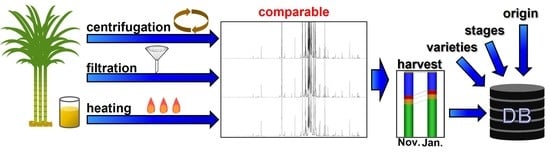
Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Preparation for NMR Measurements
2.4. NMR Measurements
2.5. Data Analysis
3. Results
3.1. Metabolomic Evaluation of Compositional Variability in Sugarcane Juice
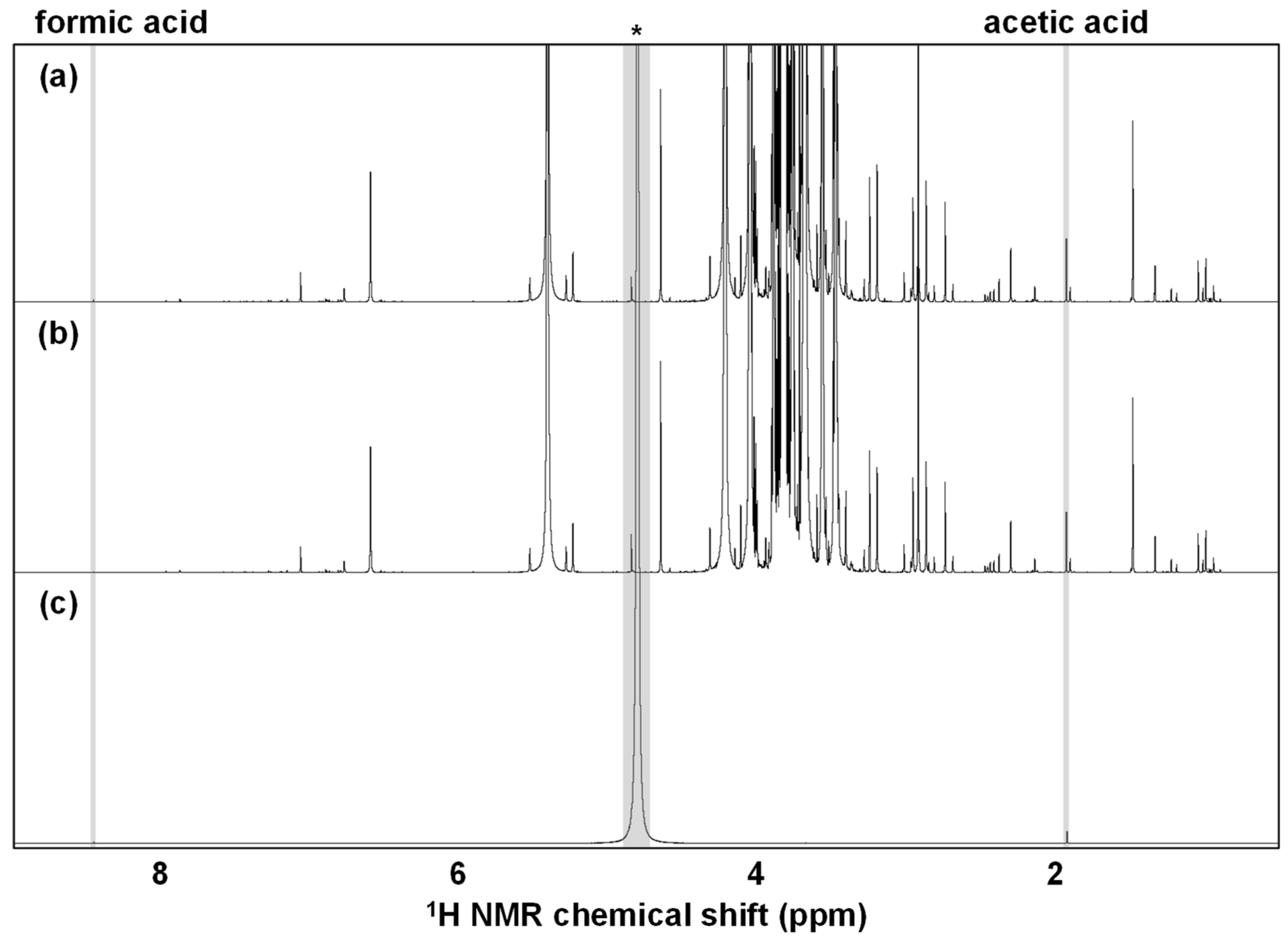
3.1.1. Filtration and Centrifugation Process-Related Effect
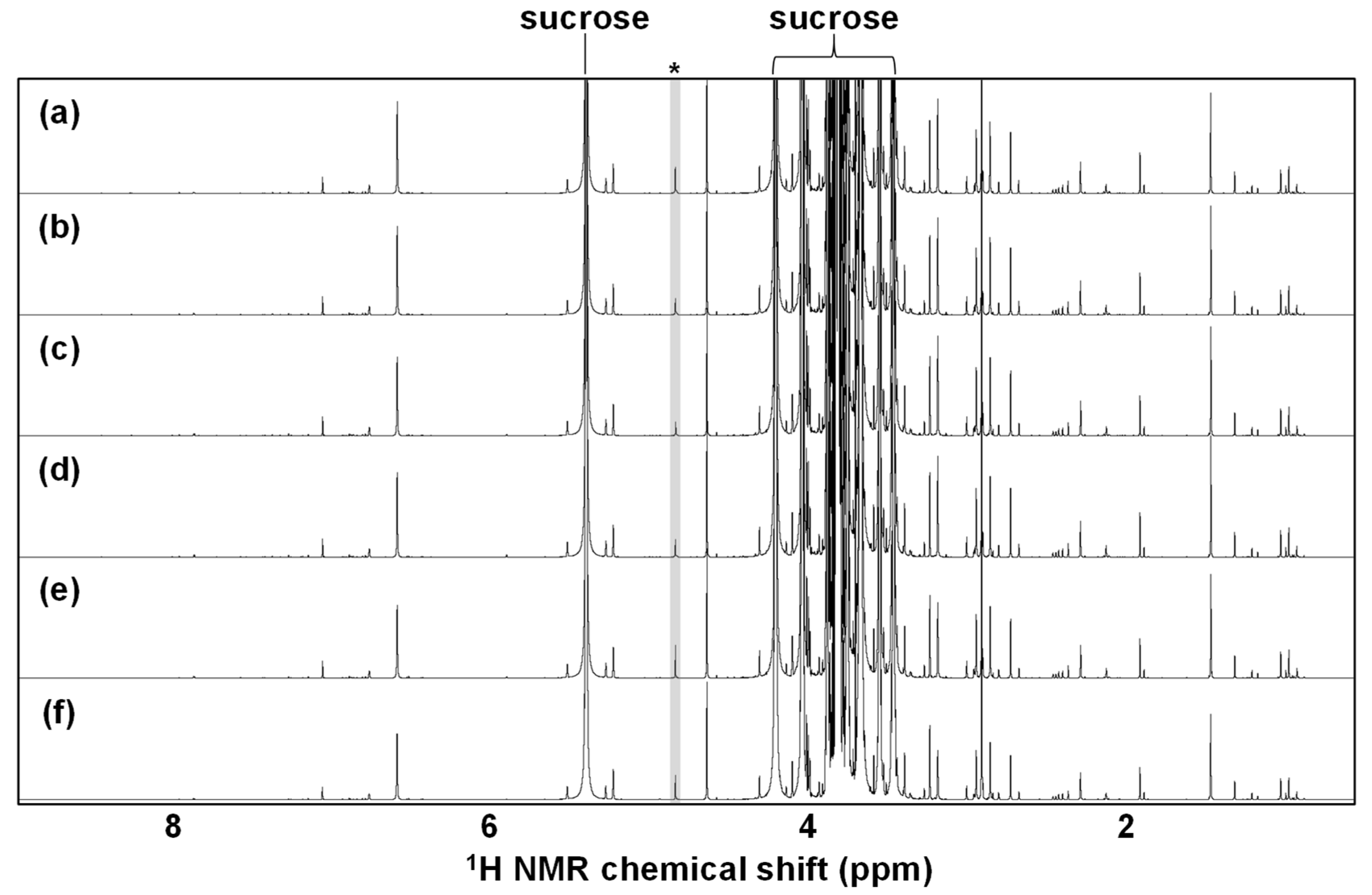
3.1.2. Thermal Treatment-Related Effect
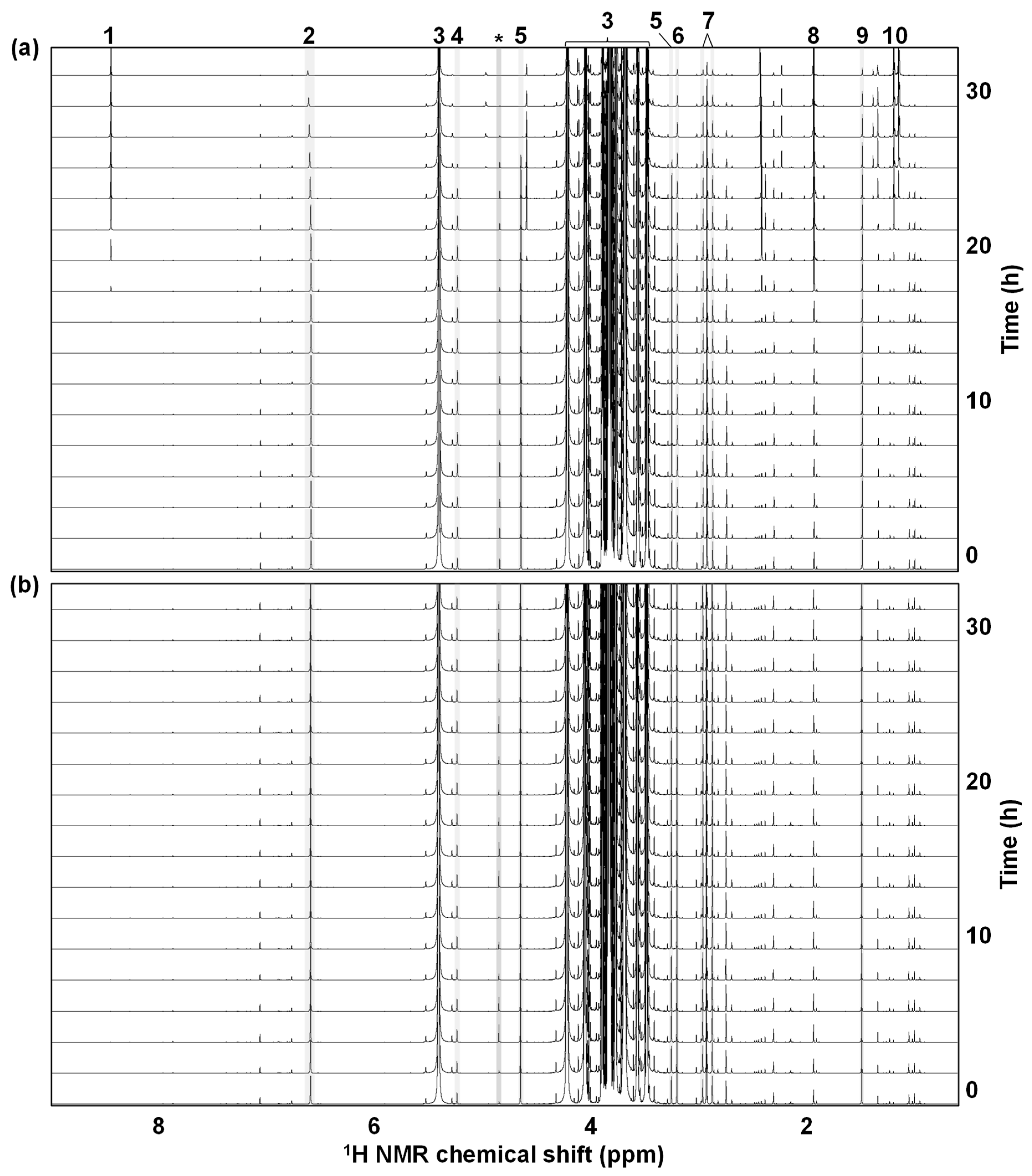
3.1.3. Metabolite Alterations during the NMR Measurement
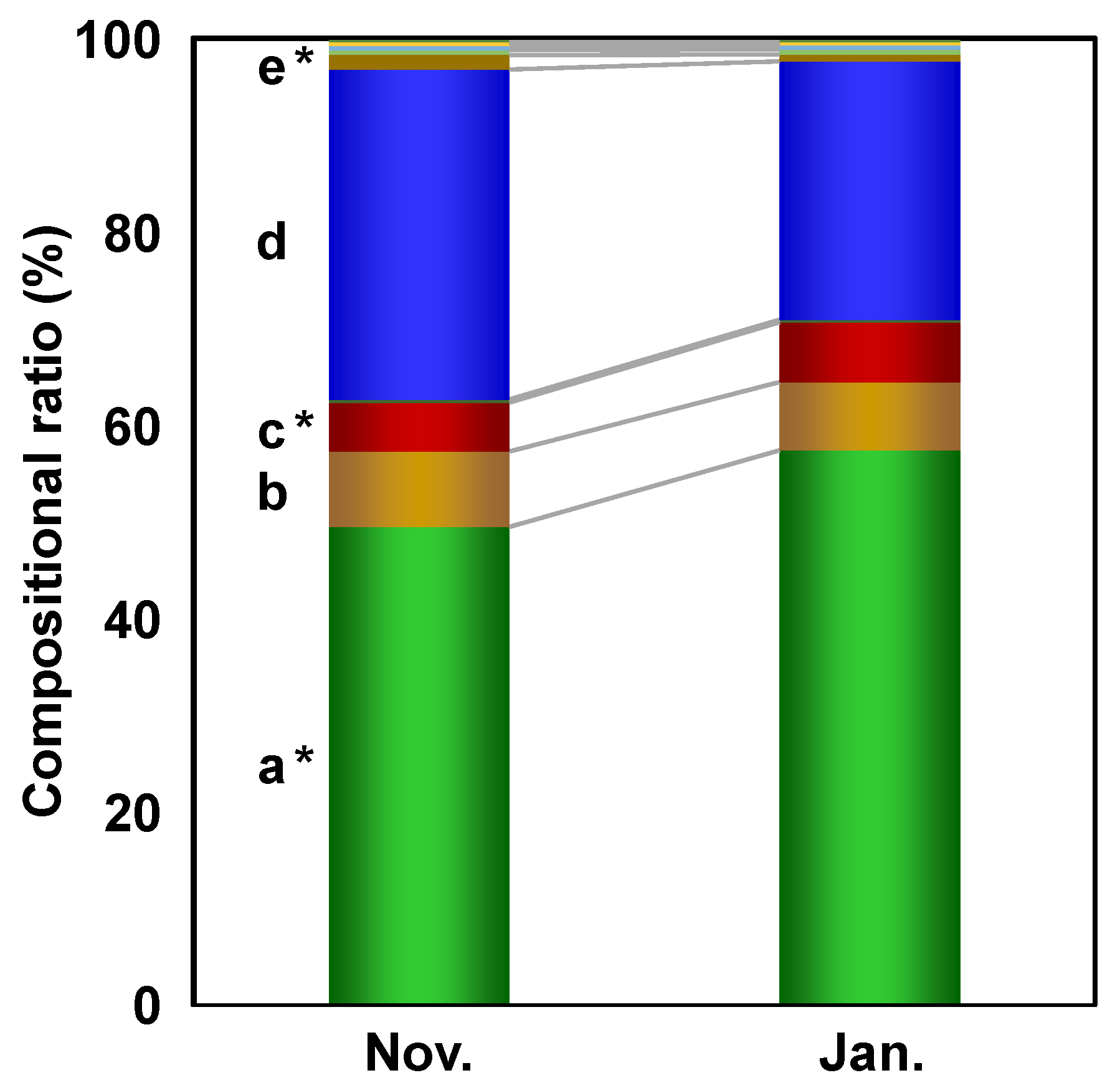
3.2. Metabolomic Characterization Based on the Harvest Period-Related Differences
3.2.1. Sugarcane Harvest Period Classification Model
3.2.2. Important Metabolites Contributed to the Harvest Period Discrimination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terajima, Y.; Hattori, T.; Shimatani, M.; Sato, M.; Takaragawa, H.; Sakaigaichi, T.; Umeda, M.; Naito, T.; Irei, S. Sugarcane breeding and supporting genetics research in Japan. Sugar Tech. 2022, 24, 134–150. [Google Scholar] [CrossRef]
