MOTS-c Functionally Prevents Metabolic Disorders
Abstract
:1. Introduction
2. An Overview of MOTS-c
2.1. Discovery of MOTS-c
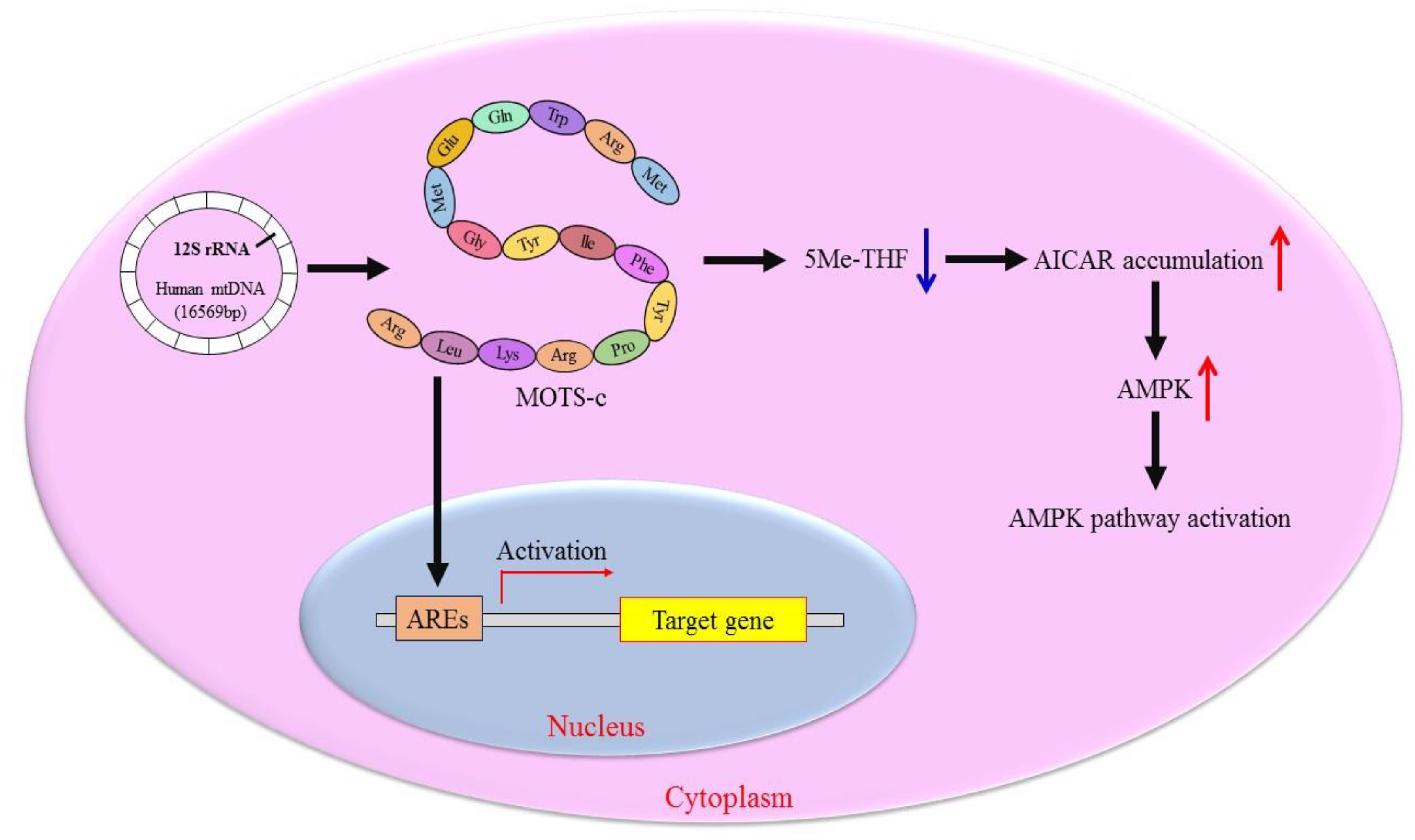
2.2. Molecular Mechanism of MOTS-c Regulating Cell Metabolism
3. MOTS-c Inhibits Pathological Metabolic Processes
3.1. MOTS-c Reduces Insulin Resistance
3.2. MOTS-c Prevents Obesity
3.3. MOTS-c Improve Muscle Function
3.4. MOTS-c Promotes Bone Metabolism
3.5. MOTS-c Enhances Immune Regulation
3.6. MOTS-c Postpone Aging
4. Clinical Application of MOTS-c
5. Conclusions
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Word or Phrase in Full |
| 5Me-THF | 5-methyltetrahydro-folate |
| ACC | acetyl-CoA carboxylase |
| AMPK | AMP-activated protein kinase |
| AICAR | 5-aminoimidazole-4-carboxamide riboside |
| AhR | aryl hydrocarbon receptor |
| AREs | antioxidant-responsive elements |
| APPL1 | adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 |
| Arg | arginine |
| ATP | adenosine triphosphate |
| AT-1 | angiotensin II type 1 |
| BAT | brown adipose tissue |
| COX4 | cytochrome c oxidase subunit 4 |
| CVD | cardiovascular diseases |
| Dio2 | deiodinase 2 |
| DMD | Duchenne muscular dystrophy |
| ERK | extracellular signal-regulated kinase |
| ErbB4 | Erb-B2 receptor tyrosine kinase 4 |
| ET-B | endothelin B |
| FOXO1 | forkhead box protein O1 |
| Gln | glutamine |
| Glu | glutamicacid |
| GLUT4 | glucose transporter 4 |
| GLP-1 | glucagon-like peptide 1 |
| Gly | glycine |
| IFN-β | interferon-β |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| IL-10 | interleukin-10 |
| Ile | isoleucine |
| JAK | janus activated kinase |
| JNK | c-Jun amino-terminal kinases |
| Leu | leucine |
| Lys | lysine |
| MDPs | mitochondrial-derived peptides |
| Met | methionine |
| MOTS-c | mitochondrial open reading frame of the 12S rRNA-c |
| mtDNA | mitochondrial DNA |
| mTORC1 | mTOR complex 1 |
| NAD | nicotinamide adenine dinucleotide |
| NRG1 | neuregulin1 |
| NRF1 | nuclear respiratory factor 1 |
| Nrf2 | nuclear factor-erythroid-2-related factor 2 |
| NF-κB | nuclear factor-kappaB |
| OPG | osteoprotegerin |
| ORF | open reading frame |
| PGC1α | peroxisome proliferator-activated receptor-γ coactivator 1α |
| Phe | phenylalanine |
| Pro | proline |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| ROS | reactive oxygen species |
| rRNA | ribosomal RNA |
| SHLP | small humanin-like peptides |
| Smad | sekelsky mothers against decapentaplegic |
| SIRT1 | sirtuin 1 |
| STAT1 | signal transducer and activator of transcription 1 |
| STAT3 | signal transducer and activator of transcription 3 |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
| TCR | T cell receptor |
| TFAM | transcription factor A for mitochondria |
| TNF-α | tumor necrosis factor-α |
| TGF-β | transforming growth factor-β |
| tRNA | transfer RNA |
| Trp | tryptophan |
| Tyr | tyrosine |
| UCP1 | uncoupling protein-1 |
| WAT | white adipose tissue |
References
