PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Rearing Conditions
2.3. Sample Collection
2.4. Crude Fat Content Determination
2.5. Plasma Biochemical Parameters Index Analysis
2.6. Hydrolyzed and Free Amino Acid Content Analysis at 2 Days
2.7. RNA Extraction, cDNA Library Construction and RNA-seq at 2 Days
2.8. De Novo Assembly, Functional Annotation, and Differentially Expressed Genes (DEGs) Analysis at 2 Days
2.9. Transcriptional Expression and Validation of Key DEGs at 2 Days
2.10. Correlation Analysis
2.11. Statistical Analysis
3. Results
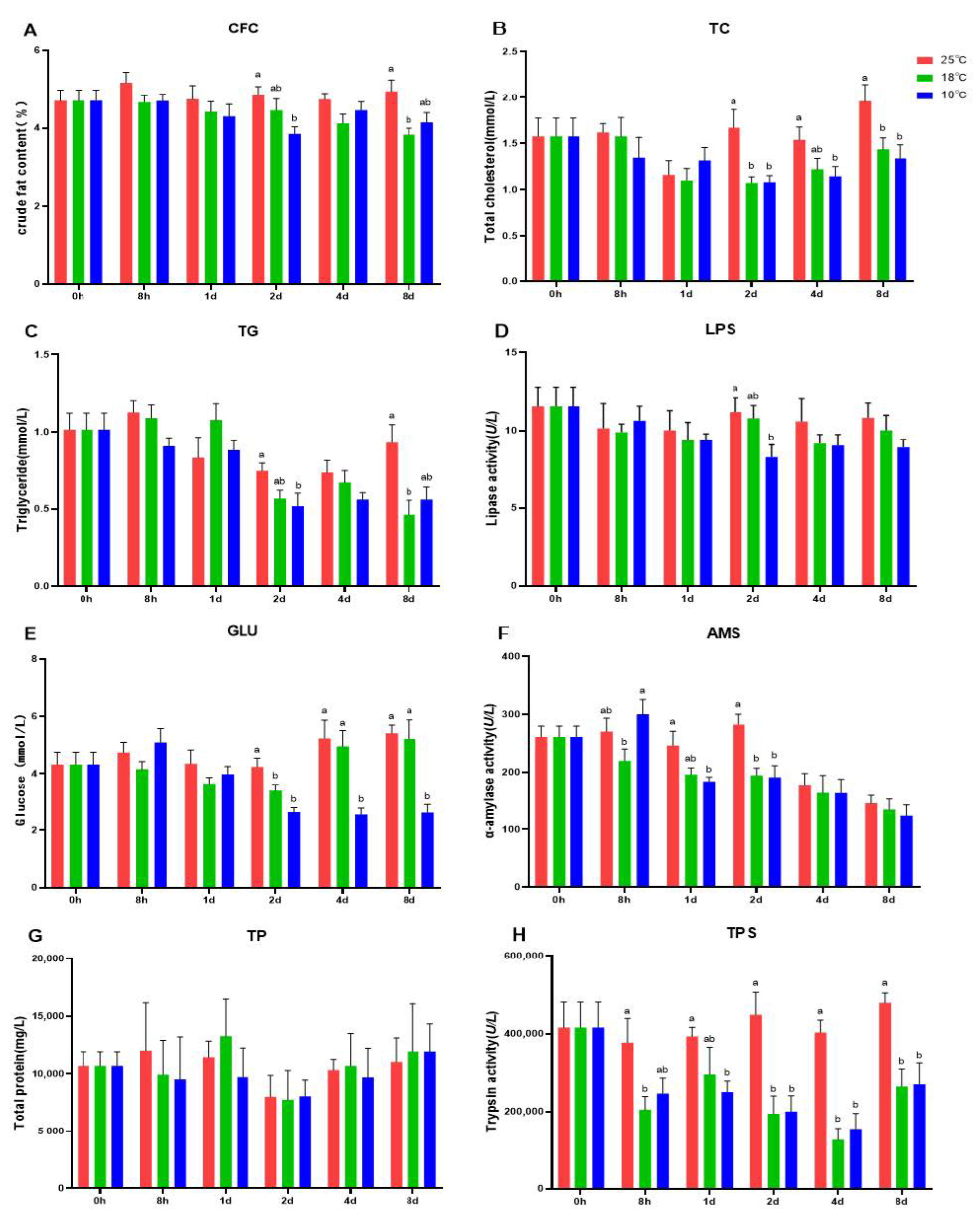
3.1. Body Composition and Plasma Biochemical Parameters Induced by Hypothermia in A. grunniens
3.2. Amino Acid Contents Induced by Hypothermia in A. grunniens
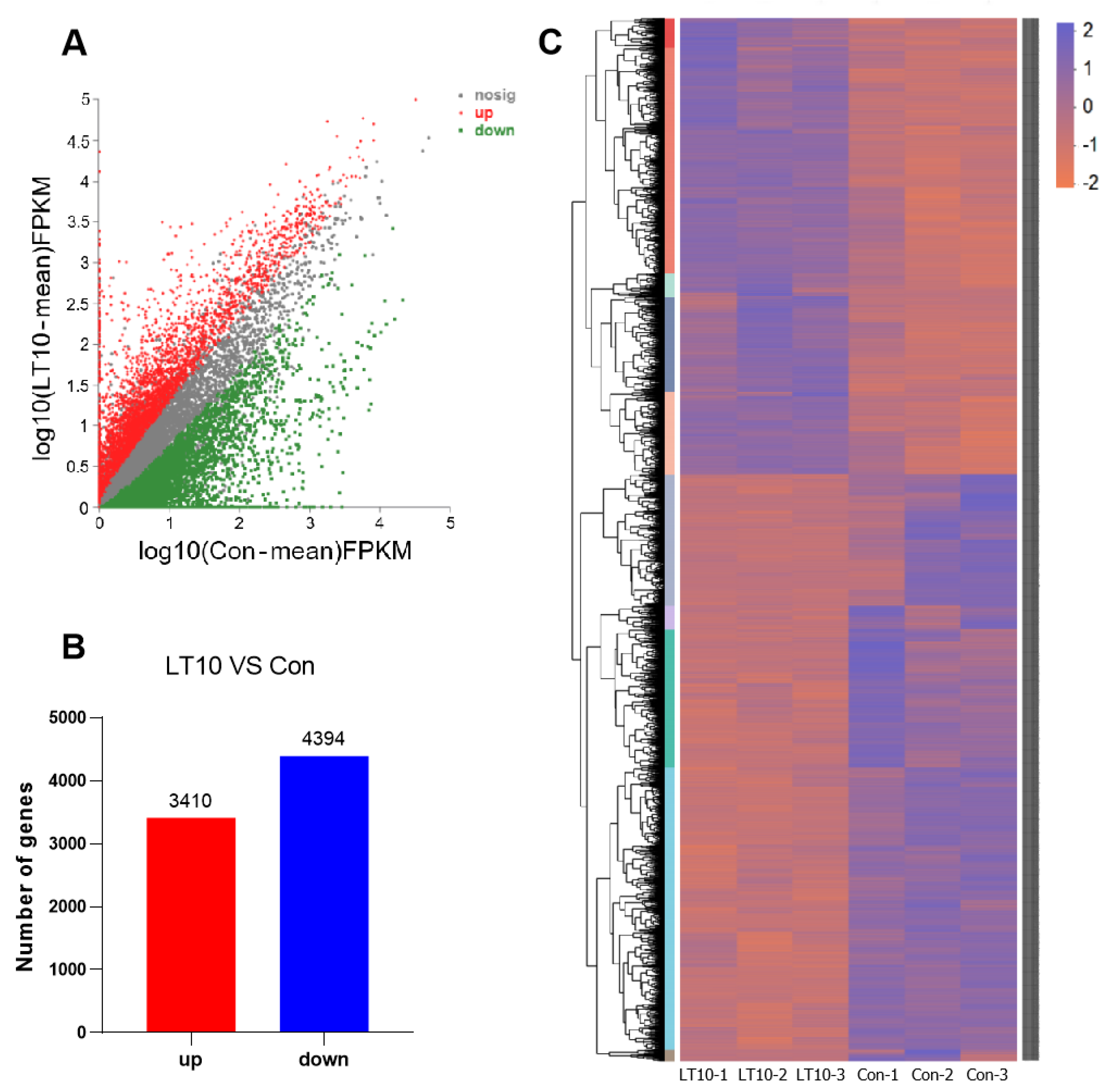
3.3. Transcriptome Profiling of DEGs Induced by Hypothermia in A. grunniens
3.4. GO and KEGG Enrichments of DEGs
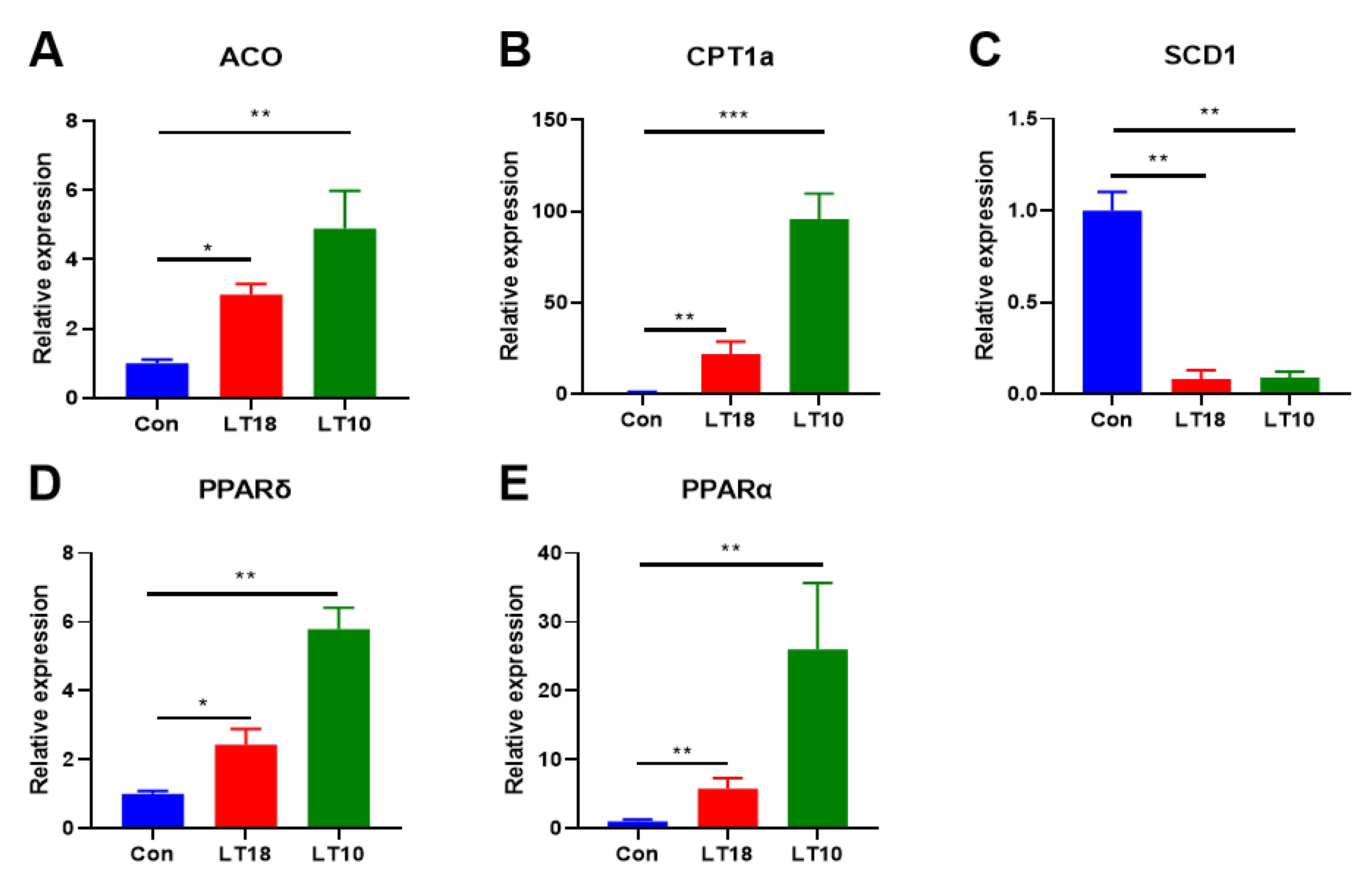
3.5. Expression of Lipid-Metabolism-Related Genes of A. grunniens under Low-Temperature Stress
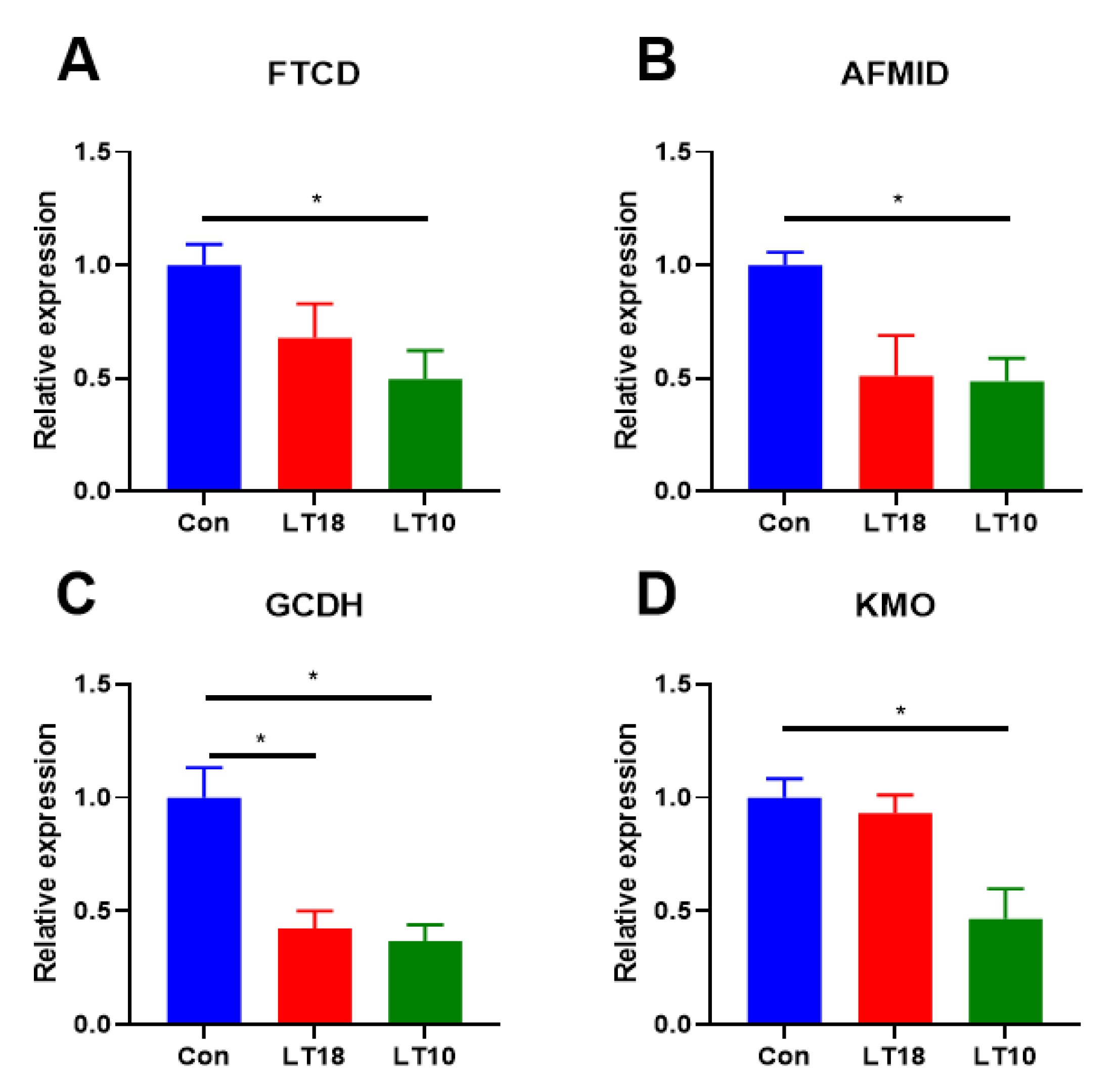
3.6. Expression of Amino Acid Metabolism-Related Genes of A. grunniens under Low-Temperature Stress
3.7. PPARs Signaling Was Involved in the Regulation of Hypothermia in A. grunniens
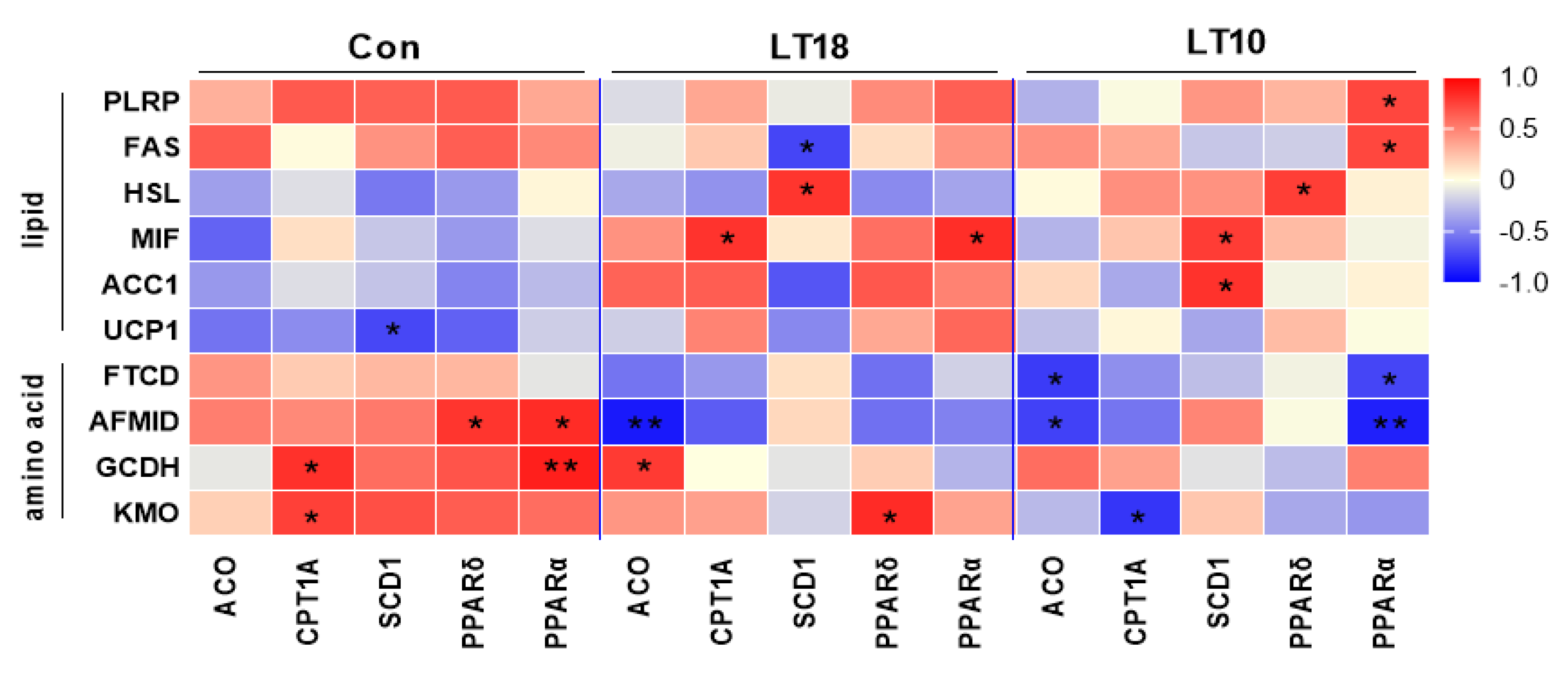
3.8. Lipid and Amino Acid Metabolism of A. grunniens Were Co-Related with PPARs Signaling under Low-Temperature Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, P.; Liu, M.; Liu, Y.; Wang, J.; Zhang, D.; Niu, H.; Jiang, S.; Wang, J.; Zhang, D.; Han, B.; et al. Transcriptome Comparison Reveals a Genetic Network Regulating the Lower Temperature Limit in Fish. Sci. Rep. 2016, 6, 28952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, H.; Li, H.; Wang, A.; Yu, H. Effect of High Temperature on Immune Response of Grass Carp (Ctenopharyngodon Idellus) by Transcriptome Analysis. Fish Shellfifish Immunol 2016, 58, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic Responses to Low Temperature Stress in the Nile Tilapia, Oreochromis Niloticus. Fish Shellfifish Immunol 2019, 84, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Cui, Y.; Liu, B.; Xie, J.; Ge, X.; Xu, P.; Ren, M.; Miao, L.; Zhou, Q.; Lin, Y. HSP60 and HSP90β from Blunt Snout Bream, Megalobrama Amblycephala: Molecular Cloning, Characterization, and Comparative Response to