Metabolomics as a Crucial Tool to Develop New Therapeutic Strategies for Neurodegenerative Diseases
Abstract
:1. Introduction
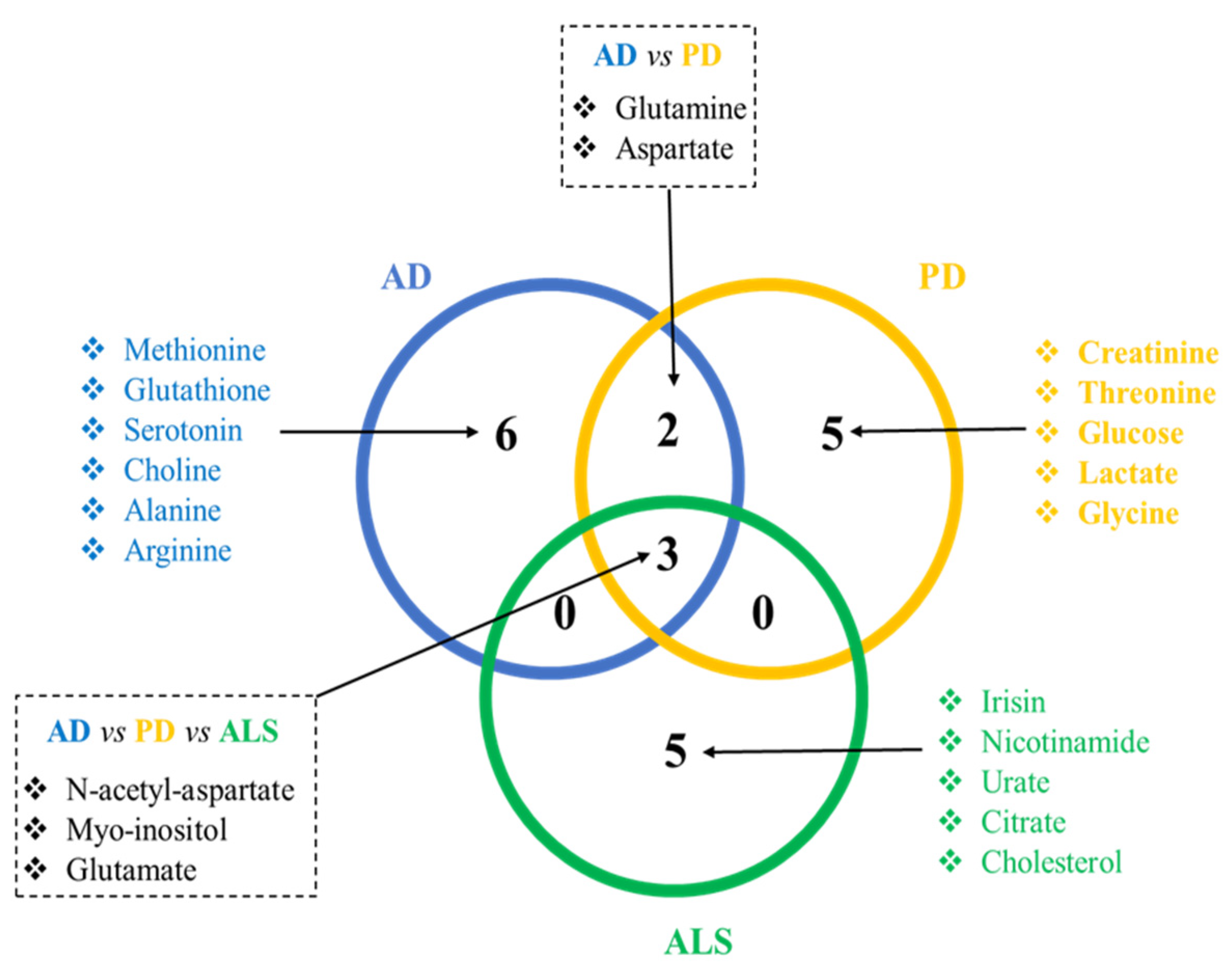
2. Common and Specific Metabolite Alterations among AD, ALS, and PD
2.1. Common Metabolite Alterations to the Three Diseases: N-acetyl-aspartate, Myo-inositol, and Glutamate
2.1.1. N-Acetyl-Aspartate (NAA)
2.1.2. Myo-Inositol (mIns)
2.1.3. Glutamate
2.2. Common between PD and AD: Glutamine and Aspartate
2.2.1. Glutamine
2.2.2. Aspartate
2.3. Sorted Metabolites for AD: Alanine, Arginine, Methionine, Glutathione, Choline, and Serotonin
2.4. Sorted Metabolites for PD: Creatinine, Threonine, Glycine, Glucose, and Lactate
2.5. Sorted Metabolites for ALS: Irisin, Nicotinamide, Urate, Citrate, and Cholesterol
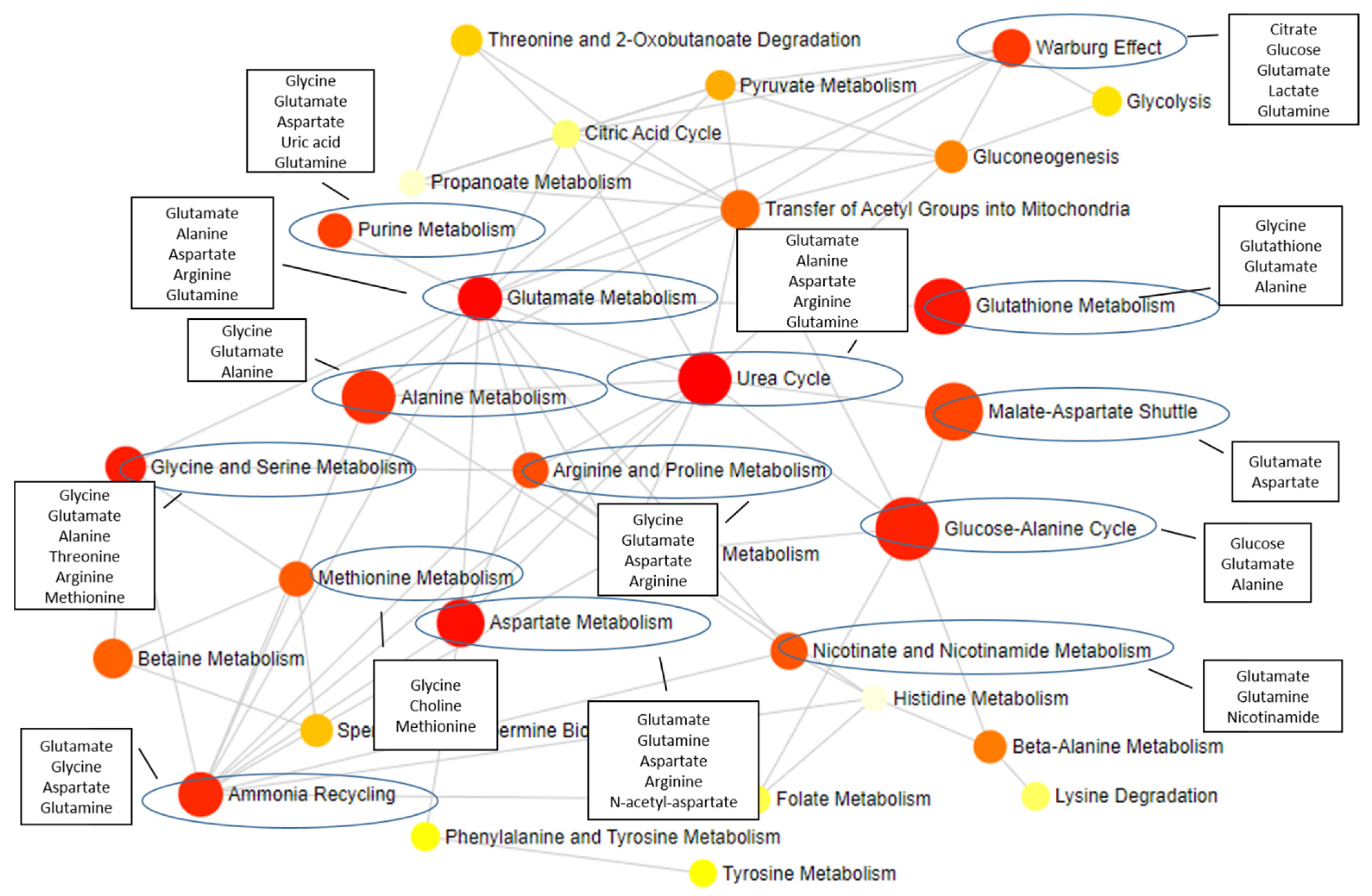
3. Correcting Metabolite Alterations by Targeted Therapy
4. Conclusions
Funding
Conflicts of Interest
References
- Vanni, S.; Baldeschi, A.C.; Zattoni, M.; Legname, G. Brain aging: A Ianus -faced player between health and neurodegeneration. J. Neurosci. Res. 2019, 98, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Gustincich, S.; Legname, G. Gene expression profiling and therapeutic interventions in neurodegenerative diseases: A comprehensive study on potentiality and limits. Expert Opin. Drug Discov. 2012, 7, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Wolska, B.; Hilbich, C.; Multhaup, G.; Martins, R.; Simms, G.; Beyreuther, K.; Masters, C.L. A4 amyloid protein deposition and the diagnosis of Alzheimer’s disease: Prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology 1988, 38, 1688. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Corcia, P.; Beltran, S.; Bakkouche, S.; Couratier, P. Therapeutic news in ALS. Rev. Neurol. 2021, 177, 544–549. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 10, a033118. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Dharshini, S.A.P.; Jemimah, S.; Taguchi, Y.H.; Gromiha, M.M. Exploring Common Therapeutic Targets for Neurodegenerative Disorders Using Transcriptome Study. Front. Genet. 2021, 12, 639160. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Kori, M.; Aydın, B.; Unal, S.; Arga, K.Y.; Kazan, D. Metabolic Biomarkers and Neurodegeneration: A Pathway Enrichment Analysis of Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. OMICS J. Integr. Biol. 2016, 20, 645–661. [Google Scholar] [CrossRef]
- Blasco, H.; Lanznaster, D.; Veyrat-Durebex, C.; Hergesheimer, R.; Vourch, P.; Maillot, F.; Andres, C.R.; Pradat, P.-F.; Corcia, P. Understanding and managing metabolic dysfunction in Amyotrophic Lateral Sclerosis. Expert Rev. Neurother. 2020, 20, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, P.R.; Castro, M.G. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail? Curr. Gene Ther. 2009, 9, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah-Daouk, R.; Zhu, H.; Sharma, S.; Bogdanov, M.; Rozen, S.G.; Matson, W.; Oki, N.O.; Motsinger-Reif, A.A.; Churchill, E.; Lei, Z.; et al. Alterations in metabolic pathways and networks in Alzheimer’s disease. Transl. Psychiatry 2013, 3, e244. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, H.C.; Greenwood, B.P.; Tolstikov, V.; Narain, N.R.; Kiebish, M.A.; Denny, C.A. Divergence in the metabolome between natural aging and Alzheimer’s disease. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Mahajan, U.V.; Varma, V.R.; Griswold, M.E.; Blackshear, C.T.; An, Y.; Oommen, A.M.; Varma, S.; Troncoso, J.C.; Pletnikova, O.; O’Brien, R.; et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study. PLOS Med. 2020, 17, e1003012. [Google Scholar] [CrossRef]
- Hao, L.; Wang, J.; Page, D.; Asthana, S.; Zetterberg, H.; Carlsson, C.; Okonkwo, O.C.; Li, L. Comparative Evaluation of MS-based Metabolomics Software and Its Application to Preclinical Alzheimer’s Disease. Sci. Rep. 2018, 8, 9291. [Google Scholar] [CrossRef]
- Tondo, M.; Wasek, B.; Escola-Gil, J.C.; de Gonzalo-Calvo, D.; Harmon, C.; Arning, E.; Bottiglieri, T. Altered Brain Metabolome Is Associated with Memory Impairment in the rTg4510 Mouse Model of Tauopathy. Metabolites 2020, 10, 69. [Google Scholar] [CrossRef]
- Zheng, H.; Niu, S.; Zhao, H.; Li, S.; Jiao, J. Donepezil improves the cognitive impairment in a tree shrew model of Alzheimer’s disease induced by amyloid-β1–40 via activating the BDNF/TrkB signal pathway. Metab. Brain Dis. 2018, 33, 1961–1974. [Google Scholar] [CrossRef]
- Kim, Y.H.; Shim, H.S.; Kim, K.H.; Lee, J.; Chung, B.C.; Kowall, N.W.; Ryu, H.; Lee, J. Metabolomic Analysis Identifies Alterations of Amino Acid Metabolome Signatures in the Postmortem Brain of Alzheimer’s Disease. Exp. Neurobiol. 2019, 28, 376–389. [Google Scholar] [CrossRef]
- Paglia, G.; Stocchero, M.; Cacciatore, S.; Lai, S.; Angel, P.; Alam, M.T.; Keller, M.; Ralser, M.; Astarita, G. Unbiased Metabolomic Investigation of Alzheimer’s Disease Brain Points to Dysregulation of Mitochondrial Aspartate Metabolism. J. Proteome Res. 2016, 15, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Bergin, D.H.; Jing, Y.; Mockett, B.G.; Zhang, H.; Abraham, W.C.; Liu, P. Altered plasma arginine metabolome precedes behavioural and brain arginine metabolomic profile changes in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. Transl. Psychiatry 2018, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pagano, M.; Marschoff, E.; González, S.; Repetto, M.; Serra, J. Enfermedad de Alzheimer y deterioro cognitivo asociado a la diabetes mellitus de tipo 2: Relaciones e hipótesis. Neurología 2013, 29, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, M.M.; Walker, D.I.; Howell, J.C.; Watts, K.D.; Jones, D.P.; Miller, G.W.; Hu, W.T. High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann. Clin. Transl. Neurol. 2019, 7, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Jonsson, P.; Öhrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and Peptide Levels in Plasma and CSF Differentiating Healthy Controls from Patients with Newly Diagnosed Parkinson’s Disease. J. Park. Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Öhman, A.; Forsgren, L. NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson’s disease and controls. Neurosci. Lett. 2015, 594, 36–39. [Google Scholar] [CrossRef]