- Mottis, A.; Herzig, S.; Auwerx, J. Mitocellular communication: Shaping health and disease. Science 2019, 366, 827–832. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2018, 208, 1673. [Google Scholar] [CrossRef] [Green Version]
- Ikegawa, N.; Kozuka, A.; Morita, N.; Murakami, M.; Sasakawa, N.; Niikura, T. Humanin derivative, HNG, enhances neurotransmitter release. Biochim. Biophys. Acta. Gen. Subj. 2022, 1866, 130204. [Google Scholar] [CrossRef]
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 2016, 8, 796–809. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowska, M.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.A.; Krauss, H.; Leciejewska, N.; Szczepankiewicz, D.; Bień, J.; Skrzypski, M.; Wilczak, M.; Sassek, M. Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders. Biology 2021, 10, 1032. [Google Scholar] [CrossRef]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018, 28, 516–524.e7. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wei, M.; Zhai, Y.; Li, Q.; Ye, Z.; Wang, L.; Luo, W.; Chen, J.; Lu, Z. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J. Mol. Med. 2019, 97, 473–485. [Google Scholar] [CrossRef]
- Ming, W.; Lu, G.; Xin, S.; Huanyu, L.; Yinghao, J.; Xiaoying, L.; Chengming, X.; Banjun, R.; Li, W.; Zifan, L. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem. Biophys. Res. Commun. 2016, 476, 412–419. [Google Scholar] [CrossRef]
- Baylan, F.A.; Yarar, E. Relationship between the mitochondria-derived peptide MOTS-c and insulin resistance in obstructive sleep apnea. Sleep Breath 2021, 25, 861–866. [Google Scholar] [CrossRef]
- Desai, R.; East, D.A.; Hardy, L.; Faccenda, D.; Rigon, M.; Crosby, J.; Alvarez, M.S.; Singh, A.; Mainenti, M.; Hussey, L.K.; et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci. Adv. 2020, 6, eabc9955. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim. Biophys. Acta. Mol. Basis. Dis. 2021, 1867, 166126. [Google Scholar] [CrossRef]
- Ferreira, V.; Folgueira, C.; Guillén, M.; Zubiaur, P.; Navares, M.; Sarsenbayeva, A.; López-Larrubia, P.; Eriksson, J.W.; Pereira, M.J.; Abad-Santos, F.; et al. Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight. Metabolism 2022, 137, 155335. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Yin, Y.; Pan, Y.; He, J.; Zhong, H.; Wu, Y.; Ji, C.; Liu, L.; Cui, X. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol. Res. 2022, 175, 105987. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell. Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Etienne, Q.; Lebrun, V.; Komuta, M.; Navez, B.; Thissen, J.P.; Leclercq, I.A.; Lanthier, N. Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites 2022, 12, 625. [Google Scholar] [CrossRef]