Intermittent Thermal Stress and Aeromonas Hydrophila Infection. Fish Shellfifish Immunol 2018, 74, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Barat, A.; Goel, C.; Tyagi, A.; Ali, S.; Sahoo, P.K. Molecular Cloning and Expression Profile of Snow Trout GPDH Gene in Response to Abiotic Stress. Mol. Biol. Rep. 2012, 39, 10843–10849. [Google Scholar] [CrossRef]
- Hassaan, M.S.; EL Nagar, A.G.; Salim, H.S.; Fitzsimmons, K.; El-Haroun, E.R. Nutritional Mitigation of Winter Thermal Stress in Nile Tilapia by Propolis-Extract: Associated Indicators of Nutritional Status, Physiological Responses and Transcriptional Response of Delta-9-Desaturase Gene. Aquaculture 2019, 511, 734256. [Google Scholar] [CrossRef]
- Soaudy, M.R.; Mohammady, E.Y.; Elashry, M.A.; Ali, M.M.; Ahmed, N.M.; Hegab, M.H.; El-Garhy, H.A.S.; El-Haroun, E.R.; Hassaan, M.S. Possibility Mitigation of Cold Stress in Nile Tilapia under Biofloc System by Dietary Propylene Glycol: Performance Feeding Status, Immune, Physiological Responses and Transcriptional Response of Delta-9-Desaturase Gene. Aquaculture 2021, 538, 736519. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Chen, Y.N.; Kuo, C.M. Physiological Responses, Desaturase Activity, and Fatty Acid Composition in Milkfish (Chanos Chanos) under Cold Acclimation. Aquaculture 2003, 220, 903–918. [Google Scholar] [CrossRef]
- Pankhurst, N.W. The Endocrinology of Stress in Fish: An Environmental Perspective. Gen. Comp. Endocrinol. 2011, 170, 265–275. [Google Scholar] [CrossRef]
- Jun, Q.; Hong, Y.; Hui, W.; Didlyn, K.M.; Jie, H.; Pao, X. Physiological Responses and HSP70 MRNA Expression in GIFT Tilapia Juveniles,Oreochromis Niloticus under Short-Term Crowding. Aquac. Res. 2013, 46, 335–345. [Google Scholar] [CrossRef]
- Salahudeen, A.K. Cold Ischemic Injury of Transplanted Kidneys: New Insights from Experimental Studies. Am. J. Physiol. Ren. Physiol. 2004, 287, F181–F187. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P.; Zhu, Z.X. Temperature Modulates Hepatic Carbohydrate Metabolic Enzyme Activity and Gene Expression in Juvenile GIFT Tilapia (Oreochromis Niloticus) Fed a Carbohydrate-Enriched Diet. J. Therm. Biol. 2014, 40, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-L.; Hu, C.-Y.; Hsu, Y.-T.; Hsieh, T.-J. Influence of Dietary Lipids on the Fatty Acid Composition and Stearoyl-CoA Desaturase Expression in Hybrid Tilapia (Oreochromis Niloticus×O. Aureus) under Cold Shock. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, B.; Røjø, C.; Grisdale-Helland, B.; Rosenlund, G.; Obach, A.; Thomassen, M.S. Influence of Temperature and High Dietary Linoleic Acid Content on Esterification, Elongation, and Desaturation of PUFA in Atlantic Salmon Hepatocytes. Lipids 2003, 38, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Vagner, M.; Santigosa, E. Characterization and Modulation of Gene Expression and Enzymatic Activity of Delta-6 Desaturase in Teleosts: A Review. Aquaculture 2011, 315, 131–143. [Google Scholar] [CrossRef]
- Ibarz, A.; Martín-Pérez, M.; Blasco, J.; Bellido, D.; de Oliveira, E.; Fernández-Borràs, J. Gilthead Sea Bream Liver Proteome Altered at Low Temperatures by Oxidative Stress. Proteomics 2010, 10, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome Proliferator-Activated Receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of Fatty Acid Oxidation in Mouse Cumulus-Oocyte Complexes during Maturation and Modulation by PPAR Agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010, 2010, 393–416. [Google Scholar] [CrossRef]
- Kersten, S.; Mandard, S.; Escher, P.; Gonzalez, F.J.; Tafuri, S.; Desvergne, B.; Wahli, W. The Peroxisome Proliferator-activated Receptor α Regulates Amino Acid Metabolism. FASEB J. 2001, 15, 1971–1978. [Google Scholar] [CrossRef]
- Ye, G.; Gao, H.; Lin, Y.; Ding, D.; Liao, X.; Zhang, H.; Chi, Y.; Dong, S. Peroxisome Proliferator-Activated Receptor A/G Reprogrammes Metabolism Associated with Lipid Accumulation in Macrophages. Metabolomics 2019, 15, 36. [Google Scholar] [CrossRef]
- Barney, R.L. The Distribution of the Fresh-Water Sheepshead, Aplodinotus Grunniens Rafinesque, in Respect to the Glacial History of North America. Ecology 1926, 7, 351–364. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Xu, P.; Tang, Y.; Su, S.; Liu, G.; Wu, N.; Xue, M.; Yu, F.; Feng, W.; et al. Hypothermia-Mediated Apoptosis and Inflammation Contribute to Antioxidant and Immune Adaption in Freshwater Drum, Aplodinotus Grunniens. Antioxidants 2022, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, C.; Wen, H.; Liu, G.; Wu, N.; Li, H.; Xue, M.; Xu, P. MiR-1/AMPK-Mediated Glucose and Lipid Metabolism under Chronic Hypothermia in the Liver of Freshwater Drum, Aplodinotus Grunniens. Metabolites 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wen, H.; Liu, G.; Ma, X.; Lv, G.; Wu, N.; Chen, J.; Xue, M.; Li, H.; Xu, P. Gut Microbes Reveal Pseudomonas Medicates Ingestion Preference via Protein Utilization and Cellular Homeostasis Under Feed Domestication in Freshwater Drum, Aplodinotus Grunniens. Front. Microbiol. 