Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use
Abstract
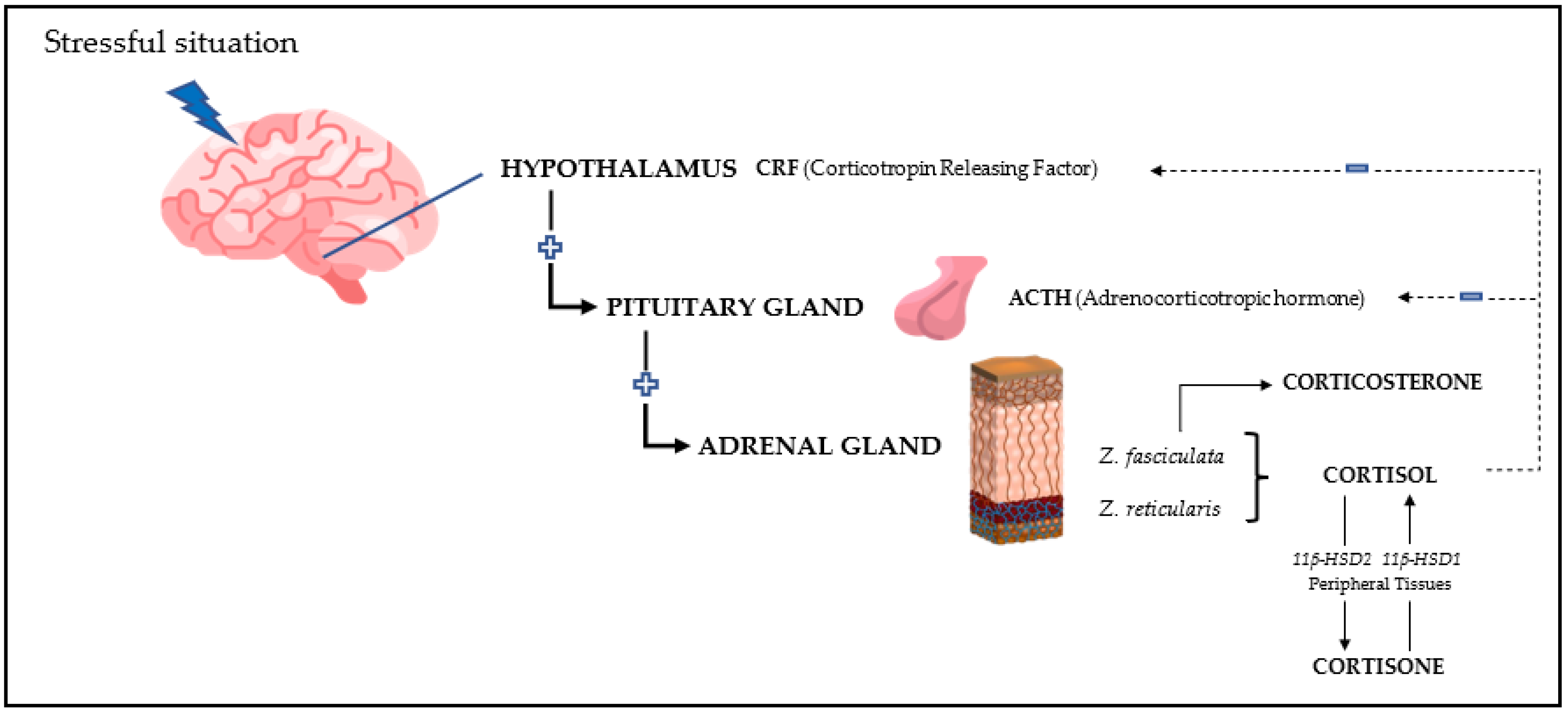
1. Introduction
2. General Characteristics of the Main Glucocorticoids
3. Measurement
- (1)
- Those using techniques based on the reaction of an antibody with the analyte to be measured, such as radioimmunoassay (RIA), enzyme immunoassay (EIA), chemiluminescence, and, more recently, bead-based luminescent amplification assays (AlphaLISA). RIA assays are currently used with less frequency due to the need of special facilities and the radioactive nature of some components.