- Graham, S.F.; Rey, N.L.; Yilmaz, A.; Kumar, P.; Madaj, Z.; Maddens, M.; Bahado-Singh, R.O.; Becker, K.; Schulz, E.; Meyerdirk, L.K.; et al. Biochemical Profiling of the Brain and Blood Metabolome in a Mouse Model of Prodromal Parkinson’s Disease Reveals Distinct Metabolic Profiles. J. Proteome Res. 2018, 17, 2460–2469. [Google Scholar] [CrossRef]
- Wuolikainen, A.; Jonsson, P.; Ahnlund, M.; Antti, H.; Marklund, S.L.; Moritz, T.; Forsgren, L.; Andersen, P.M.; Trupp, M. Multi-platform mass spectrometry analysis of the CSF and plasma metabolomes of rigorously matched amyotrophic lateral sclerosis, Parkinson’s disease and control subjects. Mol. BioSyst. 2016, 12, 1287–1298. [Google Scholar] [CrossRef]
- Mally, J.; Szalai, G.; Stone, T.W. Changes in the concentration of amino acids in serum and cerebrospinal fluid of patients with Parkinson’s disease. J. Neurol. Sci. 1997, 151, 159–162. [Google Scholar] [CrossRef]
- Gröger, A.; Kolb, R.; Schäfer, R.; Klose, U. Dopamine reduction in the substantia nigra of parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS ONE 2014, 9, e84081. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, T.; Wang, W.; Xiang, Y.; Huang, Q.; Xie, C.; Zhao, L.; Zheng, H.; Yang, Y.; Gao, H. Brain-Region Specific Metabolic Abnormalities in Parkinson’s Disease and Levodopa-Induced Dyskinesia. Front. Aging Neurosci. 2020, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Trezzi, J.-P.; Galozzi, S.; Jaeger, C.; Barkovits, K.; Brockmann, K.; Maetzler, W.; Berg, D.; Marcus, K.; Betsou, F.; Hiller, K.; et al. Distinct metabolomic signature in cerebrospinal fluid in early parkinson’s disease. Mov. Disord. 2017, 32, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.N.; Mamais, A.; Conti, M.M.; Primiani, C.T.; Kumaran, R.; Dillman, A.A.; Langston, R.G.; Beilina, A.; Garcia, J.H.; Diaz-Ruiz, A.; et al. Hexokinases link DJ-1 to the PINK1/parkin pathway. Mol. Neurodegener. 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Abe, T.; Hashiguchi, K.; Takahashi, S.; Nozaki, Y.; Kikuchi, T. A significant reduction of putative transmitter amino acids in cerebrospinal fluid of patients with Parkinson’s disease and spinocerebellar degeneration. Neurosci. Lett. 1991, 126, 155–158. [Google Scholar] [CrossRef]
- Shah, A.; Han, P.; Wong, M.-Y.; Chang, R.C.-C.; Legido-Quigley, C. Palmitate and Stearate are Increased in the Plasma in a 6-OHDA Model of Parkinson’s Disease. Metabolites 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Govind, V.; Sharma, K.R.; Maudsley, A.A.; Arheart, K.L.; Saigal, G.; Sheriff, S. Comprehensive evaluation of corticospinal tract metabolites in amyotrophic lateral sclerosis using whole-brain 1H MR spectroscopy. PLoS ONE 2012, 7, e35607. [Google Scholar] [CrossRef] [PubMed]
- Blicher, J.U.; Eskildsen, S.F.; Stærmose, T.G.; Møller, A.T.; Figlewski, K.; Near, J. Short echo-time Magnetic Resonance Spectroscopy in ALS, simultaneous quantification of glutamate and GABA at 3 T. Sci. Rep. 2019, 9, 17593. [Google Scholar] [CrossRef]
- Valbuena, G.N.; Cantoni, L.; Tortarolo, M.; Bendotti, C.; Keun, H.C. Spinal Cord Metabolic Signatures in Models of Fast- and Slow-Progressing SOD1G93A Amyotrophic Lateral Sclerosis. Front. Neurosci. 2019, 13, 1276. [Google Scholar] [CrossRef]
- Choi, J.-K.; Kã¼Stermann, E.; Dedeoglu, A.; Jenkins, B.G. Magnetic resonance spectroscopy of regional brain metabolite markers in FALS mice and the effects of dietary creatine supplementation. Eur. J. Neurosci. 2009, 30, 2143–2150. [Google Scholar] [CrossRef]
- Foerster, B.R.; Pomper, M.G.; Callaghan, B.C.; Petrou, M.; Edden, R.; Mohamed, M.; Welsh, R.C.; Carlos, R.C.; Barker, P.B.; Feldman, E. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by Use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013, 70, 1009–1016. [Google Scholar] [CrossRef]
- Kumar, A.; Bala, L.; Kalita, J.; Misra, U.; Singh, R.; Khetrapal, C.; Babu, G.N. Metabolomic analysis of serum by (1) H NMR spectroscopy in amyotrophic lateral sclerosis. Clin. Chim. Acta 2010, 411, 563–567. [Google Scholar] [CrossRef]
- Andreadou, E.; Kapaki, E.; Kokotis, P.; Paraskevas, G.P.; Katsaros, N.; Libitaki, G.; Zis, V.; Sfagos, C.; Vassilopoulos, D. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis: The effect of riluzole treatment. Clin. Neurol. Neurosurg. 2008, 110, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Spreux-Varoquaux, O.; Bensimon, G.; Lacomblez, L.; Salachas, F.; Pradat, P.F.; Le Forestier, N.; Marouan, A.; Dib, M.; Meininger, V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: A reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J. Neurol. Sci. 2002, 193, 73–78. [Google Scholar] [CrossRef]
- Fiszman, M.L.; Ricart, K.C.; Latini, A.; Rodrã guez, G.; Sica, R.E.P. In vitro neurotoxic properties and excitatory aminoacids concentration in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Relationship with the degree of certainty of disease diagnoses. Acta Neurol. Scand. 2010, 121, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Weerasekera, A.; Sima, D.; Dresselaers, T.; Van Huffel, S.; Van Damme, P.; Himmelreich, U. Non-invasive assessment of disease progression and neuroprotective effects of dietary coconut oil supplementation in the ALS SOD1G93A mouse model: A 1H-magnetic resonance spectroscopic study. NeuroImage Clin. 2018, 20, 1092–1105. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [CrossRef]