- Alsoud, L.O.; Soares, N.C.; Al-Hroub, H.M.; Mousa, M.; Kasabri, V.; Bulatova, N.; Suyagh, M.; Alzoubi, K.H.; El-Huneidi, W.; Abu-Irmaileh, B.; et al. Identification of Insulin Resistance Biomarkers in Metabolic Syndrome Detected by UHPLC-ESI-QTOF-MS. Metabolites 2022, 12, 508. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.M.; et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Miller, B.; Mehta, H.H.; Xiao, J.; Wan, J.; Arpawong, T.E.; Yen, K.; Cohen, P. The mitochondrial-derived peptide MOTS-c is a regulator of plasma metabolites and enhances insulin sensitivity. Physiol. Rep. 2019, 7, e14171. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Ma, J.; Pang, X.; Yuan, J.; Pan, Y.; Fu, Y.; Laher, I. MOTS-c and Exercise Restore Cardiac Function by Activating of NRG1-ErbB Signaling in Diabetic Rats. Front. Endocrinol. 2022, 13, 812032. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Shang, N.; Kerek, E.; Wu, K.; Wu, J. Mitofusion is required for MOTS-c induced GLUT4 translocation. Sci. Rep. 2021, 11, 14291. [Google Scholar] [CrossRef]
- Zempo, H.; Kim, S.J.; Fuku, N.; Nishida, Y.; Higaki, Y.; Wan, J.; Yen, K.; Miller, B.; Vicinanza, R.; Miyamoto-Mikami, E.; et al. A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c. Aging 2021, 13, 1692–1717. [Google Scholar] [CrossRef]
- Van Vliet, S.; Koh, H.E.; Patterson, B.W.; Yoshino, M.; LaForest, R.; Gropler, R.J.; Klein, S.; Mittendorfer, B. Obesity Is Associated with Increased Basal and Postprandial β-Cell Insulin Secretion Even in the Absence of Insulin Resistance. Diabetes 2020, 69, 2112–2119. [Google Scholar] [CrossRef]
- Yin, X.; Jing, Y.; Chen, Q.; Abbas, A.B.; Hu, J.; Xu, H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur. J. Pharmacol. 2020, 870, 172909. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Wei, M.; Gan, L.; Liu, Z.; Liu, L.; Chang, J.R.; Yin, D.C.; Cao, H.L.; Su, X.L.; Smith, W.W. Mitochondrial-Derived Peptide MOTS-c Attenuates Vascular Calcification and Secondary Myocardial Remodeling via Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway. Cardiorenal. Med. 2020, 10, 42–50. [Google Scholar] [CrossRef]
- Du, C.; Zhang, C.; Wu, W.; Liang, Y.; Wang, A.; Wu, S.; Zhao, Y.; Hou, L.; Ning, Q.; Luo, X. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr. Diabetes 2018, 19, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Tang, S.; Xue, C.; Liu, Y.; Wang, J.; Zhang, W.; Luo, W.; Chen, J. Mitochondrial-Derived Peptide MOTS-c Increases Adipose Thermogenic Activation to Promote Cold Adaptation. Int. J. Mol. Sci. 2019, 20, 2456. [Google Scholar] [CrossRef]
- Ran, N.; Lin, C.; Leng, L.; Han, G.; Geng, M.; Wu, Y.; Bittner, S.; Moulton, H.M.; Yin, H. MOTS-c promotes phosphorodiamidate morpholino oligomer uptake and efficacy in dystrophic mice. EMBO Mol. Med. 2021, 13, e12993. [Google Scholar] [CrossRef]
- Kumagai, H.; Coelho, A.R.; Wan, J.; Mehta, H.H.; Yen, K.; Huang, A.; Zempo, H.; Fuku, N.; Maeda, S.; Oliveira, P.J.; et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E680–E690. [Google Scholar] [CrossRef]
- García-Benlloch, S.; Revert-Ros, F.; Blesa, J.R.; Alis, R. MOTS-c promotes muscle differentiation in vitro. Peptides 2022, 155, 170840. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Ge, Q.; Li, J. A potential negative regulation of myostatin in muscle growth during the intermolt stage in Exopalaemon carinicauda. Gen. Comp. Endocrinol. 2021, 314, 113902. [Google Scholar] [CrossRef]
