Integration of LC-MS-Based and GC-MS-Based Metabolic Profiling to Reveal the Effects of Domestication and Boiling on the Composition of Duck Egg Yolks
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Preparation
2.2. Sample Preparation
2.3. LC-MS Analysis
2.4. GC-MS Analysis
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
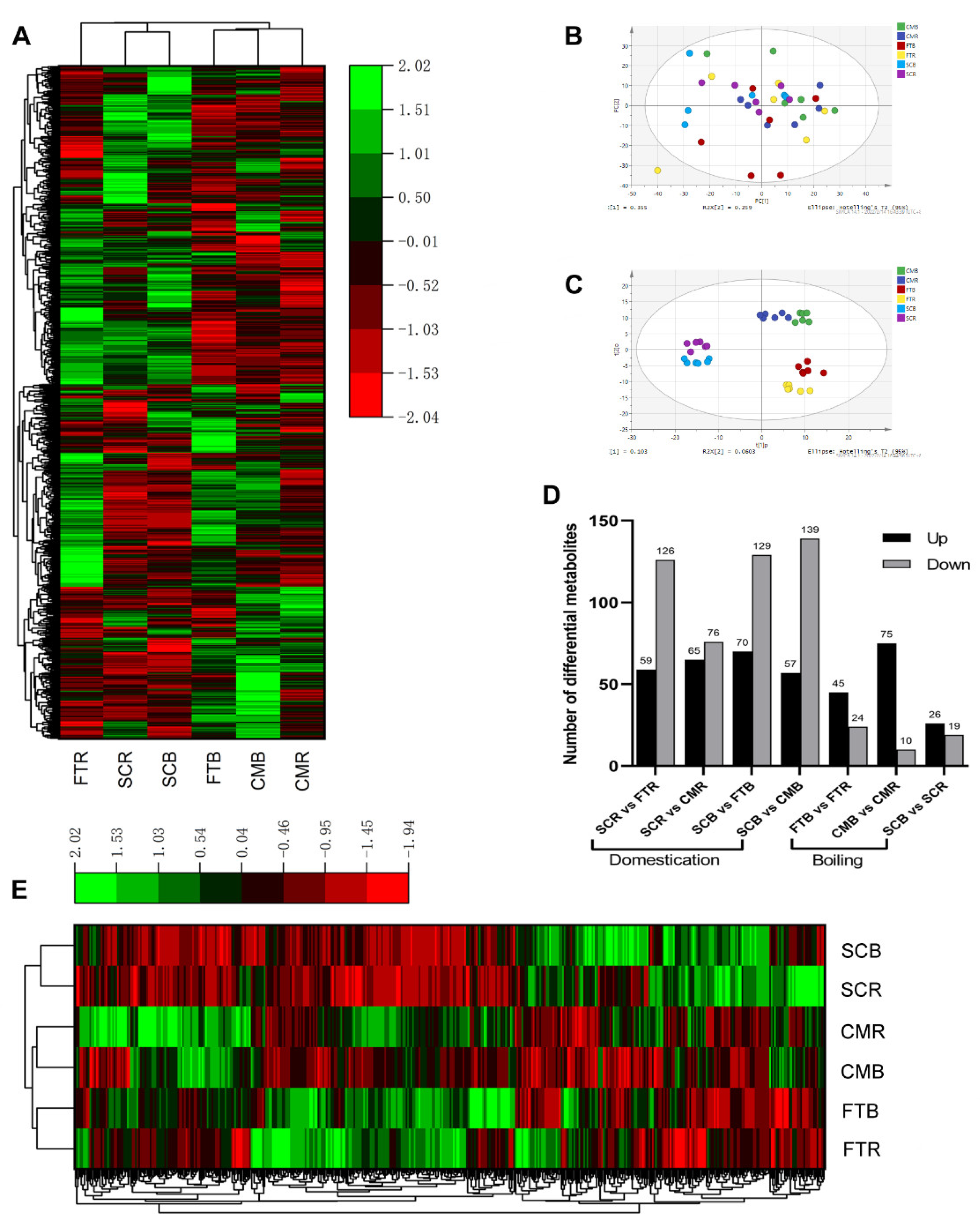
3.1. Comprehensive Profiling of Egg Yolk Metabolites
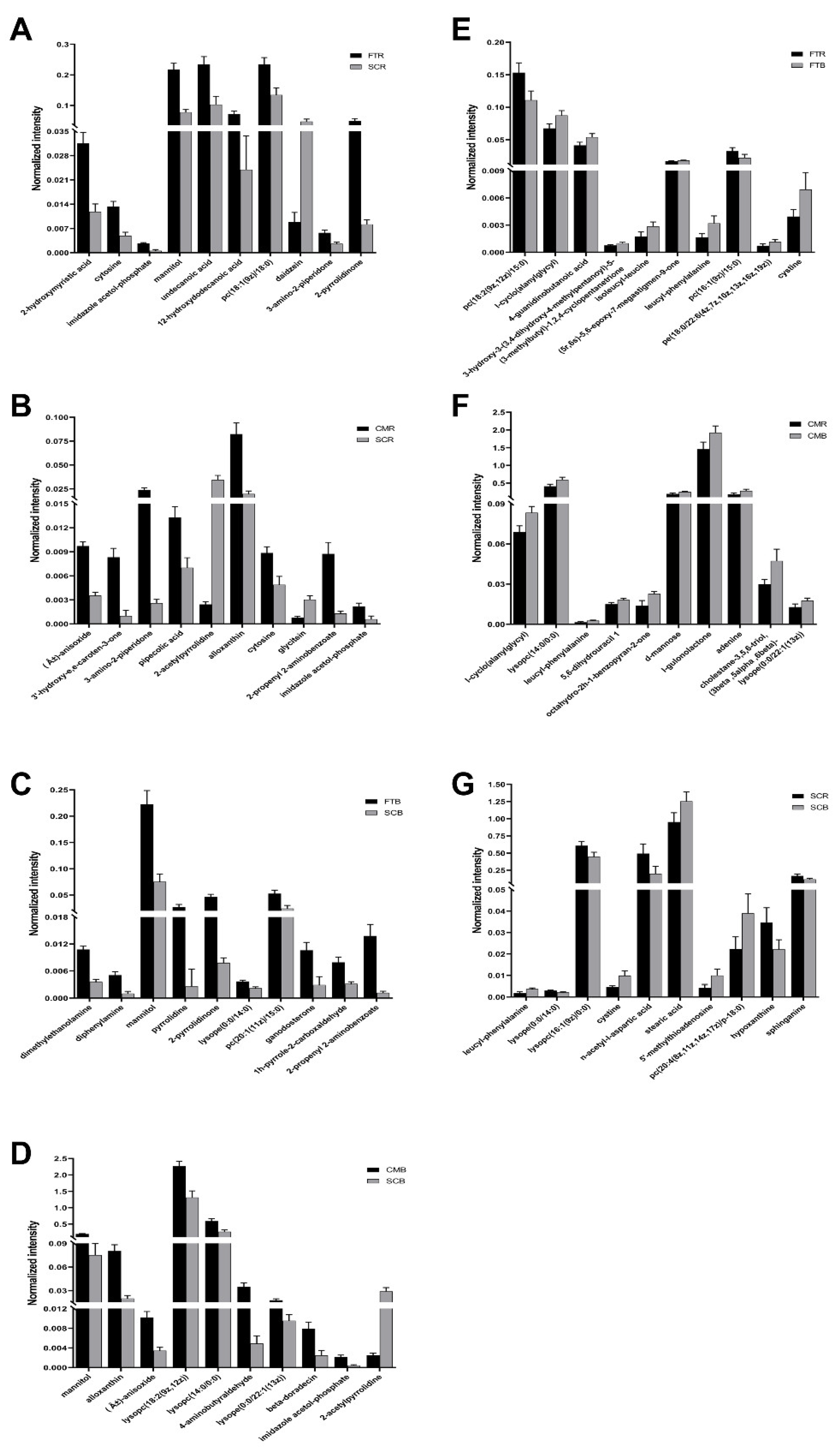
3.2. Changes in Major Metabolites in Paired Comparisons
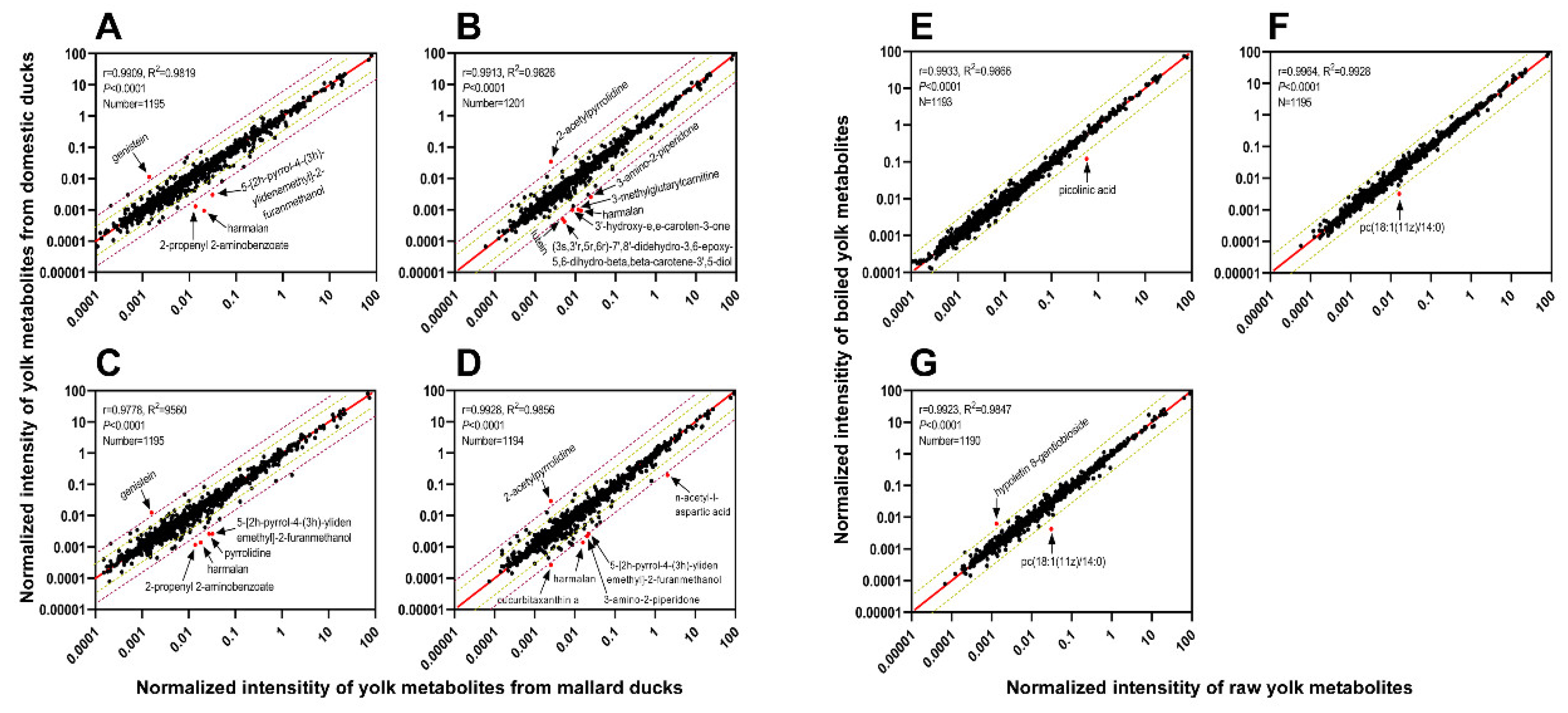
3.3. Comparison between Egg Yolk Metabolites
3.4. Metabolic Response to Domestication and Boiling
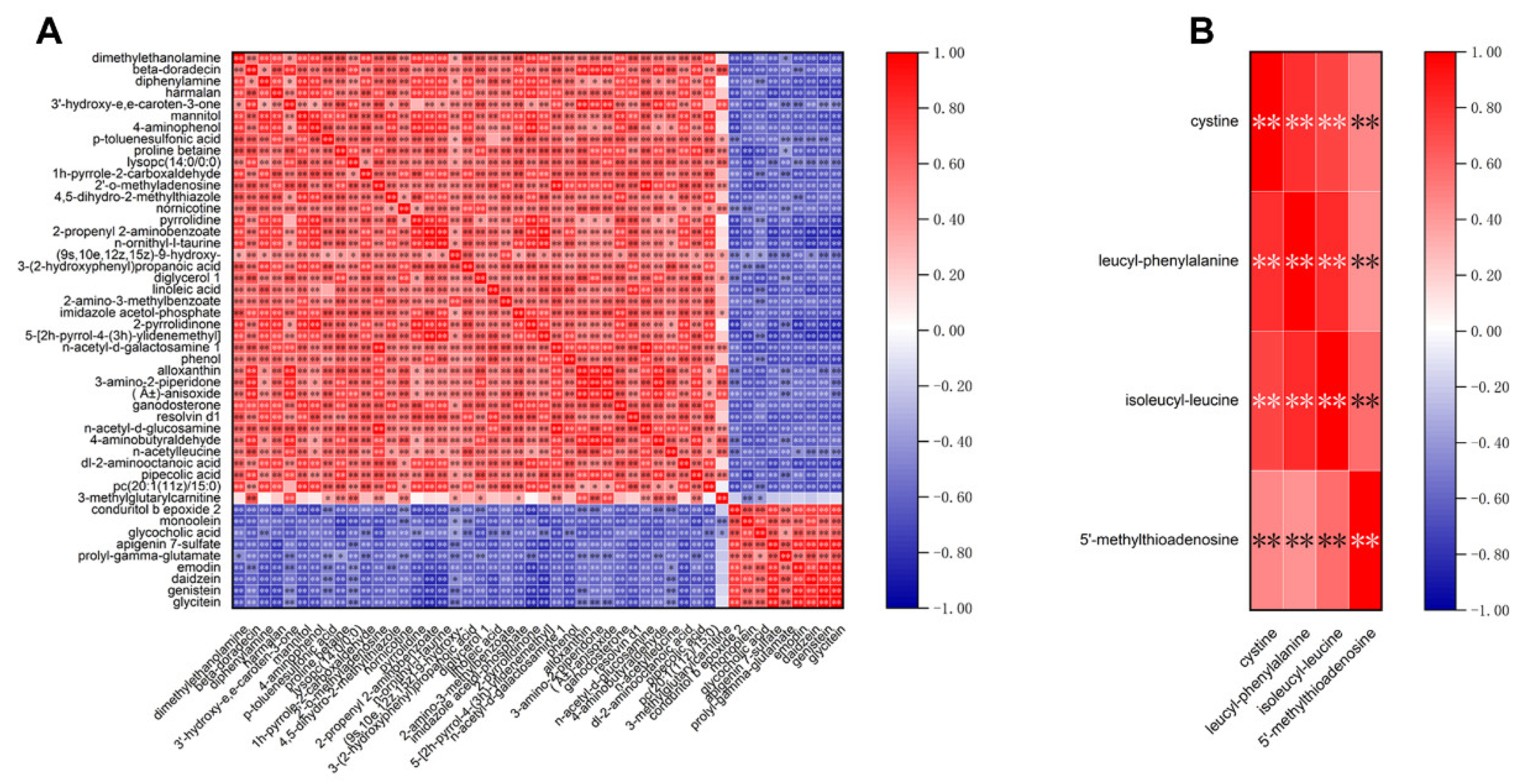
3.5. Correlation Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harlina, P.W.; Ma, M.; Shahzad, R.; Gouda, M.M.; Qiu, N. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. J. Food Sci. Technol. 2018, 55, 4719–4734. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Muir, W.; Christmann, U.; Gibbons, P.; Hancock, C.L.; Poole, C.M.; Emery, A.L.; Poovey, J.R.; Hagg, C.; Scarborough, J.H.; et al. Lipidomics of the chicken egg yolk: High-resolution mass spectrometric characterization of nutritional lipid families. Poult. Sci. 2021, 100, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.; Ricardo, F.; Alves, E.; Reis, A.; Couto, D.; Domingues, P.; Domingues, M.R.M. Lipidomic investigation of eggs’ yolk: Changes in lipid profile of eggs from different conditions. Food Res. Int. 2016, 89 Pt 1, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Knaga, S.; Winiarska-Mieczan, A.; Zięba, G. Effects of dietary alfalfa protein concentrate supplementation on performance, egg quality, and fatty acid composition of raw, freeze-dried, and hard-boiled eggs from Polbar laying hens. Poult. Sci. 2020, 99, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Wakayama, M.; Ashino, Y.; Kadowaki, R.; Sato, M.; Soga, T.; Tomita, M. Effects of feed crops and boiling on chicken egg yolk and white determined by a metabolome analysis. Food Chem. 2020, 327, 127077. [Google Scholar] [CrossRef]
- Mori, H.; Takaya, M.; Nishimura, K.; Goto, T. Breed and feed affect amino acid contents of egg yolk and eggshell color in chickens. Poult. Sci. 2020, 99, 172–178. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Cui, H.; Chen, Y.; Li, K.; Zhan, R.; Zhao, M.; Xu, Y.; Lin, Z.; Fu, Y.; He, Q.; Tang, P.C.; et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur. Heart J. 2021, 42, 4373–4385. [Google Scholar] [CrossRef]