- Camerino, G.M.; Fonzino, A.; Conte, E.; De Bellis, M.; Mele, A.; Liantonio, A.; Tricarico, D.; Tarantino, N.; Dobrowolny, G.; Musarò, A.; et al. Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in search of new therapeutic options. Sci. Rep. 2019, 9, 3185. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Bjornevik, K.; Zhang, Z.; O’Reilly, J.; Berry, J.D.; Clish, C.B.; Deik, A.; Jeanfavre, S.; Kato, I.; Kelly, R.S.; Kolonel, L.N.; et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 2019, 92, e2089–e2100. [Google Scholar] [CrossRef]
- Gray, E.; Larkin, J.R.; Claridge, T.D.W.; Talbot, K.; Sibson, N.R.; Turner, M.R. The longitudinal cerebrospinal fluid metabolomic profile of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 456–463. [Google Scholar] [CrossRef]
- Maciejek, Z.; Jazdzewski, B.; Nicpoń, K.; Ochmański, S. Plasma and erythrocyte lipids in amyotrophic lateral sclerosis. Neurol. Neurochir. Polska 1988, 22, 387–393. [Google Scholar]
- Dupuis, L.; Corcia, P.; Fergani, A.; De Aguilar, J.-L.G.; Bonnefont-Rousselot, D.; Bittar, R.; Seilhean, D.; Hauw, J.-.-J.; Lacomblez, L.; Loeffler, J.-.-P.; et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008, 70, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Shic, F.; Enriquez, C.; Ross, B.D. Reduced glutamate neurotransmission in patients with Alzheimer’s disease?an in vivo 13C magnetic resonance spectroscopy study. Magn. Reson. Mater. Physics Biol. Med. 2003, 16, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Criste, G.A.; Trapp, B.D. N-acetyl-L-aspartate in MULTIPLE sclerosis. Adv. Exp. Med. Biol. 2006, 576, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Holshouser, B.A.; Komu, M.; Möller, H.E.; Zijlmans, J.; Kolem, H.; Hinshaw, D.B.; Sonninen, P.; Vermathen, P.; Heerschap, A.; Masur, H.; et al. Localized proton NMR spectroscopy in the striatum of patients with idiopathic parkinson’s disease: A multicenter pilot study. Magn. Reson. Med. 1995, 33, 589–594. [Google Scholar] [CrossRef]
- Zheng, X.N.; Zhu, X.C.; Ruan, L.X.; Zhang, L.J.; Yuan, M.; Shang, D.S.; Liu, Y. MRS study on lentiform nucleus in idiopathic Parkinson’s disease with unilateral symptoms. J. Zhejiang Univ. Sci. 2004, 5, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; He, C.; Chen, J.; Li, J. Proton Magnetic Resonance Spectroscopy for the Early Diagnosis of Parkinson Disease in the Substantia Nigra and Globus Pallidus: A Meta-Analysis With Trial Sequential Analysis. Front. Neurol. 2022, 13, 838230. [Google Scholar] [CrossRef]
- Llumiguano, C.; Kovacs, N.; Usprung, Z.; Schwarcz, A.; Dóczi, T.; Balas, I. 1H-MRS experiences after bilateral DBS of the STN in Parkinson’s disease. Park. Relat. Disord. 2008, 14, 229–232. [Google Scholar] [CrossRef]
- Coune, P.G.; Craveiro, M.; Gaugler, M.N.; Mlynárik, V.; Schneider, B.L.; Aebischer, P.; Gruetter, R. An in vivo ultrahigh field 14.1 T1H-MRS study on 6-OHDA and α-synuclein-based rat models of Parkinson’s disease: GABA as an early disease marker. NMR Biomed. 2012, 26, 43–50. [Google Scholar] [CrossRef]
- Wang, H.; Tan, L.; Liu, Y.; Yin, R.-H.; Wang, W.-Y.; Chang, X.-L.; Jiang, T.; Yu, J.-T. Magnetic Resonance Spectroscopy in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2015, 46, 1049–1070. [Google Scholar] [CrossRef]
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503. [Google Scholar] [CrossRef]
- Shad, K.F.; Soubra, W.; Cordato, D.J. The Auditory Afferent Pathway as a Clinical Marker of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 85, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Pelliccioli, G.P.; Tarducci, R.; Chiarini, P.; Presciutti, O.; Gobbi, G.; Gallai, V. Magnetic resonance imaging and 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. Neuroradiology 2001, 43, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Usman, U.; Choi, C.; Camicioli, R.; Seres, P.; Lynch, M.; Sekhon, R.; Johnston, W.; Kalra, S. Mesial prefrontal cortex degeneration in amyotrophic lateral sclerosis: A high-field proton MR spectroscopy study. Am. J. Neuroradiol. 2011, 32, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Sako, W.; Izumi, Y.; Abe, T.; Haji, S.; Murakami, N.; Osaki, Y.; Matsumoto, Y.; Harada, M.; Kaji, R. MR spectroscopy and imaging-derived measurements in the supplementary motor area for biomarkers of amyotrophic lateral sclerosis. Neurol. Sci. 2021, 42, 4257–4263. [Google Scholar] [CrossRef]
- Ta, D.; Ishaque, A.; Srivastava, O.; Hanstock, C.; Seres, P.; Eurich, D.T.; Luk, C.; Briemberg, H.; Frayne, R.; Genge, A.L.; et al. Progressive Neurochemical Abnormalities in Cognitive and Motor Subgroups of Amyotrophic Lateral Sclerosis. Neurology 2021, 97, e803–e813. [Google Scholar] [CrossRef]
- Lazzarino, G.; Mangione, R.; Belli, A.; Di Pietro, V.; Nagy, Z.; Barnes, N.M.; Bruce, L.; Ropero, B.M.; Persson, L.I.; Manca, B.; et al. ILB® Attenuates Clinical Symptoms and Serum Biomarkers of Oxidative/Nitrosative Stress and Mitochondrial Dysfunction in Patients with Amyotrophic Lateral Sclerosis. J. Pers. Med. 2021, 11, 794. [Google Scholar] [CrossRef]
- Marat, A.L.; Haucke, V. Phosphatidylinositol 3-phosphates—At the interface between cell signalling and membrane traffic. EMBO J. 2016, 35, 561–579. [Google Scholar] [CrossRef]
- Moore, G.J.; Galloway, M.P. Magnetic resonance spectroscopy: Neurochemistry and treatment effects in affective disorders. Psychopharmacol. Bull. 2002, 36, 5–23. [Google Scholar]
- Frey, R.; Metzler, D.; Fischer, P.; Heiden, A.; Scharfetter, J.; Moser, E.; Kasper, S. Myo-inositol in depressive and healthy subjects determined by frontal 1H-magnetic resonance spectroscopy at 1.5 tesla. J. Psychiatr. Res. 1998, 32, 411–420. [Google Scholar] [CrossRef]
- Di Daniel, E.; Kew, J.N.; Maycox, P.R. Investigation of the H+–myo-inositol transporter (HMIT) as a neuronal regulator of phosphoinositide signalling. Biochem. Soc. Trans. 2009, 37, 1139–1143. [Google Scholar] [CrossRef]