- Liu, J.; Pan, M.; Huang, D.; Guo, Y.; Yang, M.; Zhang, W.; Mai, K. Myostatin-1 Inhibits Cell Proliferation by Inhibiting the mTOR Signal Pathway and MRFs, and Activating the Ubiquitin-Proteasomal System in Skeletal Muscle Cells of Japanese Flounder Paralichthys olivaceus. Cells 2020, 9, 2376. [Google Scholar] [CrossRef]
- Ligeiro Coelho, A.; Kim, S.; Wan, J.; Oliveira, P.; Cohen, P. Myostatin Modulation by Mots-C May Lead to Beneficial Effects in Muscle and Fat Tissue. Innov. Aging 2018, 2 (Suppl. 1), 886. [Google Scholar] [CrossRef]
- Qin, Q.; Delrio, S.; Wan, J.; Jay Widmer, R.; Cohen, P.; Lerman, L.O.; Lerman, A. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int. J. Cardiol. 2018, 254, 23–27. [Google Scholar] [CrossRef]
- Guo, Q.; Chang, B.; Yu, Q.L.; Xu, S.T.; Yi, X.J.; Cao, S.C. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1α. Diabetologia 2020, 63, 2675–2688. [Google Scholar] [CrossRef]
- Kumagai, H.; Natsume, T.; Kim, S.J.; Tobina, T.; Miyamoto-Mikami, E.; Shiose, K.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Miller, B.; et al. The MOTS-c K14Q polymorphism in the mtDNA is associated with muscle fiber composition and muscular performance. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130048. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef]
- Huybrechts, Y.; Van Hul, W. Osteopetrosis associated with PLEKHM1 and SNX10 genes, both involved in osteoclast vesicular trafficking. Bone 2022, 164, 116520. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, Y.; Liu, Z.; Yin, M.; Hou, M.; Zhao, Z.; Zhou, X.; Yin, L. Cytokine-scavenging nanodecoys reconstruct osteoclast/osteoblast balance toward the treatment of postmenopausal osteoporosis. Sci. Adv. 2021, 7, eabl6432. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Shaw, N.J.; Portale, A.A.; Ward, L.M.; Abrams, S.A.; Pettifor, J.M. Rickets. Nat. Rev. Dis. Primers 2017, 3, 17101. [Google Scholar] [CrossRef]
- Chan, W.L.; Steiner, M.; Witkos, T.; Egerer, J.; Busse, B.; Mizumoto, S.; Pestka, J.M.; Zhang, H.; Hausser, I.; Khayal, L.A.; et al. Impaired proteoglycan glycosylation, elevated TGF-β signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018, 14, e1007242. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.T.; Chen, W.Z. MOTS-c improves osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via TGF-β/Smad pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7156–7163. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, S.; Wang, H.; Wang, L.; Du, T.; Ye, Z.; Zhai, D.; Zhu, Z.; Tian, X.; Lu, Z.; et al. MOTS-c inhibits Osteolysis in the Mouse Calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol. Res. 2019, 147, 104381. [Google Scholar] [CrossRef]
- Horwitz, E.; Krogvold, L.; Zhitomirsky, S.; Swisa, A.; Fischman, M.; Lax, T.; Dahan, T.; Hurvitz, N.; Weinberg-Corem, N.; Klochendler, A.; et al. β-Cell DNA Damage Response Promotes Islet Inflammation in Type 1 Diabetes. Diabetes 2018, 67, 2305–2318. [Google Scholar] [CrossRef]
- Kong, B.S.; Min, S.H.; Lee, C.; Cho, Y.M. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep. 2021, 36, 109447. [Google Scholar] [CrossRef]