2022, 13, 1831. [Google Scholar] [CrossRef]
- Wen, H.; Ma, X.; Xu, P.; Zheng, B.; Yuan, X.; Zou, J.; Jin, W.; Hua, D.; Gu, R. External Morphology and Internal Anatomical Characters of Juveniles of the Freshwater Drum Aplodinotus Grunniens. J. Fish. Sci. China 2018, 25, 1161. [Google Scholar] [CrossRef]
- Liang, Z.; Haibo, W.; Bingqing, Z.; Hongxia, L.; Changyou, S.; Wu, J.; Xueyan, M.; Pao, X.; Dan, H.; Ruobo, G. Artificial Spawning and Embryonic Development of Freshwater Drum, Aplodinotus Grunniens. J. Fish. Sci. China 2021, 28, 569–578. [Google Scholar] [CrossRef]
- Hernández-Gómez, R.E.; Perera-Garcia, M.A.; Valenzuela, C.I.; Duran, M.T.; Mendoza-Carranza, M. Embryonic Development of Aplodinotus Grunniens (Perciforme: Sciaenidae) in Tenosique, Tabasco, Mexico. Int. J. Morphol. 2013, 31, 633–639. [Google Scholar] [CrossRef]
- Bodensteiner, L.R.; Lewis, W.M. Role of Temperature, Dissolved Oxygen, and Backwaters in the Winter Survival of Freshwater Drum (Aplodinotus Grunniens) in the Mississippi River. Can. J. Fish. Aquat. Sci. 1992, 49, 173–184. [Google Scholar] [CrossRef]
- Edsall, T.A. Biology of the Freshwater Drum in Western Lake Erie. Ohio J. Sci. 1967, 67, 321–340. Available online: https://kb.osu.edu/handle/1811/5342 (accessed on 1 December 2022).
- Jensen, W.B. The Origin of the Soxhlet Extractor. J. Chem. Educ. 2007, 84, 1913. [Google Scholar] [CrossRef]
- Li, H.; Qiang, J.; Song, C.; Xu, P. Transcriptome Profiling Reveal Acanthopanax Senticosus Improves Growth Performance, Immunity and Antioxidant Capacity by Regulating Lipid Metabolism in GIFT (Oreochromis Niloticus). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100784. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Smith-Unna, R.; Boursnell, C.; Patro, R.; Hibberd, J.M.; Kelly, S. TransRate: Reference-Free Quality Assessment of de Novo Transcriptome Assemblies. Genome Res. 2016, 26, 1134–1144. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python Library for Gene Ontology Analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Ge, X.; Li, H.; Liu, B.; Xu, P. MiR-34a/Notch1b Mediated Autophagy and Apoptosis Contributes to Oxidative Stress Amelioration by Emodin in the Intestine of Teleost Megalobrama Amblycephala. Aquaculture 2022, 547, 737441. [Google Scholar] [CrossRef]