- Matsuoka, M. Sugarcane cultivation and sugar industry in Japan. Sugar Tech. 2006, 8, 3–9. [Google Scholar] [CrossRef]
- Mustafa, G.; Joyia, F.A.; Anwar, S.; Parvaiz, A.; Khan, M.S. Biotechnological interventions for the improvement of sugarcane crop and sugar production. In Sugarcane—Technology and Research; IntechOpen: London, UK, 2018; pp. 113–138. [Google Scholar]
- Ali, A.; Khan, M.; Sharif, R.; Mujtaba, M.; Gao, S.J. Sugarcane Omics: An Update on the Current Status of Research and Crop Improvement. Plants 2019, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Baker, J.M.; Miller, S.J.; Deborde, C.; Maucourt, M.; Biais, B.; Rolin, D.; Moing, A.; Moco, S.; Vervoort, J.; et al. An inter-laboratory comparison demonstrates that [H]-NMR metabolite fingerprinting is a robust technique for collaborative plant metabolomic data collection. Metabolomics 2010, 6, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R. The potentiality of NMR-based metabolomics in food science and food authentication assessment. Magn. Reson. Chem. 2019, 57, 558–578. [Google Scholar] [CrossRef]
- Brennan, L. NMR-based metabolomics: From sample preparation to applications in nutrition research. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 83, 42–49. [Google Scholar] [CrossRef]
- Mahmud, I.; Shrestha, B.; Boroujerdi, A.; Chowdhury, K. NMR-based metabolomics profile comparisons to distinguish between embryogenic and non-embryogenic callus tissue of sugarcane at the biochemical level. Vitr. Cell. Dev. Biol. Plant 2015, 51, 340–349. [Google Scholar] [CrossRef]
- Mahmud, I.; Thapaliya, M.; Boroujerdi, A.; Chowdhury, K. NMR-based metabolomics study of the biochemical relationship between sugarcane callus tissues and their respective nutrient culture media. Anal. Bioanal. Chem. 2014, 406, 5997–6005. [Google Scholar] [CrossRef]