- Tekin, S.; Bir, L.S.; Avci, E.; Şenol, H.; Tekin, I.; Çınkır, U. Comparison of Serum Mitochondrial Open Reading Frame of the 12S rRNA-c (MOTS-c) Levels in Patients with Multiple Sclerosis and Healthy Controls. Cureus 2022, 18, e26981. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Feng, M.; Peng, J.; Du, X.; Chu, H.; Chen, X. The Mitochondrial-Derived Peptide MOTS-c Attenuates Oxidative Stress Injury and the Inflammatory Response of H9c2 Cells Through the Nrf2/ARE and NF-κB Pathways. Cardiovasc. Eng. Technol. 2021, 13, 651–661. [Google Scholar] [CrossRef]
- Zhai, D.; Ye, Z.; Jiang, Y.; Xu, C.; Ruan, B.; Yang, Y.; Lei, X.; Xiang, A.; Lu, H.; Zhu, Z.; et al. MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. Mol. Immunol. 2017, 92, 151–160. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Woodhead, J.S.T.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging 2020, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Fuku, N.; Pareja-Galeano, H.; Zempo, H.; Alis, R.; Arai, Y.; Lucia, A.; Hirose, N. The mitochondrial-derived peptide MOTS-c: A player in exceptional longevity? Aging Cell 2015, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e6. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, H.; Wong, L.P.; Shelton, L.; Sadreyev, R.; Kaneki, M. Farnesysltransferase Inhibitor Prevents Burn Injury-Induced Metabolome Changes in Muscle. Metabolites 2022, 12, 800. [Google Scholar] [CrossRef]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Kropotov, A.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Degradation of Extracellular NAD+ Intermediates in Cultures of Human HEK293 Cells. Metabolites 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Srihari, V.H.; Guloksuz, S.; Ferrara, M.; Tek, C.; Heninger, G.R. Neural cell-derived plasma exosome protein abnormalities implicate mitochondrial impairment in first episodes of psychosis. FASEB J. 2021, 35, e21339. [Google Scholar] [CrossRef]
- Kim, S.J.; Mehta, H.H.; Wan, J.; Kuehnemann, C.; Chen, J.; Hu, J.F.; Hoffman, A.R.; Cohen, P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging 2018, 10, 1239–1256. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Mitochondrial-Derived Peptides Exacerbate Senescence. Rejuvenation Res. 2018, 21, 369–373. [Google Scholar] [CrossRef]
- Raijmakers, R.P.H.; Jansen, A.F.M.; Keijmel, S.P.; Ter Horst, R.; Roerink, M.E.; Novakovic, B.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G.; Bleeker-Rovers, C.P. A possible role for mitochondrial-derived peptides humanin and MOTS-c in patients with Q fever fatigue syndrome and chronic fatigue syndrome. J. Transl. Med. 2019, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.R.; Woodhead, J.S.T.; Chan, A.; D’Souza, R.F.; Wan, J.; Hollingsworth, K.G.; Plank, L.D.; Cohen, P.; Poppitt, S.D.; Merry, T.L. Plasma mitochondrial derived peptides MOTS-c and SHLP2 positively associate with android and liver fat in people without diabetes. Biochim. Biophys. Acta. Gen. Subj. 2021, 1865, 129991. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneya, M.; Jerobin, J.; Bettahi, I.; Bensila, M.; Aye, M.; Siveen, K.S.; Sathyapalan, T.; Skarulis, M.; Abou-Samra, A.B.; Atkin, S.L. Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin. Endocrinol. 2019, 91, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Skuratovskaia, D.; Komar, A.; Vulf, M.; Litvinova, L. Mitochondrial destiny in type 2 diabetes: The effects of oxidative stress on the dynamics and biogenesis of mitochondria. PeerJ 2020, 8, e9741. [Google Scholar] [CrossRef]
- Cao, S.J.; Xu, S.; Wang, H.M.; Ling, Y.; Dong, J.; Xia, R.D.; Sun, X.H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, S.; Lobo, S.; Goswami, T. What is the future of noninvasive routes for protein- and peptide-based drugs? Ther. Deliv. 2016, 7, 355–357. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Chen, S.; Zeng, G.; Yuan, W.; Lu, Y.; Zhang, Y.; Huang, Q.; Xiong, X.; Xu, B.; Huang, Q. Angiogenesis-Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity. Int. J. Mol. Sci. 2022, 23, 16166. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. AMPK activation protects against diet induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Misheva, M.; Johnson, J.; McCullagh, J. Role of Oxylipins in the Inflammatory-Related Diseases NAFLD, Obesity, and Type 2 Diabetes. Metabolites 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Nuwaylati, D.; Eldakhakhny, B.; Bima, A.; Sakr, H.; Elsamanoudy, A. Low-Carbohydrate High-Fat Diet: A SWOC Analysis. Metabolites 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Andlauer, T.F.M.; Link, J.; Martin, D.; Ryner, M.; Hermanrud, C.; Grummel, V.; Auer, M.; Hegen, H.; Aly, L.; Gasperi, C.; et al. Treatment- and population-specific genetic risk factors for anti-drug antibodies against interferon-beta: A GWAS. BMC Med. 2020, 18, 298. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Rayner, C.K.; Wu, T.; Aroda, V.R.; Whittington, C.; Kanters, S.; Guyot, P.; Shaunik, A.; Horowitz, M. Gastrointestinal adverse events with insulin glargine/lixisenatide fixed-ratio combination versus glucagon-like peptide-1 receptor agonists in people with type 2 diabetes mellitus: A network meta-analysis. Diabetes Obes. Metab. 2021, 23, 136–146. [Google Scholar] [CrossRef]
- Eskian, M.; Khorasanizadeh, M.; Assa’ad, A.H.; Rezaei, N. Monoclonal Antibodies for Treatment of Eosinophilic Esophagitis. Clin. Rev. Allergy Immunol. 2018, 55, 88–98. [Google Scholar] [CrossRef]
- Klein, H.U.; Trumpff, C.; Yang, H.S.; Lee, A.J.; Picard, M.; Bennett, D.A.; De Jager, P.L. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Mol. Neurodegener. 2021, 16, 75. [Google Scholar] [CrossRef]
- Nashine, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Age-related macular degeneration (AMD) mitochondria modulate epigenetic mechanisms in retinal pigment epithelial cells. Exp. Eye Res. 2019, 189, 107701. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Y.; Ji, X.; Guo, S.; Xie, F.; Chen, Y.; Zhou, K.; Zhang, H.; Peng, F.; Wu, D.; et al. Mutational signature of mtDNA confers mechanistic insight into oxidative metabolism remodeling in colorectal cancer. Theranostics 2023, 13, 324–338. [Google Scholar] [CrossRef] [PubMed]