- Chu, P.; Wang, T.; Sun, Y.R.; Chu, M.X.; Wang, H.Y.; Zheng, X.; Yin, S. Effect of Cold Stress on the MAPK Pathway and Lipidomics on Muscle of Takifugu Fasciatus. Aquaculture 2021, 540, 736691. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Lika, K.; Anastasiadis, P.; Papandroulakis, N. Projecting Climate Change Impacts on Mediterranean Finfish Production: A Case Study in Greece. Clim. Chang. 2021, 165, 67. [Google Scholar] [CrossRef]
- Kong, X.F.; Yin, F.G.; He, Q.H.; Liu, H.J.; Li, T.J.; Huang, R.L.; Fan, M.Z.; Liu, Y.L.; Hou, Y.Q.; Li, P.; et al. Acanthopanax Senticosus Extract as a Dietary Additive Enhances the Apparent Ileal Digestibility of Amino Acids in Weaned Piglets. Livest. Sci. 2009, 123, 261–267. [Google Scholar] [CrossRef]
- Karanova, M.V. Impact of Seasonal Temperature Decrease and Cold Shock on the Composition of Free Amino Acids and Phosphomonoethers in Various Organs of Amur Sleeper Percottus Glenii (Eleotridae). J. Ichthyol. 2018, 58, 570–579. [Google Scholar] [CrossRef]
- Zeytin, S.; Schulz, C.; Ueberschär, B. Diurnal Patterns of Tryptic Enzyme Activity under Different Feeding Regimes in Gilthead Sea Bream (Sparus Aurata) Larvae. Aquaculture 2016, 457, 85–90. [Google Scholar] [CrossRef]
- Mir, I.N.; Srivastava, P.P.; Bhat, I.A.; Muralidhar, A.P.; Varghese, T.; Gireesh-Babu, P.; Jain, K.K. Expression and Activity of Trypsin and Pepsin during Larval Development of Indian Walking Catfish (Clarias Magur). Aquaculture 2018, 491, 266–272. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Kashinskaya, E.N.; Rogozhin, E.A.; Moyano, F.J. Seasonal Changes in Kinetic Parameters of Trypsin in Gastric and Agastric Fish. Fish Physiol. Biochem. 2021, 47, 381–391. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, A.-L.; Xian, J.-A. Variation of Free Amino Acid and Carbohydrate Concentrations in White Shrimp, Litopenaeus Vannamei: Effects of Continuous Cold Stress. Aquaculture 2011, 317, 182–186. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Liu, Q.; Ye, H.; Zou, C.; Xu, M.; Zhang, Y.; Ye, C. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus Coioides) under Cold Stress. Aquaculture 2019, 498, 545–555. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.V.; Xu, H.E.; Turk, J.; Semenkovich, C.F. Identification of a Physiologically Relevant Endogenous Ligand for PPARα in Liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.-J. Hormone-Sensitive Lipase Knockouts. Nutr. Metab. 2006, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chen, Z.; Zhang, W.; Li, L.; Sun, R.; Deng, C.; Fei, Z.; Sheng, Z.; Wang, L.; Sun, X.; et al. Increased Fat Mass and Insulin Resistance in Mice Lacking Pancreatic Lipase-Related Protein 1. J. Nutr. Biochem. 2011, 22, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Tang, D.; Zhu, Z.; Li, Y.; Huang, T.; Müller, R.; Yu, W.; Li, P. ACC1 (Acetyl Coenzyme A Carboxylase 1) Is a Potential Immune Modulatory Target of Cerebral Ischemic Stroke. Stroke 2019, 50, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Harmon, C.; Jedrychowski, M.P.; Xiao, H.; Garrity, R.; Tran, N.V.; Bradshaw, G.A.; Fu, A.; Szpyt, J.; Reddy, A.; et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab. 2021, 3, 604–617. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R. Role of Macrophage Migration Inhibitory Factor in Obesity, Insulin Resistance, Type 2 Diabetes, and Associated Hepatic Co-Morbidities: A Comprehensive Review of Human and Rodent Studies. Front. Immunol. 2015, 6, 306. [Google Scholar] [CrossRef]
- Solans, A.; Estivill, X.; de la Luna, S. Cloning and Characterization of Human FTCD on 21q22.3, a Candidate Gene for Glutamate Formiminotransferase Deficiency. Cytogenet. Cell Genet. 2000, 88, 43–49. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Lafita-Navarro, M.C.; Hao, Y.-H.; Kilgore, J.A.; Perez-Castro, L.; Braverman, J.; Borenstein-Auerbach, N.; Kim, M.; Lesner, N.P.; Mishra, P.; et al. MYC Promotes Tryptophan Uptake and Metabolism by the Kynurenine Pathway in Colon Cancer. Genes Dev. 2019, 33, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.; Chung, T.; Huang, W.; Hsu, C.; Liu, C.; Chiu, Y.; Huang, K.; Liao, A.T.; Lin, C. Kynurenine 3-monooxygenase (KMO), and Signal Transducer and Activator of Transcription 3 (STAT3) Expression Is Involved in Tumour Proliferation and Predicts Poor Survival in Canine Melanoma. Vet. Comp. Oncol. 2020, 19, 79–91. [Google Scholar] [CrossRef]