- (2)
4. Studies Where Cortisol and Cortisone Were Measured in Combination
4.1. Studies on Animals
4.1.1. Studies on Pigs
4.1.2. Studies on other Species
4.2. Studies on Humans
5. Studies Where Cortisol and Corticosterone Were Measured in Combination
5.1. Studies on Animals
5.1.1. Studies on Cows
5.1.2. Studies on Birds
5.1.3. Studies on Laboratory Rodents
5.1.4. Studies on Other Species
5.2. Studies on Humans
6. Studies Where Total Steroids and Selected Glucocorticoids Were Measured in Combination
6.1. Studies on Cortisol-Dominant Species
6.2. Studies on Corticosterone-Dominant Species
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, S.; Wei, F.; Li, G. The Evolution of the Concept of Stress and the Framework of the Stress System. Cell Stress 2021, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.R.; Tilbrook, A.J. INVITED REVIEW: The Usefulness of Measuring Glucocorticoids for Assessing Animal Welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Fink, G. Stress: Definition and History Introduction and Historical Outline of Several Stress Concepts; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, Consequences and Biomarkers of Stress in Swine: An Update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress in Health and Disease; Butterworth-Heinemann: Oxford, UK, 1976. [Google Scholar]
- Zulkifli, I. Review of Human-Animal Interactions and Their Impact on Animal Productivity and Welfare. J. Anim. Sci. Biotechnol. 2013, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring Cortisol, the Major Stress Hormone in Fishes. J. Fish. Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Blair, J.; Adaway, J.; Keevil, B.; Ross, R. Salivary Cortisol and Cortisone in the Clinical Setting. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 161–168. [Google Scholar] [CrossRef]
- Raff, H. CORT, Cort, B, Corticosterone, and Now Cortistatin: Enough Already! Endocrinology 2016, 157, 3307–3308. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Patterson, L.D.; Redfern, J.C.; Pleizier, N.K.; Belanger, C.B.; Midwood, J.D.; Crossin, G.T.; Cooke, S.J. Manipulating Glucocorticoids in Wild Animals: Basic and Applied Perspectives. Conserv. Physiol. 2015, 3, cov031. [Google Scholar] [CrossRef]
- Di Francesco, J.; Mastromonaco, G.F.; Rowell, J.E.; Blake, J.; Checkley, S.L.; Kutz, S. Fecal Glucocorticoid Metabolites Reflect Hypothalamic–Pituitary–Adrenal Axis Activity in Muskoxen (Ovibos moschatus). PLoS ONE 2021, 16, e0249281. [Google Scholar] [CrossRef]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A Scientific Conception of Animal Welfare That Reflects Ethical Concerns. Animal Welfare 1997, 6, 187–205. [Google Scholar]
- Perogamvros, I.; Aarons, L.; Miller, A.G.; Trainer, P.J.; Ray, D.W. Corticosteroid-Binding Globulin Regulates Cortisol Pharmacokinetics. Clin. Endocrinol. 2011, 74, 30–36. [Google Scholar] [CrossRef]
- De Guia, R.M. Stress, Glucocorticoid Signaling Pathway, and Metabolic Disorders. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1273–1280. [Google Scholar] [CrossRef]
- Steroid Definition and Examples—Biology Online Dictionary. Available online: https://www.biologyonline.com/dictionary/steroid (accessed on 21 November 2022).
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Cortisol (CHEBI:17650). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:17650 (accessed on 28 November 2022).
- Cortisone (CHEBI:16962). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:16962 (accessed on 28 November 2022).
- Corticosterone (CHEBI:16827). Available online: https://www.ebi.ac.uk/chebi/chebiOntology.do?chebiId=CHEBI:16827 (accessed on 21 November 2022).
- What Are the Differences and Similarities between Cortisol vs Corticosterone?—Brain Stuff. Available online: https://brainstuff.org/blog/differences-similarities-between-cortisol-corticosterone (accessed on 21 November 2022).
- Cortisone |C21H28O5—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cortisone (accessed on 21 November 2022).
- Tomlinson, J.W.; Stewart, P.M. Cortisol Metabolism and the Role of 11β-Hydroxysteroid Dehydrogenase. Best Pr. Res. Clin. Endocrinol. Metab. 2001, 15, 61–78. [Google Scholar] [CrossRef]
- Hydrocortisone|C21H30O5—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrocortisone (accessed on 21 November 2022).