- Lieberg, J.; Wanhainen, A.; Ottas, A.; Vähi, M.; Zilmer, M.; Soomets, U.; Björck, M.; Kals, J. Metabolomic Profile of Abdominal Aortic Aneurysm. Metabolites 2021, 11, 555. [Google Scholar] [CrossRef]
- Pereira, P.R.; Carrageta, D.F.; Oliveira, P.F.; Rodrigues, A.; Alves, M.G.; Monteiro, M.P. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med. Res. Rev. 2022, 42, 1518–1544. [Google Scholar] [CrossRef]
- Hazrati, H.; Fomsgaard, I.S.; Ding, L.; Kudsk, P. Mass spectrometry-based metabolomics unravel the transfer of bioactive compounds between rye and neighbouring plants. Plant Cell Environ. 2021, 44, 3492–3501. [Google Scholar] [CrossRef]
- Kataoka, R.; Akashi, M.; Taniguchi, T.; Kinose, Y.; Yaprak, A.E.; Turgay, O.C. Metabolomics Analyses Reveal Metabolites Affected by Plant Growth-Promoting Endophytic Bacteria in Roots of the Halophyte Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2021, 22, 11813. [Google Scholar] [CrossRef]
- Danczak, R.E.; Goldman, A.E.; Chu, R.K.; Toyoda, J.G.; Garayburu-Caruso, V.A.; Tolić, N.; Graham, E.B.; Morad, J.W.; Renteria, L.; Wells, J.R.; et al. Ecological theory applied to environmental metabolomes reveals compositional divergence despite conserved molecular properties. Sci. Total Environ. 2021, 788, 147409. [Google Scholar] [CrossRef]
- Ma, N.L.; Lam, S.D.; Che Lah, W.A.; Ahmad, A.; Rinklebe, J.; Sonne, C.; Peng, W. Integration of environmental metabolomics and physiological approach for evaluation of saline pollution to rice plant. Environ. Pollut. 2021, 286, 117214. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2022, 52, 712–734. [Google Scholar] [CrossRef]
- LeVatte, M.; Keshteli, A.H.; Zarei, P.; Wishart, D.S. Applications of Metabolomics to Precision Nutrition. Lifestyle Genom. 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Cank, K.B.; Todd, D.A.; Knowles, S.L.; Oberlies, N.H. Metabolomics-Guided Comparison of Pollen and Microalgae-Based Artificial Diets in Honey Bees. J. Agric. Food Chem. 2022, 70, 9790–9801. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Y.; Almeida, P.; Mank, J.E.; van Tuinen, M.; Wang, Q.; Jiang, Z.; Chen, Y.; Zhan, K.; Hou, S.; et al. Whole-genome resequencing reveals signatures of selection and timing of duck domestication. Gigascience 2018, 7, giy027. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Dunn, W.B.; Dobson, P.; Patel, Y.; Winder, C.L.; Francis-McIntyre, S.; Begley, P.; Carroll, K.; Broadhurst, D.; Tseng, A.; et al. Mass spectrometry tools and metabolite-specific databases for molecular identification in metabolomics. Analyst 2009, 134, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics Approach Reveals the Effects of Breed and Feed on the Composition of Chicken Eggs. Metabolites 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Asnani, A.; Gerszten, R.E. Application of metabolomics to cardiovascular biomarker and pathway discovery. J. Am. Coll. Cardiol. 2008, 52, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef]