- Mazuel, L.; Chassain, C.; Jean, B.; Pereira, B.; Cladière, A.; Speziale, C.; Durif, F. Proton MR Spectroscopy for Diagnosis and Evaluation of Treatment Efficacy in Parkinson Disease. Radiology 2016, 278, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Casjens, S.; Woitalla, D.; Dharmadhikari, S.; Edmondson, D.A.; Zella, M.A.S.; Lehnert, M.; Lotz, A.; Herrmann, L.; Muhlack, S.; et al. Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, T.; Chang, L.; Melchor, R.; Mehringer, C.M. Frontotemporal dementia and early Alzheimer disease: Differentiation with frontal lobe H-1 MR spectroscopy. Radiology 1997, 203, 829–836. [Google Scholar] [CrossRef]
- Ali, F.; Manzoor, U.; Bhattacharya, R.; Bansal, A.K.; Chandrashekharaiah, K.S.; Singh, L.R.; Saraswati, S.M.; Uversky, V.; Dar, T.A. Brain Metabolite, Myo-inositol, Inhibits Catalase Activity: A Mechanism of the Distortion of the Antioxidant Defense System in Alzheimer’s disease. ACS Omega 2022, 7, 12690–12700. [Google Scholar] [CrossRef]
- Eisen, A.; Stewart, H.; Schulzer, M.; Cameron, D. Anti-glutamate therapy in amyotrophic lateral sclerosis: A trial using lamotrigine. Can. J. Neurol. Sciences. Le J. Can. Des Sci. Neurol. 1993, 20, 297–301. [Google Scholar]
- Hanstock, C.; Sun, K.; Choi, C.; Eurich, D.; Camicioli, R.; Johnston, W.; Kalra, S. Spectroscopic markers of neurodegeneration in the mesial prefrontal cortex predict survival in ALS. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A.C. Book Review: GABA and Glutamate in the Human Brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Masu, M. Molecular diversity and functions of glutamate receptors. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 319–348. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A. Cerebrospinal and blood levels of amino acids as potential biomarkers for Parkinson’s disease: Review and meta-analysis. Eur. J. Neurol. 2020, 27, 2336–2347. [Google Scholar] [CrossRef]
- Manyevitch, R.; Protas, M.; Scarpiello, S.; DeLiso, M.; Bass, B.; Nanajian, A.; Chang, M.; Thompson, S.M.; Khoury, N.; Gonnella, R.; et al. Evaluation of Metabolic and Synaptic Dysfunction Hypotheses of Alzheimer’s Disease (AD): A Meta-Analysis of CSF Markers. Curr. Alzheimer Res. 2018, 15, 164–181. [Google Scholar] [CrossRef]
- Niebroj-Dobosz, I.; Jamrozik, Z.; Janik, P.; Hausmanowa-Petrusewicz, I.; Kwiecinski, H. Anti-neural antibodies in serum and cerebrospinal fluid of amyotrophic lateral sclerosis (ALS) patients. Acta Neurol. Scand. 2009, 100, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Lanznaster, D.; de Assis, D.R.; Corcia, P.; Pradat, P.-F.; Blasco, H. Metabolomics Biomarkers: A Strategy Toward Therapeutics Improvement in ALS. Front. Neurol. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Pioro, E.P.; Majors, A.W.; Mitsumoto, H.; Nelson, D.R.; Ng, T.C. 1H-MRS evidence of neurodegeneration and excess glutamate glutamine in ALS medulla. Neurology 1999, 53, 71. [Google Scholar] [CrossRef] [PubMed]
- Atassi, N.; Xu, M.; Triantafyllou, C.; Keil, B.; Lawson, R.; Cernasov, P.; Ratti, E.; Long, C.J.; Paganoni, S.; Murphy, A.; et al. Ultra high-field (7tesla) magnetic resonance spectroscopy in Amyotrophic Lateral Sclerosis. PLoS ONE 2017, 12, e0177680. [Google Scholar] [CrossRef]
- Cheong, I.; Marjanska, M.; Deelchand, D.K.; Eberly, L.; Walk, D.; Oz, G. Ultra-High Field Proton MR Spectroscopy in Early-Stage Amyotrophic Lateral Sclerosis. Neurochem. Res. 2017, 42, 1833–1844. [Google Scholar] [CrossRef]
- Perry, T.L.; Krieger, C.; Ba, S.H.; Eisen, A. Amyotrophic lateral sclerosis: Amino acid levels in plasma and cerebrospinal fluid. Ann. Neurol. 1990, 28, 12–17. [Google Scholar] [CrossRef]
- Ilzecka, J.; Stelmasiak, Z.; Solski, J.; Wawrzycki, S.; Szpetnar, M. Plasma amino acids concentration in amyotrophic lateral sclerosis patients. Amino Acids 2003, 25, 69–73. [Google Scholar] [CrossRef]
- Zeydan, B.; Kantarci, K. Decreased glutamine and glutamate: An early biomarker of neurodegeneration. Int. Psychogeriatr. 2021, 33, 1–2. [Google Scholar] [CrossRef]
- McKenna, M.C. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- Dal-Cim, T.; Martins, W.C.; Thomaz, D.T.; Coelho, V.; Poluceno, G.G.; Lanznaster, D.; Vandresen-Filho, S.; Tasca, C.I. Neuroprotection Promoted by Guanosine Depends on Glutamine Synthetase and Glutamate Transporters Activity in Hippocampal Slices Subjected to Oxygen/Glucose Deprivation. Neurotox. Res. 2016, 29, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, R.; Avidan, N.; Avisar, N.; Shaked, I.; Vardimon, L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc. Natl. Acad. Sci. USA 1997, 94, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, Y.-X.; Dou, F.-F.; Lü, H.-Z.; Ma, Z.-W.; Lu, P.-H.; Xu, X.-M. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int. 2010, 56, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Hensley, K.; Cole, P.; Subramaniam, R.; Aksenov, M.; Aksenova, M.; Bummer, P.M.; Haley, B.E.; Carney, J.M. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: Relevance to Alzheimer’s disease. J. Neurochem. 2002, 68, 2451–2457. [Google Scholar] [CrossRef]
- Le Prince, G.; Delaere, P.; Fages, C.; Lefrançois, T.; Touret, M.; Salanon, M.; Tardy, M. Glutamine synthetase (GS) expression is reduced in senile dementia of the Alzheimer type. Neurochem. Res. 1995, 20, 859–862. [Google Scholar] [CrossRef]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: Mechanism for deficient glutamatergic transmission? Mol. Neurodegener. 2011, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.R. Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem. Int. 2000, 36, 471–482. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Aksenova, M.V.; Carney, J.M.; Buterfield, D.A. Oxidative modification of glutamine synthetase by amyloid beta peptide. Free Radic. Res. 1997, 27, 267–281. [Google Scholar] [CrossRef]
- Zipp, F.; Demisch, L.; Derouiche, A.; Fischer, P.A. Glutamine synthetase actiity in patients with Parkinson’s disease. Acta Neurol. Scand. 1998, 97, 300–302. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Molina, J.; Vargas, C.; Gómez, P.; Navarro, J.; Benito-Leon, J.; Ortí-Pareja, M.; Gasalla, T.; Cisneros, E.; Arenas, J. Neurotransmitter amino acids in cerebrospinal fluid of patients with Parkinson’s disease. J. Neurol. Sci. 1996, 141, 39–44. [Google Scholar] [CrossRef]