- Terao, M.; Katayama, I. Local Cortisol/Corticosterone Activation in Skin Physiology and Pathology. J. Dermatol. Sci. 2016, 84, 11–16. [Google Scholar] [CrossRef]
- Ruis, M.A.; Te Brake, J.H.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. The Circadian Rhythm of Salivary Cortisol in Growing Pigs: Effects of Age, Gender, and Stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Weitzman, E.D.; Fukushima, D.; Nogeire, C.; Roffwarg, H.; Gallagher, T.F.; Hellman, L. Twenty-Four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J. Clin. Endocrinol. Metab. 1971, 33, 14–22. [Google Scholar] [CrossRef]
- Wood, P.J.; Barth, J.H.; Freedman, D.B.; Perry, L.; Sheridan, B. Evidence for the Low Dose Dexamethasone Suppression Test to Screen for Cushing’s Syndrome-Recommendations for a Protocol for Biochemistry Laboratories. Ann. Clin. Biochem. 1997, 34, 222–229. [Google Scholar] [CrossRef]
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brooks, H.L. The Adrenal Medulla & Adrenal Cortex. In Ganong’s Review of Medical Physiology, 25e; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Palme, R. Non-Invasive Measurement of Glucocorticoids: Advances and Problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Majelantle, T.L.; Mcintyre, T.; Ganswindt, A. Monitoring the Effects of Land Transformation on African Clawless Otters (Aonyx capensis) Using Fecal Glucocorticoid Metabolite Concentrations as a Measure of Stress. Integr. Zool. 2020, 15, 293–306. [Google Scholar] [CrossRef]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of Corticosterone Metabolites in Birds’ Droppings: An Analytical Approach. Ann. N. Y. Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A.; Monfort, S.L.; Whitney, T.K.; Mechref, Y.S.; Novotny, M.; McClintock, M.K. Frequent Serial Fecal Corticoid Measures from Rats Reflect Circadian and Ovarian Corticosterone Rhythms. J. Endocrinol. 2005, 184, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Altmann, J.; Isani, S.S.; Yu, J. A Matter of Time: Evaluating the Storage of Fecal Samples for Steroid Analysis. Gen. Comp. Endocrinol. 2002, 128, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wolf, T.E.; Mangwiro, N.; Fasina, F.O.; Ganswindt, A. Non-Invasive Monitoring of Adrenocortical Function in Female Domestic Pigs Using Saliva and Faeces as Sample Matrices. PLoS ONE 2020, 15, e0234971. [Google Scholar] [CrossRef] [PubMed]
- De Palo, E.F.; Antonelli, G.; Benetazzo, A.; Prearo, M.; Gatti, R. Human Saliva Cortisone and Cortisol Simultaneous Analysis Using Reverse Phase HPLC Techn.nique. Clin. Chim. Acta 2009, 405, 60–65. [Google Scholar] [CrossRef]
- Okumura, T.; Nakajima, Y.; Takamatsu, T.; Matsuoka, M. Column-Switching High.-Performance Liquid Chromatographic System with a Laser-Induced Fluorimetric Detector for Direct, Automated Assay of Salivary Cortisol. J. Chromatogr. B Biomed. Sci. Appl. 1995, 670, 11–20. [Google Scholar] [CrossRef]
- Carrozza, C.; Lapolla, R.; Gervasoni, J.; Rota, C.A.; Locantore, P.; Pontecorvi, A.; Zuppi, C.; Persichilli, S. Assessment of Salivary Free Cortisol Levels by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) in Patients Treated with Mitotane. Hormones 2012, 11, 344–349. [Google Scholar] [CrossRef]
- Jonsson, B.A.G.; Malmberg, B.; Amilon, A.; Garde, A.H.; Ørbaek, P.D. Etermination of Cortisol in Human Saliva Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. J. Chromatogr. B 2003, 784, 63–68. [Google Scholar] [CrossRef]
- Agusti, C.; Carbajal, A.; Olvera-Maneu, S.; Domingo, M.; Lopez-Bejar, M. Blubber and Serum Cortisol Concentrations as Indicators of the Stress Response and Overall Health Status in Striped Dolphins. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 272, 111268. [Google Scholar] [CrossRef]
- Prims, S.; vanden Hole, C.; van Cruchten, S.; van Ginneken, C.; van Ostade, X.; Casteleyn, C. Hair or Salivary Cortisol Analysis to Identify Chronic Stress in Piglets? Vet. J. 2019, 252, 105357. [Google Scholar] [CrossRef]
- Naskar, S.; Borah, S.; Vashi, Y.; Thomas, R.; Sarma, D.K.; Goswami, J.; Dhara, S.K. Steroid and Metabolic Hormonal Profile of Porcine Serum Vis-à-Vis Ovarian Follicular Fluid. Vet. World 2016, 9, 1320–1323. [Google Scholar] [CrossRef]