- Nemutlu, E.; Zhang, S.; Gupta, A.; Juranic, N.O.; Macura, S.I.; Terzic, A.; Jahangir, A.; Dzeja, P. Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted 3¹P NMR and mass spectrometry. Physiol. Genom. 2012, 44, 386–402. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K. Spectroscopic and statistical techniques for information recovery in metabonomics and metabolomics. Annu. Rev. Anal. Chem. 2008, 1, 45–69. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zhang, S.; Ward, L.E.; Karakelides, H.; Raftery, D.; Nair, K.S. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS ONE 2010, 5, e10538. [Google Scholar] [CrossRef]
- Dunn, W.B. Current trends and future requirements for the mass spectrometric investigation of microbial, mammalian and plant metabolomes. Phys. Biol. 2008, 5, 011001. [Google Scholar] [CrossRef]
- Nemutlu, E.; Zhang, S.; Xu, Y.Z.; Terzic, A.; Zhong, L.; Dzeja, P.D.; Cha, Y.M. Cardiac resynchronization therapy induces adaptive metabolic transitions in the metabolomic profile of heart failure. J. Card. Fail. 2015, 21, 460–469. [Google Scholar] [CrossRef]
- Villas-Bôas, S.G.; Bruheim, P. The potential of metabolomics tools in bioremediation studies. OMICS 2007, 11, 305–313. [Google Scholar] [CrossRef]
- Wu, L.; Huang, X.; Liu, S.; Liu, J.; Guo, Y.; Sun, Y.; Lin, J.; Guo, Y.; Wei, S. Understanding the formation mechanism of oolong tea characteristic non-volatile chemical constitutes during manufacturing processes by using integrated widely-targeted metabolome and DIA proteome analysis. Food Chem. 2020, 310, 125941. [Google Scholar] [CrossRef]
- Liu, H.; Ding, P.; Tong, Y.; He, X.; Yin, Y.; Zhang, H.; Song, Z. Metabolomic analysis of the egg yolk during the embryonic development of broilers. Poult. Sci. 2021, 100, 101014. [Google Scholar] [CrossRef]
- El-Hefny, M.; Ashmawy, N.A.; Salem, M.Z.M.; Salem, A.Z.M. Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathog. 2017, 113, 348–356. [Google Scholar] [CrossRef]