- Ondarza, R.; Velasco, F.; Velasco, M.; Aceves, J.; Flores, G. Neurotransmitter levels in cerebrospinal fluid in relation to severity of symptoms and response to medical therapy in Parkinson’s disease. Ster. Funct. Neurosurg. 1994, 62, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.H.; D’Aniello, A.; Vetere, A.; Padula, L.; Cusano, G.P.; Man, E.H. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res. Bull. 1991, 26, 983–985. [Google Scholar] [CrossRef]
- Fisher, G.H.; D’Aniello, A.; Vetere, A.; Cusano, G.P.; Chávez, M.; Petrucelli, L. Quantitfication of d-aspartate in normal and Alzheimer brains. Neurosci. Lett. 1992, 143, 215–218. [Google Scholar] [CrossRef]
- Rosko, L.; Smith, V.N.; Yamazaki, R.; Huang, J.K. Oligodendrocyte Bioenergetics in Health and Disease. Neurosci. 2018, 25, 334–343. [Google Scholar] [CrossRef]
- D’Aniello, A.; Vetere, A.; Fisher, G.H.; Cusano, G.; Chavez, M.; Petrucelli, L. Presence of d-alanine in proteins of normal and Alzheimer human brain. Brain Res. 1992, 592, 44–48. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, H.-T.; Lane, H.-Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef]
- Ratner, S. Enzymes of Arginine and Urea Systhesis. Adv. Enzymol. Relat. Areas Mol. Biol. 2006, 39, 1–90. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Hatcher, G.R.J.; Bennett, B.M.; Reynolds, J.N. NO chimeras as therapeutic agents in Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 237–245. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Katusic, Z.S. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ. Res. 2010, 107, 1498–1502. [Google Scholar] [CrossRef]
- Law, A. Say NO to Alzheimer’s disease: The putative links between nitric oxide and dementia of the Alzheimer’s type. Brain Res. Rev. 2001, 35, 73–96. [Google Scholar] [CrossRef]
- Yi, J.; Horky, L.L.; Friedlich, A.L.; Shi, Y.; Rogers, J.T.; Huang, X. L-arginine and Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2009, 2, 211–238. [Google Scholar]
- Malinski, T. Nitric oxide and Nitroxidative stress in Alzheimer’s disease. J. Alzheimer’s Dis. 2007, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’Oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, srep02364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Fleete, M.S.; Jing, Y.; Collie, N.D.; Curtis, M.; Waldvogel, H.; Faull, R.; Abraham, W.C.; Zhang, H. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol. Aging 2014, 35, 1992–2003. [Google Scholar] [CrossRef]
- Ibáñez, C.; Simó, C.; Martín-Álvarez, P.J.; Kivipelto, M.; Winblad, B.; Cedazo-Mínguez, A.; Cifuentes, A. Toward a Predictive Model of Alzheimer’s Disease Progression Using Capillary Electrophoresis–Mass Spectrometry Metabolomics. Anal. Chem. 2012, 84, 8532–8540. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2006, 32, 213–224. [Google Scholar] [CrossRef]
- Onozato, M.; Uta, A.; Magarida, A.; Fukuoka, N.; Ichiba, H.; Tsujino, N.; Funatogawa, T.; Tagata, H.; Nemoto, T.; Mizuno, M.; et al. Alterations in methionine to homocysteine ratio in individuals with first-episode psychosis and those with at-risk mental state. Clin. Biochem. 2020, 77, 48–53. [Google Scholar] [CrossRef]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef]
- Hooshmand, B.; Refsum, H.; Smith, A.D.; Kalpouzos, G.; Mangialasche, F.; Von Arnim, C.A.F.; Kåreholt, I.; Kivipelto, M.; Fratiglioni, L. Association of Methionine to Homocysteine Status With Brain Magnetic Resonance Imaging Measures and Risk of Dementia. JAMA Psychiatry 2019, 76, 1198–1205. [Google Scholar] [CrossRef]
- McCampbell, A.; Wessner, K.; Marlatt, M.W.; Wolffe, C.; Toolan, D.; Podtelezhnikov, A.; Yeh, S.; Zhang, R.; Szczerba, P.; Tanis, K.Q.; et al. Induction of Alzheimer’s-like changes in brain of mice expressing mutant APP fed excess methionine. J. Neurochem. 2010, 116, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Pi, T.; Wei, S.; Jiang, Y.; Shi, J.-S. High Methionine Diet-Induced Alzheimer’s Disease like Symptoms Are Accompanied by 5-Methylcytosine Elevated Levels in the Brain. Behav. Neurol. 2021, 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Fithian, A.T.; Gylys, K.; Cummings, J.L.; Coppola, G.; Elashoff, D.; Pratico, D.; Moskovitz, J.; Bitan, G. Plasma Methionine sulfoxide in persons with familial Alzheimer’s disease mutations. Dement. Geriatr. Cogn. Disord. 2012, 33, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Lindsay, C.B.; Montecinos-Oliva, C.; Arrazola, M.S.; Retamales, R.M.; Bunout, D.; Hirsch, S.; Inestrosa, N.C. Is L-methionine a trigger factor for Alzheimer’s-like neurodegeneration?: Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol. Neurodegener. 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, P.; Bosco, P.; Ferri, R.; Perri, C.; Suriano, R.; Costantini, B.; Macrì, F.; Proto, C.; Cento, R.M.; Lanzone, A. Fasting and Post-methionine Homocysteine Levels in Alzheimer’s Disease and Vascular Dementia. Int. J. Vitam. Nutr. Res. 2009, 79, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Kalani, K.; Kamat, P.K.; Chaturvedi, P.; Tyagi, N. A high methionine, low folate and vitamin B6/B12 containing diet can be associated with memory loss by epigenetic silencing of netrin-1. Neural Regen. Res. 2019, 14, 1247–1254. [Google Scholar] [CrossRef]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Alachkar, A.; Agrawal, S.; Baboldashtian, M.; Nuseir, K.; Salazar, J.; Agrawal, A. L-methionine enhances neuroinflammation and impairs neurogenesis: Implication for Alzheimer’s disease. J. Neuroimmunol. 2022, 366, 577843. [Google Scholar] [CrossRef]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. In Protein Misfolding and Cellular Stress in Disease and Aging; Humana Press: Totowa, NJ, USA, 2010; Volume 648, pp. 269–277. [Google Scholar] [CrossRef]