| Nucleotide Position | Nucleotide Sequence | Amino Acid (3-letter code) | Amino Acid Position | Mutation Type |
|---|---|---|---|---|
| 1343 | atg | Met | 1 | |
| 1346 | agg | Arg | 2 | |
| 1349 | tgg | Trp | 3 | |
| 1352 | caa | Gln | 4 | |
| 1355 | gaa | Glu | 5 | |
| 1358 | atg | Met | 6 | |
| 1361 | ggc | Gly | 7 | |
| 1364 | tac | Tyr | 8 | |
| 1367 | att | Ile | 9 | |
| 1370 | ttc | Phe | 10 | |
| 1373 | ta[C>T] | Tyr | 11 | synonymous substitution |
| 1376 | ccc | Pro | 12 | |
| 1379 | aga | Arg | 13 | |
| 1382 | [A>C]aa | Lys>Gln | 14 | missense substitution |
| 1385 | cta | Leu | 15 | |
| 1388 | cga | Arg | 16 | |
| 1391 | tag | Stop |
| The Functions of MOTS-c | The Disease Associated to MOTS-c Dysfunction | References |
|---|---|---|
| Reduces insulin resistance | type 2 diabetes, diabetes-induced abnormal cardiac structures and functions | [5,23] |
| Prevents obesity | obesity, vascular calcification | [5,8,29] |
| Improves muscle function | duchenne muscular dystrophy, energy-deficient muscle illnesses | [31,32,33] |
| Promotes bone metabolism | osteoporosis | [45,46] |
| Enhances immune regulation | type 1 diabetes, multiple sclerosis, inflammation-related disorders, methicillin-resistant staphylococcus aureus infection | [48,49] |
| Postpones aging | aging-related diseases | [52,53,54] |
| The Functions of MOTS-c | The Genes and Pathways Involved in the Functions of MOTS-c | References |
|---|---|---|
| Reduces insulin resistance | AMPK, NRG1, ErbB4, TFAM, COX4, NRF1, GLUT4 | [5,22,23,24] |
| Prevents obesity | AMPK, GLUT4, IL-6, TNF-α, AT-1, ET-B, PGC1α, UCP1, Dio2, ERK | [5,23,26,28,30] |
| MOTS-c improves muscle function | AMPK, STAT3, FOXO1, APPL1, SIRT1, PGC1α | [5,32,33,38] |
| Promotes bone metabolism | AMPK, TGF-β, SMAD7, OPG, RANKL, NFκB, STAT1 | [45,46] |
| Enhances immune regulation | TCR, mTORC1, NF-κB, Nrf2, TNF-α, IL-1β, IL-6, IL-10, ERK, JNK, P38, AhR, Stat3 | [26,48,50,51] |
| Postpones aging | JAK | [5,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wei, X.; Wei, P.; Lu, H.; Zhong, L.; Tan, J.; Liu, H.; Liu, Z. MOTS-c Functionally Prevents Metabolic Disorders. Metabolites 2023, 13, 125. https://doi.org/10.3390/metabo13010125
Gao Y, Wei X, Wei P, Lu H, Zhong L, Tan J, Liu H, Liu Z. MOTS-c Functionally Prevents Metabolic Disorders. Metabolites. 2023; 13(1):125. https://doi.org/10.3390/metabo13010125
Chicago/Turabian StyleGao, Yue, Xinran Wei, Pingying Wei, Huijie Lu, Luying Zhong, Jie Tan, Hongbo Liu, and Zheng Liu. 2023. "MOTS-c Functionally Prevents Metabolic Disorders" Metabolites 13, no. 1: 125. https://doi.org/10.3390/metabo13010125
APA StyleGao, Y., Wei, X., Wei, P., Lu, H., Zhong, L., Tan, J., Liu, H., & Liu, Z. (2023). MOTS-c Functionally Prevents Metabolic Disorders. Metabolites, 13(1), 125. https://doi.org/10.3390/metabo13010125