- Pace, L.R.; Harrison, Z.L.; Brown, M.N.; Haggard, W.O.; Jennings, J.A. Characterization and Antibiofilm Activity of Mannitol-Chitosan-Blended Paste for Local Antibiotic Delivery System. Mar. Drugs 2019, 17, 517. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, J.; Richey, G.; Wang, M.; Hong, S.M.C.; Hong, S.H. Undecanoic Acid, Lauric Acid, and N-Tridecanoic Acid Inhibit Escherichia coli Persistence and Biofilm Formation. J. Microbiol. Biotechnol. 2021, 31, 130–136. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Rajkumar, M.; Vincent, J.D.; Gunasekaran, P.; Anbazhagan, C.; Kaveri, K.; Kannan, S. Antioxidant and in vitro anticancer effect of 2-pyrrolidinone rich fraction of Brassica oleracea var. capitata through induction of apoptosis in human cancer cells. Phytother. Res. 2013, 27, 1664–1670. [Google Scholar] [CrossRef]
- Yu, K.; Liu, H.; Kachroo, P. Pipecolic Acid Quantification Using Gas Chromatography-coupled Mass Spectrometry. Bio. Protoc. 2020, 10, e3841. [Google Scholar] [CrossRef]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Maoka, T.; Miyashita, K. Suppressive effects of alloxanthin and diatoxanthin from Halocynthia roretzi on LPS-induced expression of pro-inflammatory genes in RAW264.7 cells. J. Oleo Sci. 2008, 57, 181–189. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Si, S.; Li, H.; Song, G. A simple and efficient synthesis of fused morpholine pyrrolidines/piperdines with potential insecticidal activities. Mol. Divers 2014, 18, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Alsamarrai, A.S.H.; Abdulghani, S.S. Microwave-Assisted Synthesis, Structural Characterization and Assessment of the Antibacterial Activity of Some New Aminopyridine, Pyrrolidine, Piperidine and Morpholine Acetamides. Molecules 2021, 26, 533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Guan, T.; Jia, S.; Liu, Y.; Zhao, X. Effects of quercetin on cadmium-induced toxicity in rat urine using metabonomics techniques. Hum. Exp. Toxicol. 2020, 39, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.B.; Das, A.; Chaudhuri, D.; Panja, S.; Mandal, N. Sundew plant, a potential source of anti-inflammatory agents, selectively induces G2/M arrest and apoptosis in MCF-7 cells through upregulation of p53 and Bax/Bcl-2 ratio. Cell Death Discov. 2016, 2, 15062. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mei, J.; Tan, M.; Xie, J. Effect of CO2 on the spoilage potential of Shewanella putrefaciens target to flavour compounds. Food Chem. 2022, 397, 133748. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Dong, L.; Huan, Y.; Yoshimoto, M.; Zhu, Y.; Tada, I.; Wang, X.; Zhao, S.; Zhang, F.; et al. Comprehensive Metabolomic Comparison of Five Cereal Vinegars Using Non-Targeted and Chemical Isotope Labeling LC-MS Analysis. Metabolites 2022, 12, 427. [Google Scholar] [CrossRef]