- Bains, J.S.; A Shaw, C. Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Rev. 1997, 25, 335–358. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2008, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Glutathione metabolism and oxidative stress in neurodegeneration. JBIC J. Biol. Inorg. Chem. 2000, 267, 4903. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, P.K.; Saharan, S.; Tripathi, M.; Murari, G. Brain glutathione levels—A novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol. Psychiatry 2015, 78, 702–710. [Google Scholar] [CrossRef]
- Ding, Q.; Markesbery, W.R.; Chen, Q.; Li, F.; Keller, J. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 2005, 25, 9171–9175. [Google Scholar] [CrossRef]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Flor, L.; López-Nogueroles, M.; García, L.; Ferrer, I.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Plasma alterations in cholinergic and serotonergic systems in early Alzheimer Disease: Diagnosis utility. Clin. Chim. Acta 2019, 500, 233–240. [Google Scholar] [CrossRef]
- Ganz, A.B.; Cohen, V.V.; Swersky, C.C.; Stover, J.; Vitiello, G.A.; Lovesky, J.; Chuang, J.C.; Shields, K.; Fomin, V.G.; Lopez, Y.S.; et al. Genetic Variation in Choline-Metabolizing Enzymes Alters Choline Metabolism in Young Women Consuming Choline Intakes Meeting Current Recommendations. Int. J. Mol. Sci. 2017, 18, 252. [Google Scholar] [CrossRef]
- Göthert, M. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part I. General background and discovery of serotonin as a basis for 5-HT receptor identification. Pharmacol. Rep. 2013, 65, 771–786. [Google Scholar] [CrossRef]
- Yabuki, Y.; Matsuo, K.; Hirano, K.; Shinoda, Y.; Moriguchi, S.; Fukunaga, K. Combined Memantine and Donepezil Treatment Improves Behavioral and Psychological Symptoms of Dementia-Like Behaviors in Olfactory Bulbectomized Mice. Pharmacology 2017, 99, 160–171. [Google Scholar] [CrossRef]
- Hasselbalch, S.; Madsen, K.; Svarer, C.; Pinborg, L.; Holm, S.; Paulson, O.; Waldemar, G.; Knudsen, G. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol. Aging 2008, 29, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Pimenova, A.A.; Thathiah, A.; De Strooper, B.; Tesseur, I. Regulation of Amyloid Precursor Protein Processing by Serotonin Signaling. PLoS ONE 2014, 9, e87014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H.; et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.J.; Noristani, H.N.; Verkhratsky, A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 2012, 99, 15–41. [Google Scholar] [CrossRef]
- Molina, J.A.; Jiménez-Jiménez, F.J.; Gómez, P.; Vargas, C.; Navarro, J.A.; Ortí-Pareja, M.; Gasalla, T.; Benito-León, J.; Bermejo, F.; Arenas, J. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson’s disease. J. Neurol. Sci. 1997, 150, 123–127. [Google Scholar] [CrossRef]
- D’Ascenzo, N.; Antonecchia, E.; Angiolillo, A.; Bender, V.; Camerlenghi, M.; Xie, Q.; Di Costanzo, A. Metabolomics of blood reveals age-dependent pathways in Parkinson’s Disease. Cell Biosci. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Figura, M.; Kuśmierska, K.; Bucior, E.; Szlufik, S.; Koziorowski, D.; Jamrozik, Z.; Janik, P. Serum amino acid profile in patients with Parkinson’s disease. PLoS ONE 2018, 13, e0191670. [Google Scholar] [CrossRef]
- Hernandes, M.S.; Troncone, L.R.P. Glycine as a neurotransmitter in the forebrain: A short review. J. Neural Transm. 2009, 116, 1551–1560. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ikeda, K.; Shiojima, T.; Kinoshita, M. Increased plasma concentrations of aspartate, glutamate and glycine in Parkinson’s disease. Neurosci. Lett. 1992, 145, 175–177. [Google Scholar] [CrossRef]
- Hare, T.; Beasley, B.; Chambers, R.; Boehme, D.; Vogel, W. DOPA and amino acid levels in plasma and cerebrospinal fluid of patients with parkinson’s disease before and during treatment with L-dOPA. Clin. Chim. Acta 1973, 45, 273–280. [Google Scholar] [CrossRef]
- Beal, M.F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann. Neurol. 1992, 31, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gjessing, L.; Gjesdahl, P.; Dietrichson, P.; Presthus, J. Free Amino Acids in the Cerebrospinal Fluid in Old Age and in Parkinson’s Disease. Eur. Neurol. 1974, 12, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Perrone, R.D.; E Madias, N. Serum Creatinine and Renal Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Cui, C.; Sun, J.; Pawitan, Y.; Piehl, F.; Chen, H.; Ingre, C.; Wirdefeldt, K.; Evans, M.; Andersson, J.; Carrero, J.-J.; et al. Creatinine and C-reactive protein in amyotrophic lateral sclerosis, multiple sclerosis and Parkinson’s disease. Brain Commun. 2020, 2, fcaa152. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.-J.; Liu, L.-Y.; Peng, W.-B.; Rao, J.; Liu, Z.; Cui, L.-L. The effectiveness of creatine treatment for Parkinson’s disease: An updated meta-analysis of randomized controlled trials. BMC Neurol. 2017, 17, 105. [Google Scholar] [CrossRef]
- Sandyk, R. The relationship between diabetes mellitus and Parkinson’s disease. Int. J. Neurosci. 1993, 69, 125–130. [Google Scholar] [CrossRef]
- Lu, L.; Fu, D.-L.; Li, H.-Q.; Liu, A.-J.; Li, J.-H.; Zheng, G.-Q. Diabetes and Risk of Parkinson’s Disease: An Updated Meta-Analysis of Case-Control Studies. PLoS ONE 2014, 9, e85781. [Google Scholar] [CrossRef]
- Boyd, I.A.E.; Lebovitz, H.E.; Feldman, J.M. Endocrine function and glucose metabolism in patients with Parkinson’s disease and their alteration byl-Dopa1. J. Clin. Endocrinol. Metab. 1971, 33, 829–837. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol. Med. 2013, 19, 176–186. [Google Scholar] [CrossRef]