- Zhao, F.; Wei, Q.-W.; Li, B.-J.; Weng, Q.-N.; Jiang, Y.; Ning, C.-B.; Liu, K.-Q.; Wu, W.-J.; Liu, H.-L. Impact of Adrenocorticotropin Hormone Administration on the Endocrinology, Estrus Onset, and Ovarian Function of Weaned Sows. Endocr. J. 2022, 69, 23–33. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Validation of an Automated Chemiluminescent Immunoassay for Salivary Cortisol Measurements in Pigs. J. Vet. Diagn Investig. 2012, 24, 918–923. [Google Scholar] [CrossRef]
- López-Arjona, M.; Tecles, F.; Mateo, S.V.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Cerón, J.J.; Martínez-Subiela, S. Measurement of Cortisol, Cortisone and 11β-Hydroxysteroid Dehydrogenase Type 2 Activity in Hair of Sows during Different Phases of the Reproductive Cycle. Vet. J. 2020, 259–260. [Google Scholar] [CrossRef]
- Aydin, E.; Drotleff, B.; Noack, H.; Derntl, B.; Lämmerhofer, M. Fast Accurate Quantification of Salivary Cortisol and Cortisone in a Large-Scale Clinical Stress Study by Micro-UHPLC-ESI-MS/MS Using a Surrogate Calibrant Approach. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1182, 122939. [Google Scholar] [CrossRef]
- Puglisi, S.; Leporati, M.; Amante, E.; Parisi, A.; Pia, A.R.; Berchialla, P.; Terzolo, M.; Vincenti, M.; Reimondo, G. Limited Role of Hair Cortisol and Cortisone Measurement for Detecting Cortisol Autonomy in Patients with Adrenal Incidentalomas. Front. Endocrinol. 2022, 13, 833514. [Google Scholar] [CrossRef]
- Chiesa, L.; Pavone, S.; Pasquale, E.; Pavlovic, R.; Panseri, S.; Valiani, A.; Arioli, F.; Manuali, E. Study on Cortisol, Cortisone and Prednisolone Presence in Urine of Chianina Cattle Breed. J. Anim. Physiol. Anim. Nutr. 2017, 101, 893–903. [Google Scholar] [CrossRef]
- Lang, J.; Stickel, S.; Gaum, P.M.; Habel, U.; Bertram, J.; Eickhoff, S.B.; Chechko, N. Predicting Hair Cortisol and Cortisone Concentration in Postpartum Women through Repeated Measurements of Perceived Stress. Metabolites 2021, 11, 815. [Google Scholar] [CrossRef]
- Scharlau, F.; Pietzner, D.; Vogel, M.; Gaudl, A.; Ceglarek, U.; Thiery, J.; Kratzsch, J.; Hiemisch, A.; Kiess, W. Evaluation of Hair Cortisol and Cortisone Change during Pregnancy and the Association with Self-Reported Depression, Somatization, and Stress Symptoms. Stress 2018, 21, 43–50. [Google Scholar] [CrossRef]
- Hamilton, J.; Allard, S.; Racine, H.; Skalican Guthrie, K.; Hill, T.; Loughman, Z. Impact of Indigestible Materials on the Efficiency of Fecal Corticosterone Immunoassay Testing in Pituophis Species. Animals 2022, 12, 1410. [Google Scholar] [CrossRef]
- Rowell, M.K.; Santymire, R.M.; Rymer, T.L. Corticosterone Metabolite Concentration Is Not Related to Problem Solving in the Fawn-Footed Mosaic-Tailed Rat Melomys Cervinipes. Animals 2021, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.G.F.; de Araujo, L.D.; Roa, S.L.R.; Bueno, A.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Moreira, A.C.; Elias, L.L.K.; de Castro, M.; Martins, C.S. Restricted Feeding Modulates Peripheral Clocks and Nutrient Sensing Pathways in Rats. Arch. Endocrinol. Metab. 2021, 65, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zietek, M.; Sochaczewska, D.; Swiatkowska-Freund, M.; Celewicz, Z.; Szczuko, M. The Possible Role of Corticosterone in Regulating Sodium and Potassium Concentrations in Human Milk. Ginekol. Pol. 2021, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, E.M.; Scheun, J.; Dehnhard, M.; Kumar, V.; Umapathy, G.; Ganswindt, A. Non-Invasive Monitoring of Glucocorticoid Metabolite Concentrations in Native Indian, as Well as Captive and Re-Wilded Tigers in South Africa. Gen. Comp. Endocrinol. 2021, 308, 113783. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; McWhorter, T.J. Fecal Glucocorticoid Metabolite Concentration as a Tool for Assessing Impacts of Interventions in Humboldt Penguins (Spheniscus humboldti). Birds 2021, 2, 106–113. [Google Scholar] [CrossRef]
- Yarnell, K.; Purcell, R.S.; Walker, S.L. Fecal Glucocorticoid Analysis: Non-Invasive Adrenal Monitoring in Equids. J. Vis. Exp. 2016, 110, 53479. [Google Scholar] [CrossRef]
- Washburn, B.E.; Millspaugh, J.J.; Schulz, J.H.; Jones, S.B.; Mong, T. Using Fecal Glucocorticoids for Stress Assessment in Mourning Doves. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1516&context=icwdm_usdanwrc (accessed on 16 November 2022).