- Parker, C.A.; Anderson, N.J.; Robinson, E.S.; Price, R.; Tyacke, R.J.; Husbands, S.M.; Dillon, M.P.; Eglen, R.M.; Hudson, A.L.; Nutt, D.J.; et al. Harmane and harmalan are bioactive components of classical clonidine-displacing substance. Biochemistry 2004, 43, 16385–16392. [Google Scholar] [CrossRef]
- Fekkes, D.; Bode, W.T. Occurrence and partition of the beta-carboline norharman in rat organs. Life Sci. 1993, 52, 2045–2054. [Google Scholar] [CrossRef]
- Kim, D.C.; Quang, T.H.; Yoon, C.S.; Ngan, N.T.T.; Lim, S.I.; Lee, S.Y.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced BV2 microglial cells. Food Chem. 2016, 213, 69–75. [Google Scholar] [CrossRef]
- Torquetti, C.; Ferreira, P.O.; de Almeida, A.C.; Fernandes, R.P.; Caires, F.J. Thermal study and characterization of new cocrystals of ciprofloxacin with picolinic acid. J. Therm. Anal. Calorim. 2022, 147, 1299–1306. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Song, H.; Xia, L. Aroma extract dilution analysis of a beef flavouring prepared from flavour precursors and enzymatically hydrolysed beef. Flavour Fragr. J. 2008, 23, 185–193. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Ruan, L.; Jiang, L.; Zhao, W.; Meng, H.; Zheng, Q.; Wang, J. Hepatotoxicity or hepatoprotection of emodin? Two sides of the same coin by 1H-NMR metabolomics profiling. Toxicol. Appl. Pharmacol. 2021, 431, 115734. [Google Scholar] [CrossRef]
- Kim, M.; Im, S.; Cho, Y.K.; Choi, C.; Son, Y.; Kwon, D.; Jung, Y.S.; Lee, Y.H. Anti-Obesity Effects of Soybean Embryo Extract and Enzymatically-Modified Isoquercitrin. Biomolecules 2020, 10, 1394. [Google Scholar] [CrossRef]
- Heinen, C.A.; Reuss, S.; Amidon, G.L.; Langguth, P. Ion pairing with bile salts modulates intestinal permeability and contributes to food-drug interaction of BCS class III compound trospium chloride. Mol. Pharm. 2013, 10, 3989–3996. [Google Scholar] [CrossRef]
- Grebenstein, P.E.; Erickson, P.; Grace, M.; Kotz, C.M. Anatabine, Nornicotine, and Anabasine Reduce Weight Gain and Body Fat through Decreases in Food Intake and Increases in Physical Activity. J. Clin. Med. 2022, 11, 481. [Google Scholar] [CrossRef]
- Den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Mohan Krishna, K.; Inturi, B.; Pujar, G.V.; Purohit, M.N.; Vijaykumar, G.S. Design, synthesis and 3D-QSAR studies of new diphenylamine containing 1,2,4-triazoles as potential antitubercular agents. Eur. J. Med. Chem. 2014, 84, 516–529. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, C.; Zhang, W.; Wang, W.; He, B.; Hu, B.; Jiang, G.; Wang, Y.; Hong, J.; Li, S.; et al. Stachydrine prevents LPS-induced bone loss by inhibiting osteoclastogenesis via NF-κB and Akt signalling. J. Cell. Mol. Med. 2019, 23, 6730–6743. [Google Scholar] [CrossRef] [PubMed]