- Batisse-Lignier, M.; Rieu, I.; Guillet, C.; Pujos, E.; Morio, B.; Lemaire, J.-J.; Durif, F.; Boirie, Y. Deep Brain Stimulation of the Subthalamic Nucleus Regulates Postabsorptive Glucose Metabolism in Patients With Parkinson’s Disease. J. Clin. Endocrinol. Metab. 2013, 98, E1050–E1054. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Dutheil, F.; Durand, E.; Rieu, I.; Mulliez, A.; Fantini, M.L.; Boirie, Y.; Durif, F. Glucose dysregulation in Parkinson’s disease: Too much glucose or not enough insulin? Park. Relat. Disord. 2018, 55, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Xiromerisiou, G.; Hadjigeorgiou, G.M.; Papadimitriou, A.; Katsarogiannis, E.; Gourbali, V.; Singleton, A.B. Association between AKT1 gene and Parkinson’s disease: A protective haplotype. Neurosci. Lett. 2008, 436, 232–234. [Google Scholar] [CrossRef]
- Jain, D.; Jain, R.; Eberhard, D.; Eglinger, J.; Bugliani, M.; Piemonti, L.; Marchetti, P.; Lammert, E. Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J. Mol. Cell Biol. 2012, 4, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Corti, O.; Lesage, S.; Brice, A. What Genetics Tells us About the Causes and Mechanisms of Parkinson’s Disease. Physiol. Rev. 2011, 91, 1161–1218. [Google Scholar] [CrossRef]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Erkut, C.; Pan-Montojo, F.; Boland, S.; Stewart, M.P.; Müller, D.J.; Wurst, W.; Hyman, A.A.; Kurzchalia, T.V. Products of the Parkinson’s disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol. Open 2014, 3, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Aleyasin, H.; Seifert, E.L.; Hewitt, S.J.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.C.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2008, 87, 123–129. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Song, J.; Kwon, K.; Jang, S.; Kim, C.; Baek, K.; Kim, J.; Park, C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012, 21, 3215–3225. [Google Scholar] [CrossRef]
- Castellani, R.; Smith, M.A.; Richey, P.L.G.; Perry, G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996, 737, 195–200. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2018, 175, 1756-1768.e17. [Google Scholar] [CrossRef]
- Panati, K.; Narala, V.R.; Narasimha, V.R.; Derangula, M.; Tatireddigari, V.R.A.; Yeguvapalli, S. Expression, purification and biological characterisation of recombinant human irisin (12.5 kDa). J. Genet. Eng. Biotechnol. 2018, 16, 459–466. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-S.; Li, D.; Sun, W.-P.; Guo, M.; Lun, Y.-Z.; Zhou, Y.-M.; Xiao, F.-C.; Jing, L.-X.; Sun, S.-X.; Zhang, L.-B.; et al. Nicotinamide overload may play a role in the developmentof type 2 diabetes. World J. Gastroenterol. 2009, 15, 5674–5684. [Google Scholar] [CrossRef]
- Williams, A.; Ramsden, D. Nicotinamide: A double edged sword. Park. Relat. Disord. 2005, 11, 413–420. [Google Scholar] [CrossRef]
- Tang, B.L. Could Sirtuin Activities Modify ALS Onset and Progression? Cell. Mol. Neurobiol. 2016, 37, 1147–1160. [Google Scholar] [CrossRef]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol Improves Motoneuron Function and Extends Survival in SOD1G93A ALS Mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.; Salvatori, I.; Gerbino, V.; Rossi, S.; Palamiuc, L.; Rene, F.; Carri, M.T. Tissue-specific deregulation of selected HDACs characterizes ALS progression in mouse models: Pharmacological characterization of SIRT1 and SIRT2 pathways. Cell Death Dis. 2014, 5, e1296. [Google Scholar] [CrossRef] [PubMed]
- Harlan, B.A.; Killoy, K.M.; Pehar, M.; Liu, L.; Auwerx, J.; Vargas, M.R. Evaluation of the NAD+ biosynthetic pathway in ALS patients and effect of modulating NAD+ levels in hSOD1-linked ALS mouse models. Exp. Neurol. 2020, 327, 113219. [Google Scholar] [CrossRef] [PubMed]
- Körner, S.; Böselt, S.; Thau, N.; Rath, K.J.; Dengler, R.; Petri, S. Differential Sirtuin Expression Patterns in Amyotrophic Lateral Sclerosis (ALS) Postmortem Tissue: Neuroprotective or Neurotoxic Properties of Sirtuins in ALS? Neurodegener. Dis. 2012, 11, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Bayer, H.; Lindenberg, K.S.; Hanselmann, J.; Pasquarelli, N.; Ludolph, A.C.; Weydt, P.; Witting, A. Comparison of Sirtuin 3 Levels in ALS and Huntington’s Disease—Differential Effects in Human Tissue Samples vs. Transgenic Mouse Models. Front. Mol. Neurosci. 2017, 10, 156. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Ehinger, J.K.; Morota, S.; Hansson, M.J.; Paul-Visse, G.; Elmér, E. Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. J. Neurol. 2015, 262, 1493–1503. [Google Scholar] [CrossRef]
- Lanznaster, D.; Bruno, C.; Bourgeais, J.; Emond, P.; Zemmoura, I.; Lefèvre, A.; Reynier, P.; Eymieux, S.; Blanchard, E.; Vourc’H, P.; et al. Metabolic Profile and Pathological Alterations in the Muscle of Patients with Early-Stage Amyotrophic Lateral Sclerosis. Biomedicines 2022, 10, 1307. [Google Scholar] [CrossRef]
- Hyndman, D.; Liu, S.; Miner, J.N. Urate Handling in the Human Body. Curr. Rheumatol. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.-J.; Russell, R.M.; Krinsky, N.I.; Aldini, G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 2004, 430, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of Plasma Uric Acid on Antioxidant Capacity, Oxidative Stress, and Insulin Sensitivity in Obese Subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Paganoni, S.; Schwarzschild, M.A. Urate as a Marker of Risk and Progression of Neurodegenerative Disease. Neurotherapeutics 2016, 14, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.A.; Brown, M.V.; Alexander, D.; Li, Z.; Wulff, J.E.; Lawson, R.; Jaffa, M.; Milburn, M.V.; Ryals, J.A.; Bowser, R.; et al. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, S.; Zhang, M.; Zárate, A.Q.; Jaffa, M.; Yu, H.; Cudkowicz, M.E.; Wills, A.-M. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J. Neurol. 2012, 259, 1923–1928. [Google Scholar] [CrossRef]
- Keizman, D.; Ish-Shalom, M.; Berliner, S.; Maimon, N.; Vered, Y.; Artamonov, I.; Tsehori, J.; Nefussy, B.; Drory, V. Low uric acid levels in serum of patients with ALS: Further evidence for oxidative stress? J. Neurol. Sci. 2009, 285, 95–99. [Google Scholar] [CrossRef]