- Young, A.M.; Hallford, D.M. Validation of a Fecal Glucocorticoid Metabolite Assay to Assess Stress in the Budgerigar (Melopsittacus undulatus). Zoo Biol. 2013, 32, 112. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol Determination in Hair and Faeces from Domestic Cats and Dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Bechshøft, T.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol Levels in Hair of East Greenland Polar Bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [CrossRef]
- Romero, L.M.; Fairhurst, G.D. Measuring Corticosterone in Feathers: Strengths, Limitations, and Suggestions for the Future. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 202, 112–122. [Google Scholar] [CrossRef]
- Skarlandtová, H.; Bičíková, M.; Neužil, P.; Mlček, M.; Hrachovina, V.; Svoboda, T.; Medová, E.; Kudlička, J.; Dohnalová, A.; Havránek, Š.; et al. The Cortisol to Cortisone Ratio during Cardiac Catheterisation in Sows. Prague Med. Rep. 2015, 116, 279–289. [Google Scholar] [CrossRef]
- Giergiel, M.; Olejnik, M.; Jabłoński, A.; Posyniak, A. The Markers of Stress in Swine Oral Fluid. J. Vet. Res. 2021, 65, 487–495. [Google Scholar] [CrossRef]
- Wiseman, S.; Thomas, J.K.; McPhee, L.; Hursky, O.; Raine, J.C.; Pietrock, M.; Giesy, J.P.; Hecker, M.; Janz, D.M. Attenuation of the Cortisol Response to Stress in Female Rainbow Trout Chronically Exposed to Dietary Selenomethionine. Aquat. Toxicol. 2011, 105, 643–651. [Google Scholar] [CrossRef]
- Ellis, T.; James, J.D.; Stewart, C.; Scott, A.P. A Non-Invasive Stress Assay Based upon Measurement of Free Cortisol Released into the Water by Rainbow Trout. J. Fish. Biol 2004, 65, 1233–1252. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Bohlin, J.; Dahl, E.; Knappe-Poindecker, M.; Fjeldaas, T.; Lepschy, M.; Palme, R.; Langbein, J.; Ropstad, E. Assessment of Chronic Stress in Sheep (Part I): The Use of Cortisol and Cortisone in Hair as Non-Invasive Biological Markers. Small Rumin. Res. 2015, 132, 25–31. [Google Scholar] [CrossRef]
- Bae, Y.J.; Reinelt, J.; Netto, J.; Uhlig, M.; Willenberg, A.; Ceglarek, U.; Villringer, A.; Thiery, J.; Gaebler, M.; Kratzsch, J. Salivary Cortisone, as a Biomarker for Psychosocial Stress, Is Associated with State Anxiety and Heart Rate. Psychoneuroendocrinology 2019, 101, 35–41. [Google Scholar] [CrossRef]
- Elder, C.J.; Harrison, R.F.; Cross, A.S.; Vilela, R.; Keevil, B.G.; Wright, N.P.; Ross, R.J. Use of Salivary Cortisol and Cortisone in the High- and Low-Dose Synacthen Test. Clin. Endocrinol. 2018, 88, 772–778. [Google Scholar] [CrossRef]
- Harrison, R.F.; Debono, M.; Whitaker, M.J.; Keevil, B.G.; Newell-Price, J.; Ross, R.J. Salivary Cortisone to Estimate Cortisol Exposure and Sampling Frequency Required Based on Serum Cortisol Measurements. J. Clin. Endocrinol. Metab. 2018, 104, 765–772. [Google Scholar] [CrossRef]
- Del Corral, P.; Schurman, R.C.; Kinza, S.S.; Fitzgerald, M.J.; Kordick, C.A.; Rusch, J.L.; Nadolski, J.B. Salivary but Not Plasma Cortisone Tracks the Plasma Cortisol Response to Exercise: Effect of Time of Day. J. Endocrinol. Investig. 2016, 39, 315–322. [Google Scholar] [CrossRef]
- Davison, B.; Singh, G.R.; McFarlane, J. Hair Cortisol and Cortisone as Markers of Stress in Indigenous and Non-Indigenous Young Adults. Stress 2019, 22, 210–220. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Hu, K.; Yang, J. Evidence of the Moderating Role of Hair Cortisol and Hair Cortisone in the Relationship between Work Stress and Depression Symptoms among Chinese Fishermen. J. Affect. Disord. 2021, 294, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Brossaud, J.; Charret, L.; de Angeli, D.; Haissaguerre, M.; Ferriere, A.; Puerto, M.; Gatta-Cherifi, B.; Corcuff, J.-B.; Tabarin, A. Hair Cortisol and Cortisone Measurements for the Diagnosis of Overt and Mild Cushing’s Syndrome. Eur. J. Endocrinol. 2021, 184, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, F.; Settanni, F.; Parasiliti-Caprino, M.; Rumbolo, F.; Nonnato, A.; Ricciardo, M.; Amante, E.; Priolo, G.; Vitali, S.; Anfossi, L.; et al. Reference Ranges of Late-Night Salivary Cortisol and Cortisone Measured by LC-MS/MS and Accuracy for the Diagnosis of Cushing’s Syndrome. J. Endocrinol. Investig. 