- Sabino, A.R.; Tavares, S.S.; Riffel, A.; Li, J.V.; Oliveira, D.J.; Feres, C.I.; Henrique, L.; Oliveira, J.S.; Correia, G.D.; Sabino, A.R. 1H NMR metabolomic approach reveals chlorogenic acid as a response of sugarcane induced by exposure to Diatraea saccharalis. Ind. Crops Prod. 2019, 140, 111651. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Baker, J.M.; Ward, J.L.; Beale, M.H.; Creste, S.; Cavalheiro, A.J. Metabolite Profiling of Sugarcane Genotypes and Identification of Flavonoid Glycosides and Phenolic Acids. J. Agric. Food Chem. 2016, 64, 4198–4206. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lal, U.R.; Mukhtar, H.M.; Singh, P.S.; Shah, G.; Dhawan, R.K. Phytochemical profile of sugarcane and its potential health aspects. Pharmacogn. Rev. 2015, 9, 45–54. [Google Scholar] [CrossRef]
- Hattori, T.; Terajima, Y.; Sakaigaichi, T.; Terauchi, T.; Tarumoto, Y.; Adachi, K.; Hayano, M.; Tanaka, M.; Ishikawa, S.; Umeda, M.; et al. High Ratoon Yield Sugarcane Cultivar “Harunoogi” Developed for Kumage Region by Using an Interspecific Hybrid between a Commercial Cultivar and Saccharum spontaneum L. J. NARO Res. Dev. 2019, 2, 21–44. [Google Scholar]