- Molaei, E.; Molaei, A.; Hayes, A.W.; Karimi, G. Resolvin D1, therapeutic target in acute respiratory distress syndrome. Eur. J. Pharmacol. 2021, 911, 174527. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Sparks, D.L.; Knott, K.K.; Willard, S.; Brown, A. Simultaneous choice bioassays accompanied by physiological changes identify civetone and decanoic acid as pheromone candidates for giant pandas. Zoo Biol. 2020, 39, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B. The role of GlcNAc in formation and function of extracellular matrices. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 215–226. [Google Scholar] [CrossRef]
- Xue, Y.; Nestor, G. Determination of Amide cis/trans Isomers in N-Acetyl-d-glucosamine: Tailored NMR Analysis of the N-Acetyl Group Conformation. ChemBioChem 2022, 23, e202200338. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Gao, J.; Li, X.; Zhang, R.; Jin, X.; Hu, Z.; Zheng, B.; Persson, S.; Chen, P. The 2′-O-methyladenosine nucleoside modification gene OsTRM13 positively regulates salt stress tolerance in rice. J. Exp. Bot. 2017, 68, 1479–1491. [Google Scholar] [CrossRef]

| Metabolites | Class 1 | Relative Area (Yolk) | SEM | p-Value | Trend 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fenghua Teal | Captive Mallard | Shaoxing Duck | ||||||||||
| Wild | Wild | Domestic | ||||||||||
| Raw | Boiled | Raw | Boiled | Raw | Boiled | Domestication | Boiling | Domestication × Boiling | ||||
| Conduritol b epoxide 2 | / | 0.0039 | 0.0035 | 0.0047 | 0.0046 | 0.0110 | 0.0096 | 0.0006 | <0.001 | 0.187 | 0.393 | Up |
| Dimethylethanolamine | Amines | 0.0096 | 0.0108 | 0.0091 | 0.0082 | 0.0044 | 0.0035 | 0.0005 | <0.001 | 0.560 | 0.477 | Down |
| Beta-doradecin | Prenol lipids | 0.0063 | 0.0056 | 0.0079 | 0.0079 | 0.0025 | 0.0025 | 0.0004 | <0.001 | 0.733 | 0.761 | Down |
| Monoolein | Glycerolipids | 0.0015 | 0.0013 | 0.0014 | 0.0013 | 0.0022 | 0.0024 | 0.0001 | <0.001 | 0.900 | 0.161 | Up |
| Diphenylamine | Benzene and substituted derivatives | 0.0055 | 0.0051 | 0.0031 | 0.0030 | 0.0012 | 0.0010 | 0.0003 | <0.001 | 0.618 | 0.886 | Down |
| Harmalan | Harmala alkaloids | 0.0206 | 0.0181 | 0.0137 | 0.0158 | 0.0009 | 0.0014 | 0.0015 | <0.001 | 0.951 | 0.832 | Down |
| 3′-Hydroxy-e,e-caroten-3-one | Prenol lipids | 0.0022 | 0.0025 | 0.0083 | 0.0081 | 0.0010 | 0.0013 | 0.0005 | <0.001 | 0.890 | 0.886 | Down |