2020, 43, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in Hair and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Shen, K.; Liu, P.; Ye, K.; Wang, Y.; Li, C.; Kang, X.; Song, Y. Increased Cortisol and Cortisone Levels in Overweight Children. Med. Sci. Monit. Basic Res. 2017, 23, 25. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Z.; Chen, S.; Yu, T.; Wang, J.; Wang, W.; Deng, H. Correlations of Hair Level with Salivary Level in Cortisol and Cortisone. Life Sci. 2018, 193, 57–63. [Google Scholar] [CrossRef]
- Venkataseshu, G.K.; Estergreen, V.L. Cortisol and Corticosterone in Bovine Plasma and the Effect of Adrenocorticotropin. J. Dairy Sci. 1970, 53, 480–483. [Google Scholar] [CrossRef]
- Gross, J.J.; Schwinn, A.C.; Bruckmaier, R.M. Free and Bound Cortisol, Corticosterone, and Metabolic Adaptations during the Early Inflammatory Response to an Intramammary Lipopolysaccharide Challenge in Dairy Cows. Domest. Anim. Endocrinol. 2021, 74, 106554. [Google Scholar] [CrossRef]
- Koren, L.; Nakagawa, S.; Burke, T.; Soma, K.K.; Wynne-Edwards, K.E.; Geffen, E. Non-Breeding Feather Concentrations of Testosterone, Corticosterone and Cortisol Are Associated with Subsequent Survival in Wild House Sparrows. Proc. R. Soc. B Biol. Sci. 2012, 279, 1560–1566. [Google Scholar] [CrossRef]
- Schmidt, K.L.; Chin, E.H.; Shah, A.H.; Soma, K.K. Cortisol and Corticosterone in Immune Organs and Brain of European Starlings: Developmental Changes, Effects of Restraint Stress, Comparison with Zebra Finches. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 42–51. [Google Scholar] [CrossRef]
- Tetel, V.; van Wyk, B.; Fraley, G.S. Sex Differences in Glucocorticoid Responses to Shipping Stress in Pekin Ducks. Poult. Sci. 2022, 101, 101534. [Google Scholar] [CrossRef]
- Flament, A.; Delleur, V.; Poulipoulis, A.; Marlier, D. Corticosterone, Cortisol, Triglycerides, Aspartate Aminotransferase and Uric Acid Plasma Concentrations during Foie Gras Production in Male Mule Ducks (Anas platyrhynchos × Cairina moschata). Br. Poult. Sci. 2012, 53, 408–413. [Google Scholar] [CrossRef]
- Romero, L.M.; Meister, C.J.; Cyr, N.E.; Kenagy, G.J.; Wingfield, J.C. Seasonal Glucocorticoid Responses to Capture in Wild Free-Living Mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 614–622. [Google Scholar] [CrossRef]
- Kenagy, G.J.; Place, N.J. Seasonal Changes in Plasma Glucocorticosteroids of Free-Living Female Yellow-Pine Chipmunks: Effects of Reproduction and Capture and Handling. Gen. Comp. Endocrinol. 2000, 117, 189–199. [Google Scholar] [CrossRef]
- Vera, F.; Antenucci, C.D.; Zenuto, R.R. Cortisol and Corticosterone Exhibit Different Seasonal Variation and Responses to Acute Stress and Captivity in Tuco-Tucos (Ctenomys Talarum). Gen. Comp. Endocrinol. 2011, 170, 550–557. [Google Scholar] [CrossRef]
- Gong, S.; Miao, Y.-L.; Jiao, G.-Z.; Sun, M.-J.; Li, H.; Lin, J.; Luo, M.-J.; Tan, J.-H. Dynamics and Correlation of Serum Cortisol and Corticosterone under Different Physiological or Stressful Conditions in Mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef]
- Ottenweller, J.E.; Tapp, W.N.; Burke, J.M.; Natelson, B.H. Plasma Cortisol and Corticosterone Concentrations in the Golden Hamster, (Mesocricetus auratus). Life Sci. 1985, 37, 1551–1558. [Google Scholar] [CrossRef]
- Vera, F.; Antenucci, C.D.; Zenuto, R.R. Different Regulation of Cortisol and Corticosterone in the Subterranean Rodent Ctenomys Talarum: Responses to Dexamethasone, Angiotensin II, Potassium, and Diet. Gen. Comp. Endocrinol. 2019, 273, 108–117. [Google Scholar] [CrossRef]
- Forsburg, Z.R.; Goff, C.B.; Perkins, H.R.; Robicheaux, J.A.; Almond, G.F.; Gabor, C.R. Validation of Water-Borne Cortisol and Corticosterone in Tadpoles: Recovery Rate from an Acute Stressor, Repeatability, and Evaluating Rearing Methods. Gen. Comp. Endocrinol. 2019, 281, 145–152. [Google Scholar] [CrossRef]
- Raubenheimer, P.J.; Young, E.A.; Andrew, R.; Seckl, J.R. The Role of Corticosterone in Human Hypothalamic- Pituitary-Adrenal Axis Feedback. Clin. Endocrinol. 2006, 65, 22–26. [Google Scholar] [CrossRef]