- Wajner, M.; Amaral, A.U.; Leipnitz, G.; Seminotti, B. Pathogenesis of Brain Damage in Glutaric Acidemia Type I: Lessons from the Genetic Mice Model. Int. J. Dev. Neurosci 2019, 78, 215–221. [Google Scholar] [CrossRef]
- Mandard, S.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor a Target Genes. Cell. Mol. Life Sci. 2004, 61, 393–416. [Google Scholar] [CrossRef]
- Shimada, M.; Hibi, M.; Nakagawa, T.; Hayakawa, T.; Field, C.J. High-Fructose Diet-Induced Hepatic Expression of the Scd1 Gene Is Associated with Increased Acetylation of Histones H3 and H4 and the Binding of ChREBP at the Scd1 Promoter in Rats. Biomed. Res. 2021, 42, 85–88. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.L.; Yu, D.H.; Lv, C.H.; Luo, H.Y.; Wang, Z.Y. Molecular Cloning and Expression Analysis of Scd1 Gene from Large Yellow Croaker Larimichthys Crocea under Cold Stress. Gene 2015, 568, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sblano, S.; Cerchia, C.; Laghezza, A.; Piemontese, L.; Brunetti, L.; Leuci, R.; Gilardi, F.; Thomas, A.; Genovese, M.; Santi, A.; et al. A Chemoinformatics Search for Peroxisome Proliferator-Activated Receptors Ligands Revealed a New Pan-Agonist Able to Reduce Lipid Accumulation and Improve Insulin Sensitivity. Eur. J. Med. Chem. 2022, 235, 114240. [Google Scholar] [CrossRef] [PubMed]


| Amino Acids | Hydrolyzed Amino Acid | Free Amino Acid | ||||
|---|---|---|---|---|---|---|
| 25 °C | 18 °C | 10 °C | 25 °C | 18 °C | 10 °C | |
| Arginine | 2.61 ± 0.08 a | 2.95 ± 0.13 a | 2.85 ± 0.29 a | 0.0157 ± 0.00639 a | 0.005 ± 0.00298 b | 0.0024 ± 0.00017 b |
| Histidine | 1.00 ± 0.04 a | 0.92 ± 0.05 a | 0.90 ± 0.10 a | 0.1236 ± 0.01007 a | 0.0107 ± 0.01044 b | 0.0348 ± 0.01737 b |
| Isoleucine | 1.8- ± 0.02 a | 2.07 ± 0.12 a | 2.00 ± 0.23 a | 0.0062 ± 0.00316 a | 0.0045 ± 0.00066 a | 0.0044 ± 0.00031 a |
| Leucine | 2.91 ± 0.04 a | 3.34 ± 0.18 a | 3.21 ± 0.36 a | 0.0168 ± 0.00251 a | 0.0095 ± 0.00135 a | 0.0051 ± 0.00252 a |
| Lysine | 3.48 ± 0.08 a | 3.78 ± 0.20 a | 3.80 ± 0.43 a | 0.1586 ± 0.01514 a | 0.0543 ± 0.01039 b | 0.1242 ± 0.01722 ab |
| Methionine | 0.85 ± 0.05 a | 0.87 ± 0.07 a | 0.82 ± 0.09 a | 0.0074 ± 0.00178 a | 0.0161 ± 0.00744 a | 0.0178 ± 0.01332 a |
| Phenylalanine | 1.65 ± 0.02 a | 1.84 ± 0.09 a | 1.78 ± 0.19 a | 0.0084 ± 0.00149 a | 0.0043 ± 0.00118 b | 0.0041 ± 0.00029 b |
| Threonine | 1.36 ± 0.02 a | 1.53 ± 0.07 a | 1.46 ± 0.15 a | 0.0681 ± 0.00878 a | 0.0344 ± 0.00307 b | 0.0427 ± 0.00361 b |