| Mannitol | Organooxygen compounds | 0.2176 | 0.2227 | 0.1794 | 0.1986 | 0.0783 | 0.0753 | 0.0109 | <0.001 | 0.576 | 0.353 | Down |
| Glycocholic acid | Steroids and steroid derivatives | 0.0009 | 0.0008 | 0.0006 | 0.0006 | 0.0025 | 0.0035 | 0.0002 | <0.001 | 0.114 | 0.091 | Up |
| 4-aminophenol | Benzene and substituted derivatives | 0.0057 | 0.0065 | 0.0028 | 0.0028 | 0.0020 | 0.0017 | 0.0004 | <0.001 | 0.910 | 0.565 | Down |
| p-Toluenesulfonic acid | Organic sulfuric acids and derivatives | 0.4382 | 0.4090 | 0.4130 | 0.4116 | 0.3208 | 0.2970 | 0.0129 | <0.001 | 0.353 | 0.838 | Down |
| Proline betaine | Carboxylic acids and derivatives | 0.6744 | 0.7447 | 0.7691 | 0.8050 | 0.2035 | 0.1280 | 0.0548 | <0.001 | 0.862 | 0.320 | Down |
| Lysopc(14:0/0:0) | Glycerophospholipids | 0.4477 | 0.4533 | 0.4105 | 0.5938 | 0.3419 | 0.2679 | 0.0210 | <0.001 | 0.745 | 0.011 | Down |
| Apigenin 7-sulfate | Flavonoids | 0.0002 | 0.0002 | 0.0003 | 0.0002 | 0.0014 | 0.0013 | 0.0001 | <0.001 | 0.677 | 0.733 | Up |
| 1h-Pyrrole-2-carboxaldehyde | Organooxygen compounds | 0.0088 | 0.0079 | 0.0074 | 0.0079 | 0.0029 | 0.0032 | 0.0005 | <0.001 | 0.995 | 0.697 | Down |
| 2′-o-Methyladenosine | Purine nucleosides | 0.0156 | 0.0257 | 0.0215 | 0.0427 | 0.0083 | 0.0062 | 0.0026 | <0.001 | 0.088 | 0.027 | Down |
| 3-Methyl-glutarylcarnitine | Fatty acyls | 0.0014 | 0.0013 | 0.0118 | 0.0170 | 0.0011 | 0.0015 | 0.0012 | 0.008 | 0.535 | 0.646 | Down |
| 4,5-Dihydro-2-methylthiazole | Azolines | 0.1286 | 0.1348 | 0.1234 | 0.1276 | 0.1145 | 0.1097 | 0.0018 | <0.001 | 0.940 | 0.068 | Down |
| Nornicotine | Pyridines and derivatives | 0.0060 | 0.0038 | 0.0045 | 0.0048 | 0.0025 | 0.0025 | 0.0003 | <0.001 | 0.321 | 0.373 | Down |
| Pyrrolidine | Pyrrolidines | 0.0259 | 0.0273 | 0.0078 | 0.0073 | 0.0041 | 0.0022 | 0.0018 | <0.001 | 0.820 | 0.718 | Down |
| 2-Propenyl 2-aminobenzoate | Benzene and substituted derivatives | 0.0135 | 0.0138 | 0.0087 | 0.0086 | 0.0013 | 0.0012 | 0.0009 | <0.001 | 0.976 | 0.937 | Down |
| n-Ornithyl-l-taurine | Carboxylic acids and derivatives | 0.0261 | 0.0278 | 0.0163 | 0.0168 | 0.0083 | 0.0080 | 0.0014 | <0.001 | 0.856 | 0.719 | Down |
| (9s,10e,12z,15z)-9-Hydroxy-10,12,15-octadecatrienoic acid | Lineolic acids and derivatives | 0.0179 | 0.0194 | 0.0183 | 0.0196 | 0.0144 | 0.0160 | 0.0005 | <0.001 | 0.066 | 0.904 | Down |
| 3-(2-Hydroxyphenyl)propanoic acid | Phenylpropanoic acids | 0.1464 | 0.1264 | 0.0822 | 0.0840 | 0.0241 | 0.0283 | 0.0109 | <0.001 | 0.897 | 0.732 | Down |