- Stevenson, E.T.; Gese, E.M.; Neuman-Lee, L.A.; French, S.S. Levels of Plasma and Fecal Glucocorticoid Metabolites Following an ACTH Challenge in Male and Female Coyotes (Canis latrans). J. Comp. Physiol. B 2018, 188, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Carlin, E.; Teren, G.; Ganswindt, A. Non-Invasive Assessment of Body Condition and Stress-Related Fecal Glucocorticoid Metabolite Concentrations in African Elephants (Loxodonta africana) Roaming in Fynbos Vegetation. Animals 2020, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Hunninck, L.; Palme, R.; Sheriff, M.J. Stress as a Facilitator? Territorial Male Impala Have Higher Glucocorticoid Levels than Bachelors. Gen. Comp. Endocrinol. 2020, 297, 113553. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.; Fichtel, C.; Heistermann, M.; Kappeler, P.M. Dynamics and Determinants of Glucocorticoid Metabolite Concentrations in Wild Verreaux’s Sifakas. Horm. Behav. 2020, 124, 104760. [Google Scholar] [CrossRef]
- Dalerum, F.; Ganswindt, A.; Palme, R.; Bettega, C.; Delgado, M.D.M.; Dehnhard, M.; Freire, S.; González, R.G.; Marcos, J.; Miranda, M.; et al. Methodological Considerations for Using Fecal Glucocorticoid Metabolite Concentrations as an Indicator of Physiological Stress in the Brown Bear (Ursus arctos). Physiol. Biochem. Zool. 2020, 93, 227–234. [Google Scholar] [CrossRef]
- Young, C.; Ganswindt, A.; McFarland, R.; de Villiers, C.; van Heerden, J.; Ganswindt, S.; Barrett, L.; Henzi, S.P. Faecal Glucocorticoid Metabolite Monitoring as a Measure of Physiological Stress in Captive and Wild Vervet Monkeys. Gen. Comp. Endocrinol. 2017, 253, 53–59. [Google Scholar] [CrossRef]
- Lavin, S.R.; Woodruff, M.C.; Atencia, R.; Cox, D.; Woodruff, G.T.; Setchell, J.M.; Wheaton, C.J. Biochemical and Biological Validations of a Faecal Glucocorticoid Metabolite Assay in Mandrills (Mandrillus sphinx). Conserv. Physiol. 2019, 7, coz032. [Google Scholar] [CrossRef]
- Fauteux, D.; Gauthier, G.; Berteaux, D.; Bosson, C.; Palme, R.; Boonstra, R. Assessing Stress in Arctic Lemmings: Fecal Metabolite Levels Reflect Plasma Free Corticosterone Levels. Physiol. Biochem. Zool. 2017, 90, 370–382. [Google Scholar] [CrossRef]
| Cortisol | Cortisone | Corticosterone | |
|---|---|---|---|
| Formula | 11β,17α,21-trihydroxypregn-4-ene-3,20-dione [17] 1  | 17-hydroxy-11-dehydrocorticosterone [18] 2  | 11β,21-dihydroxypregn-4-ene-3,20-dione [19] 3  |
| Structural differences | An extra hydroxyl group attached to the 17th carbon [20]. | A ketone group attached to the 17th carbon [21]. | No extra hydroxyl group on the 17th carbon [20]. |
| Metabolism | Synthesised from pregnenolone in adrenal gland. Inactivated mainly in the kidney by 11β-hydroxysteroid dehydrogenase (11β-HSD) type 2 into cortisone [22,23]. | Transformation in the liver, lungs, ovaries, and central nervous system by 11β-HSD type 1 into cortisol [24]. | Derived from pregnenolone in adrenal gland [19]. |
| Activity | Active molecule [25] | Inactive molecule | Active molecule |
| Half-life | In plasma: 66 min In tissues: 12 h [26,27] | In plasma: 90 min [21] | In plasma: 60–90 min [28] |
| Predominant species | It is the main glucocorticoid in most mammals [29] | Same species as cortisol | It is the main glucocorticoid in rats, mice, birds, and reptiles, due to a lack of the enzyme 17-α hydroxylase [9] |
| Analyte | Analytical Method | Reference |
|---|---|---|
| Cortisol | EIA | [34,39,40] |
| RIA | [41,42] | |
| Chemiluminescence | [43] | |
| AlphaLISA | [44] | |
| HPLC | [35,36] | |
| LC-MS/MS | [37,38] | |
| Cortisone | AlphaLISA | [44] |
| UHPLC-MS/MS | [45,46] | |
| LC-MS/MS | [47] | |
| LC-MS3 | [48,49] | |
| Corticosterone | EIA | [50,51] |
| RIA | [52,53] | |
| Total steroids | EIA | [54,55,56] |
| RIA | [57,58] |
| Species | Study | Cohort | Analytical Method | Stressor | Matrix | Values (Plasma/Saliva: ng/mL; Hair: pg/mg; Water-Borne: ng/L−1) | ||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Before Stressor | After Stressor | ||||||