- Nagase, M.; Yamamoto, Y.; Miyazaki, Y.; Yoshino, H. Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep. 2016, 21, 1–9. [Google Scholar] [CrossRef]
- O’Reilly, J.; Liu, D.; Johns, D.R.; Cudkowicz, M.E.; Paganoni, S.; Schwarzschild, M.A.; Leitner, M.; Ascherio, A. Serum urate at trial entry and ALS progression in EMPOWER. Amyotroph. Lateral Scler. Front. Degener. 2016, 18, 120–125. [Google Scholar] [CrossRef]
- Moore, C.C.; Miller, W.L. The role of transcriptional regulation in steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1991, 40, 517–525. [Google Scholar] [CrossRef]
- Dorst, J.; Kühnlein, P.; Hendrich, C.; Kassubek, J.; Sperfeld, A.D.; Ludolph, A.C. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J. Neurol. 2010, 258, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Wuolikainen, A.; Ačimovič, J.; Lövgren-Sandblom, A.; Parini, P.; Andersen, P.M.; Björkhem, I. Cholesterol, oxysterol, triglyceride, and coenzyme Q homeostasis in ALS. evidence against the hypothesis that elevated 27-hydroxycholesterol is a pathogenic factor. PLoS ONE 2014, 9, e113619. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Guo, X.; Chen, X.; Zheng, Z.; Wei, Q.; Cao, B.; Zeng, Y.; Shang, H. The serum lipid profiles of amyotrophic lateral sclerosis patients: A study from south-west China and a meta-analysis. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Cember, A.T.J.; Nanga, R.P.R.; Reddy, R. Glutamate-weighted CEST (gluCEST) imaging for mapping neurometabolism: An update on the state of the art and emerging findings from in vivo applications. NMR Biomed. 2022, e4780, Online ahead of print. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Nakaya, J. Effect of oral administration of L-arginine on senile dementia. Am. J. Med. 2000, 108, 439. [Google Scholar] [CrossRef]
- Russin, K.J.; Nair, K.S.; Montine, T.J.; Baker, L.D.; Craft, S. Diet Effects on Cerebrospinal Fluid Amino Acids Levels in Adults with Normal Cognition and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 84, 843–853. [Google Scholar] [CrossRef]
- Hurson, M.; Regan, M.C.; Kirk, S.J.; Wasserkrug, H.L.; Barbul, A. Metabolic Effects of Arginine in a Healthy Elderly Population. J. Parenter. Enter. Nutr. 1995, 19, 227–230. [Google Scholar] [CrossRef]
- Polis, B.; Srikanth, K.D.; Elliott, E.; Gil-Henn, H.; Samson, A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics 2018, 15, 1036–1054. [Google Scholar] [CrossRef]
- Chmiela, T.; Węgrzynek, J.; Kasprzyk, A.; Waksmundzki, D.; Wilczek, D.; Gorzkowska, A. If Not Insulin Resistance so What?–Comparison of Fasting Glycemia in Idiopathic Parkinson’s Disease and Atypical Parkinsonism. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2022, 15, 1451–1460. [Google Scholar] [CrossRef]
- Lin, K.-J.; Wang, T.-J.; Chen, S.-D.; Lin, K.-L.; Liou, C.-W.; Lan, M.-Y.; Chuang, Y.-C.; Chuang, J.-H.; Wang, P.-W.; Lee, J.-J.; et al. Two Birds One Stone: The Neuroprotective Effect of Antidiabetic Agents on Parkinson Disease—Focus on Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors. Antioxidants 2021, 10, 1935. [Google Scholar] [CrossRef]
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat. Rev. Neurosci. 2018, 20, 34–48. [Google Scholar] [CrossRef] [PubMed]
- De La Rubia, J.E.; Drehmer, E.; Platero, J.L.; Benlloch, M.; Caplliure-Llopis, J.; Villaron-Casales, C.; De Bernardo, N.; Alarcón, J.; Fuente, C.; Carrera, S.; et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
| Alterations in Patients | Preclinical Studies |
|---|---|
| AD | |
| Methionine: increased in CSF [13] | [14] |
| Glutathione: increased in CSF [13]; increased in brain tissue [15] | [14] |
| Serotonin: decreased in CSF [16]; increased in CSF [13] | [17] |
| Choline: decreased in brain tissue [15] | [18] |
| N-acetyl-aspartate: decreased in brain tissue [15,19]; increased in brain tissue [20] | [14,21,22] |
| Glutamine: increased in CSF [23]; increased in brain tissue [20] | [17,18] |
| Alanine: increased in brain tissue [19] | [18] |
| Myo-inositol: increased in brain tissue [19] | [18] |
| Glutamate: increased in brain tissue [20] | [21,22] |
| Arginine: increased in brain tissue [20] | [14,17,21] |
| Aspartate: decreased in brain tissue [20] | [18] |
| PD | |
| Creatinine: decreased in CSF [24]; increased in CSF [25] | [26] |
| Threonine: increased in CSF [27] | [26] |
| Glutamine: increased in CSF [27,28]; increased in SN [29] | [26,30] |
| Glucose: increased in CSF [25,31] | [32] |
| Lactate: increased in CSF [25] | [26] |
| Glutamate: decreased in CSF [28,33]; increased in SN [29] | [30] |
| Aspartate: decreased in CSF [33] | [26,30] |
| Glycine: decreased in CFS [33] | [26] |
| N-acetyl-aspartate: decreased in SN [29] | [34] |
| Myo-inositol: decreased in SN [29] | [26] |
| ALS | |
| N-acetyl-aspartate: decreased in motor cortex [35,36] | [37,38] |
| Myo-inositol: increased in motor cortex [39] | [37,38] |
| Glutamate: increased in blood [40,41]; increased in CSF [42,43] | [38,44] |
| Irisin: increased in serum [45] | [46] |
| Nicotinamide: decreased systemic and in CSF [47] | [47] |
| Urate: decreased in plasma [48] | [37] |
| Citrate: increased in CSF [49] | [37] |
| Cholesterol: increased in plasma [50,51] | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanznaster, D.; Dingeo, G.; Samey, R.A.; Emond, P.; Blasco, H. Metabolomics as a Crucial Tool to Develop New Therapeutic Strategies for Neurodegenerative Diseases. Metabolites 2022, 12, 864. https://doi.org/10.3390/metabo12090864
Lanznaster D, Dingeo G, Samey RA, Emond P, Blasco H. Metabolomics as a Crucial Tool to Develop New Therapeutic Strategies for Neurodegenerative Diseases. Metabolites. 2022; 12(9):864. https://doi.org/10.3390/metabo12090864
Chicago/Turabian StyleLanznaster, Débora, Giulia Dingeo, Rayhanatou Altine Samey, Patrick Emond, and Hélène Blasco. 2022. "Metabolomics as a Crucial Tool to Develop New Therapeutic Strategies for Neurodegenerative Diseases" Metabolites 12, no. 9: 864. https://doi.org/10.3390/metabo12090864