- Sakaigaichi, T.; Terauchi, T.; Matsuoka, M.; Terajima, Y.; Hattori, T.; Irei, S.; Ujihara, K.; Sugimoto, A.; Ishikawa, S.; Tanaka, M.; et al. Stalk Weight Type Sugarcane Variety “KTn03-54” with Early Maturing in the Kumage Region of Kagoshima Prefecture. Bull. NARO Agric. Res. Kyushu Okinawa Reg. 2017, 66, 1–20. [Google Scholar]
- Lewis, I.A.; Schommer, S.C.; Markley, J.L. rNMR: Open source software for identifying and quantifying metabolites in NMR spectra. Magn. Reson. Chem. 2009, 47 (Suppl. S1), S123–S126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 August 2022).
- Date, Y.; Kikuchi, J. Application of a Deep Neural Network to Metabolomics Studies and Its Performance in Determining Important Variables. Anal. Chem. 2018, 90, 1805–1810. [Google Scholar] [CrossRef]
- R Studio Team. R Studio: Integrated Development Environment for R; R Studio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 1 August 2022).
- Kikuchi, J.; Tsuboi, Y.; Komatsu, K.; Gomi, M.; Chikayama, E.; Date, Y. SpinCouple: Development of a Web Tool for Analyzing Metabolite Mixtures via Two-Dimensional J-Resolved NMR Database. Anal. Chem. 2016, 88, 659–665. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic. Acids. Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Ogura, T.; Date, Y.; Masukujane, M.; Coetzee, T.; Akashi, K.; Kikuchi, J. Improvement of physical, chemical, and biological properties of aridisol from Botswana by the incorporation of torrefied biomass. Sci. Rep. 2016, 6, 28011. [Google Scholar] [CrossRef]
- Sekiyama, Y.; Okazaki, K.; Kikuchi, J.; Ikeda, S. NMR-Based Metabolic Profiling of Field-Grown Leaves from Sugar Beet Plants Harbouring Different Levels of Resistance to Cercospora Leaf Spot Disease. Metabolites 2017, 7, 4. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
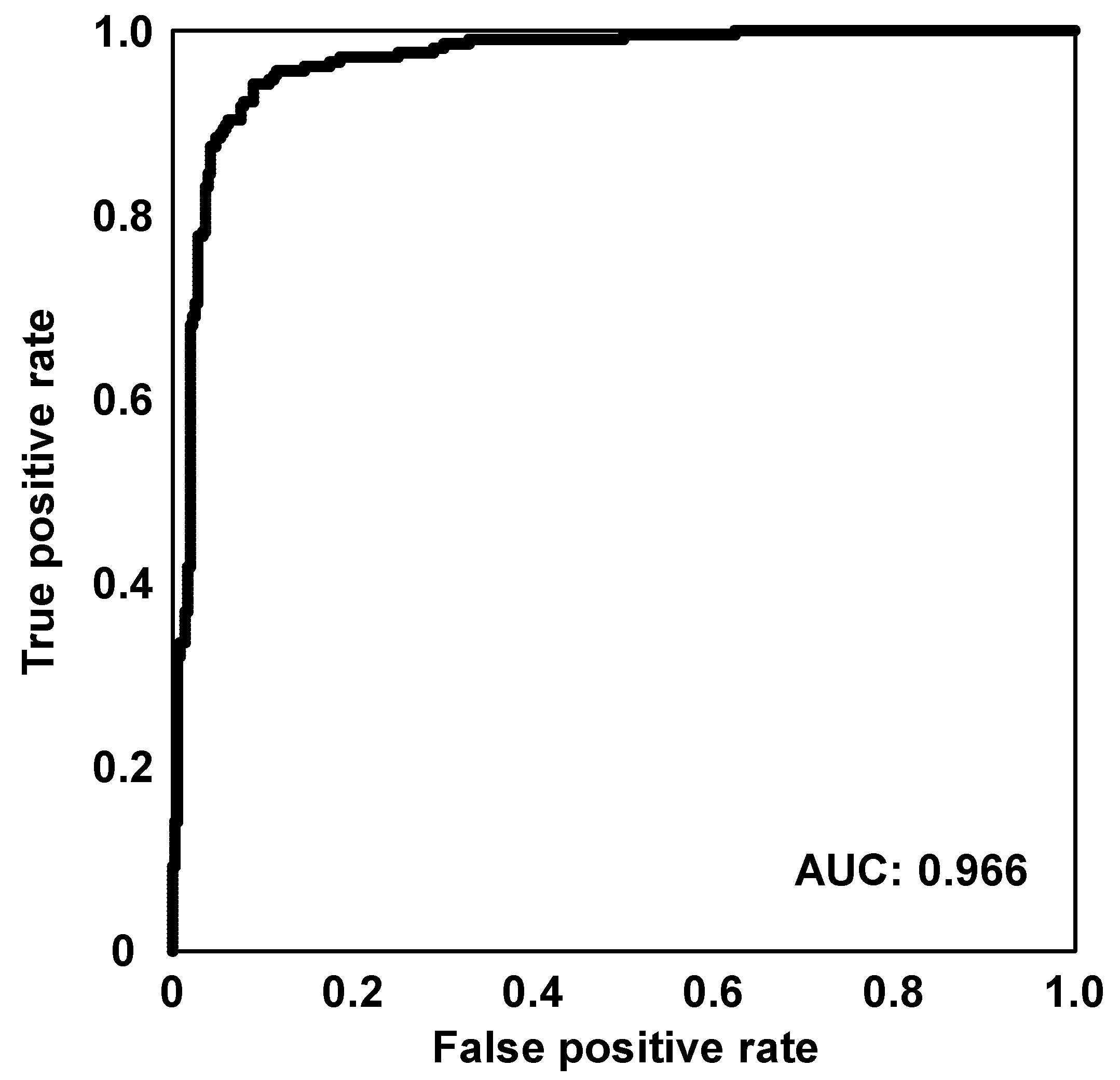

| Predict\Correct | November | January |
|---|---|---|
| November | 334.3 | 21.7 |
| January | 21.7 | 184.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Date, Y.; Ishikawa, C.; Umeda, M.; Tarumoto, Y.; Okubo, M.; Tamura, Y.; Ono, H. Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements. Metabolites 2022, 12, 862. https://doi.org/10.3390/metabo12090862
Date Y, Ishikawa C, Umeda M, Tarumoto Y, Okubo M, Tamura Y, Ono H. Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements. Metabolites. 2022; 12(9):862. https://doi.org/10.3390/metabo12090862
Chicago/Turabian StyleDate, Yasuhiro, Chiaki Ishikawa, Makoto Umeda, Yusuke Tarumoto, Megumi Okubo, Yasuaki Tamura, and Hiroshi Ono. 2022. "Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements" Metabolites 12, no. 9: 862. https://doi.org/10.3390/metabo12090862
APA StyleDate, Y., Ishikawa, C., Umeda, M., Tarumoto, Y., Okubo, M., Tamura, Y., & Ono, H. (2022). Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements. Metabolites, 12(9), 862. https://doi.org/10.3390/metabo12090862