| Pig | [63] | 14 | LC-MS/MS | Nasal snare | Saliva (Sl) Plasma (P) | Cortisol | Sl: 0.06–0.25 * P: 100 * | Sl: 1–4 * P: 60–140 * |
| Cortisone | Sl: 0.01–0.125 * P: 19 * | Sl: 0.25-1 * P: 17–33 * | ||||||
| [44] | 32 | AlphaLISA | Farrowing | Hair | Cortisol | 31.9 | 33.7 | |
| Cortisone | 119.9 | 527.2 | ||||||
| [62] | 25 | LC-MS/MS | Catheterisation | Serum (S) | Cortisol | 42.8 | 71 | |
| Cortisone | 1.8 | 19 | ||||||
| Human | [71] | 197 | LC/MS | Emotional stress | Hair | Cortisol | 3.2 | 3.7 |
| Cortisone | 5.9 | 7.4 | ||||||
| [49] | 239 | HPLC | Pregnancy | Hair | Cortisol | ND | 3.75 | |
| Cortisone | ND | 14 | ||||||
| [72] | 229 | LC/MS | Ocean-going fishing 1–3 months | Hair | Cortisol | 12.8 | 10.5 | |
| Cortisone | 3.3 | 4.9 | ||||||
| [67] | 67 | LC-MS/MS | Trier Social Stress Test | Saliva Serum | Cortisol | S: 2.2 Sl: 0.7 | S: 17.5 Sl: 0.41 | |
| Cortisone | 4.2 | Sl: 9 | ||||||
| [70] | 12 | EIA (cortisol) Chemiluminescence (cortisone) | GXT (morning) | Plasma Saliva | Cortisol | P: 170 Sl: 2.6 | P: 250 Sl: 4.9 | |
| Cortisone | P: 37.5 Sl: 13.6 | P: 72.1 Sl: 21.1 | ||||||
| Others species | [64] | 120 | LC-MS/MS | Air exposure | Plasma | Cortisol | 10 * | 55 * |
| Cortisone | 10 * | 40 * | ||||||
| [65] | 12 | RIA | Air exposure | Water-borne | Cortisol | 1.1 | 25.2 | |
| Cortisone | 0.7 | 8 * | ||||||
| [66] | 24 | EIA | Bacterial inoculation | Hair | Cortisol | 9 * | 2 * | |
| Cortisone | 100 * | 170 * | ||||||
| Species | Study | Cohort (n) | Analytical Method | Stressor | Matrix | Values (Plasma/Saliva: ng/mL; Faces/Tissues: ng/g; Feathers: ng/g; Water-Borne: pg/g) | ||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Before Stressor | After Stressor | ||||||
| Cow | [78] | 18 | IDMS (Isotope dilution and spectrophotometry) | Injection of ACTH | Serum | Cortisol | 3–6 | 4.1–8.9 |
| Corticosterone | 2.4–3.5 | 3–4.1 | ||||||
| [79] | 10 | RIA (cortisol) EIA (corticosterone) | LPS infection | Plasma (P) | Cortisol | 0.5 | 18 | |
| Corticosterone | 0.4 | 2.8 | ||||||
| Birds | [80] | LC-MS/MS | Moult | Plasma Feathers (F) | Cortisol | P: 0.17 | P: 0 F: 4.4–75.5 | |
| Corticosterone | P: 8.6 | P: 13–17 F: 4.1–372.9 | ||||||
| [81] | 70 | EIA (cortisol) RIA (corticosterone) | Restraining (P10) | Plasma Tissues (T) | Cortisol | P: 0.9 * T: 0.5–1.5 * | P: 1.5 * T: 1–2.2 * | |
| Corticosterone | P: 11 * T: 2–8 * | P: 30 * T: 5–20 * | ||||||
| Rodents | [88] | Not specified | RIA | Acute (A): supine restraint Chronic (C): cold restraint (2–4/day) | Plasma | Cortisol | 4 * | A: 60 * C: 15 * |
| Corticosterone | 12 * | A: 65 * C: 15 * | ||||||
| [87] | 6 | RIA (cortisol) EIA (corticosterone) | Acute: restraint, forced swimming Chronic: restraint | Serum | Cortisol | 8–14 * | A: 30–35 * C: 15–30 * | |
| Corticosterone | 40–160 * | A: 800–1200 * C: 200–1000 * | ||||||
| Others species | [90] | 17 tadpoles | EIA | ACTH injection | Water-borne | Cortisol | 4–5 * | 3–3.5 * |
| Corticosterone | 100–180 * | 180–350 * | ||||||
| Human | [70] | 12 | EIA | Graded exercise test | Plasma | Cortisol | 170 * | 250 * |
| Corticosterone | 14 * | 47 * | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botía, M.; Escribano, D.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Tecles, F.; López-Arjona, M.; Cerón, J.J. Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use. Metabolites 2023, 13, 106. https://doi.org/10.3390/metabo13010106
Botía M, Escribano D, Martínez-Subiela S, Tvarijonaviciute A, Tecles F, López-Arjona M, Cerón JJ. Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use. Metabolites. 2023; 13(1):106. https://doi.org/10.3390/metabo13010106
Chicago/Turabian StyleBotía, María, Damián Escribano, Silvia Martínez-Subiela, Asta Tvarijonaviciute, Fernando Tecles, Marina López-Arjona, and José J. Cerón. 2023. "Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use" Metabolites 13, no. 1: 106. https://doi.org/10.3390/metabo13010106
APA StyleBotía, M., Escribano, D., Martínez-Subiela, S., Tvarijonaviciute, A., Tecles, F., López-Arjona, M., & Cerón, J. J. (2023). Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use. Metabolites, 13(1), 106. https://doi.org/10.3390/metabo13010106