| Valine | 2.08 ± 0.02 a | 2.32 ± 0.12 a | 2.25 ± 0.25 a | 0.0428 ± 0.00213 a | 0.0215 ± 0.00957 a | 0.0227 ± 0.00926 a |
| total EAA | 17.73 ± 0.33 a | 19.62 ± 1.02 a | 19.07 ± 3.62 a | 0.4476 ± 0.00548 a | 0.1603 ± 0.01095 c | 0.2581 ± 0.02536 b |
| Alanine | 2.86 ± 0.09 a | 3.13 ± 0.12 a | 3.05 ± 0.26 a | 0.082 ± 0.00739 a | 0.0541 ± 0.02722 a | 0.0681 ± 0.00964 a |
| Aspartic acid | 4.19 ± 0.07 a | 4.73 ± 0.39 a | 4.57 ± 0.48 a | 0.0288 ± 0.00435 a | 0.0166 ± 0.00088 b | 0.0203 ± 0.00239 ab |
| Cysteine | 0.05 ± 0.00 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.0006 ± 0.00019 a | 0.0006 ± 0.00035 a | 0.0003 ± 0.00009 a |
| Glutamic acid | 6.49 ± 0.11 a | 7.20 ± 0.35 a | 7.01 ± 0.73 a | 0.12757 ± 0.01672 a | 0.06913 ± 0.00636 b | 0.08979 ± 0.00538 ab |
| Glycine | 3.97 ± 0.23 a | 4.16 ± 0.09 a | 4.12 ± 0.35 a | 0.3315 ± 0.02239 a | 0.2898 ± 0.02433 a | 0.329 ± 0.05891 a |
| Proline | 2.20 ± 0.09 a | 2.31 ± 0.06 a | 2.40 ± 0.26 a | 0.0899 ± 0.01459 a | 0.0346 ± 0.01009 b | 0.053 ± 0.00871 ab |
| Serine | 1.46 ± 0.03 a | 1.65 ± 0.07 a | 1.57 ± 0.17 a | 0.0051 ± 0.00199 a | 0.0112 ± 0.00702 a | 0.0043 ± 0.00183 a |
| Tyrosine | 0.93 ± 0.03 a | 1.14 ± 0.06 a | 1.03 ± 0.12 a | 0.0075 ± 0.00165 a | 0.0014 ± 0.00127 b | 0.0003 ± 0.00006 b |
| Total NEAA | 22.04 ± 0.59 a | 24.39 ± 1.67 a | 23.78 ± 2.37 a | 0.6730 ± 0.06312 a | 0.4692 ± 0.06114 a | 0.5652 ± 0.07076 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Wen, H.; Xu, P.; Chen, J.; Xue, M.; Li, J.; Wang, M.; Song, C.; Li, H. PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens). Metabolites 2023, 13, 102. https://doi.org/10.3390/metabo13010102
Wu N, Wen H, Xu P, Chen J, Xue M, Li J, Wang M, Song C, Li H. PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens). Metabolites. 2023; 13(1):102. https://doi.org/10.3390/metabo13010102
Chicago/Turabian StyleWu, Ningyuan, Haibo Wen, Pao Xu, Jianxiang Chen, Miaomiao Xue, Jianlin Li, Meiyao Wang, Changyou Song, and Hongxia Li. 2023. "PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens)" Metabolites 13, no. 1: 102. https://doi.org/10.3390/metabo13010102
APA StyleWu, N., Wen, H., Xu, P., Chen, J., Xue, M., Li, J., Wang, M., Song, C., & Li, H. (2023). PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens). Metabolites, 13(1), 102. https://doi.org/10.3390/metabo13010102