| Prolyl-gamma-glutamate | Carboxylic acids and derivatives | 0.0033 | 0.0027 | 0.0044 | 0.0030 | 0.0069 | 0.0060 | 0.0003 | <0.001 | 0.056 | 0.923 | Up |
| Emodin | Anthracenes | 0.0177 | 0.0213 | 0.0235 | 0.0311 | 0.0725 | 0.0838 | 0.0048 | <0.001 | 0.056 | 0.512 | Up |
| Diglycerol 1 | / | 0.0040 | 0.0039 | 0.0045 | 0.0041 | 0.0032 | 0.0032 | 0.0001 | <0.001 | 0.239 | 0.447 | Down |
| Linoleic acid | Fatty acyls | 18.9801 | 17.9344 | 16.0182 | 20.3844 | 12.5212 | 13.2524 | 0.6534 | <0.001 | 0.264 | 0.661 | Down |
| 2-Amino-3-methylbenzoate | Benzene and substituted derivatives | 0.0115 | 0.0119 | 0.0110 | 0.0126 | 0.0096 | 0.0101 | 0.0002 | <0.001 | 0.062 | 0.447 | Down |
| Metabolites | ||||||||||||
| Imidazole acetol-phosphate | Organic phosphoric acids and derivatives | 0.0027 | 0.0021 | 0.0022 | 0.0022 | 0.0006 | 0.0004 | 0.0002 | <0.001 | 0.106 | 0.513 | Down |
| 2-Pyrrolidinone | Pyrrolidines | 0.0494 | 0.0464 | 0.0158 | 0.0140 | 0.0082 | 0.0078 | 0.0030 | <0.001 | 0.786 | 0.851 | Down |
| 5-[2h-Pyrrol-4-(3h)-ylidenemethyl]-2-furanmethanol | Heteroaromatic compounds | 0.0312 | 0.0322 | 0.0207 | 0.0216 | 0.0030 | 0.0026 | 0.0022 | <0.001 | 0.922 | 0.793 | Down |
| n-Acetyl-d-galactosamine 1 | / | 0.0137 | 0.0174 | 0.0166 | 0.0266 | 0.0078 | 0.0065 | 0.0014 | <0.001 | 0.174 | 0.050 | Down |
| Phenol | Phenols | 0.0027 | 0.0042 | 0.0025 | 0.0030 | 0.0021 | 0.0019 | 0.0002 | 0.001 | 0.258 | 0.057 | Down |
| Alloxanthin | Prenol lipids | 0.0480 | 0.0501 | 0.0825 | 0.0804 | 0.0198 | 0.0200 | 0.0044 | <0.001 | 0.984 | 0.990 | Down |
| 3-Amino-2-piperidone | Carboxylic acids and derivatives | 0.0057 | 0.0056 | 0.0240 | 0.0202 | 0.0026 | 0.0022 | 0.0015 | <0.001 | 0.660 | 0.764 | Down |
| Daidzein | Isoflavonoids | 0.0089 | 0.0082 | 0.0101 | 0.0098 | 0.0479 | 0.0479 | 0.0032 | <0.001 | 0.887 | 0.924 | Up |
| (±)-anisoxide | / | 0.0064 | 0.0069 | 0.0097 | 0.0102 | 0.0035 | 0.0035 | 0.0005 | <0.001 | 0.748 | 0.654 | Down |
| Genistein | Isoflavonoids | 0.0014 | 0.0016 | 0.0019 | 0.0020 | 0.0113 | 0.0125 | 0.0009 | <0.001 | 0.266 | 0.365 | Up |
| Ganodosterone | Steroids and steroid derivatives | 0.0114 | 0.0106 | 0.0097 | 0.0103 | 0.0041 | 0.0029 | 0.0006 | <0.001 | 0.374 | 0.449 | Down |
| Resolvin d1 | Fatty acyls | 0.3247 | 0.2843 | 0.2387 | 0.2931 | 0.1821 | 0.1774 | 0.0114 | <0.001 | 0.946 | 0.732 | Down |
| Glycitein | Isoflavonoids | 0.0005 | 0.0006 | 0.0008 | 0.0007 | 0.0030 | 0.0027 | 0.0002 | <0.001 | 0.307 | 0.269 | Up |
| n-Acetyl-d-glucosamine | Organooxygen compounds | 0.0187 | 0.0249 | 0.0236 | 0.0398 | 0.0100 | 0.0089 | 0.0022 | <0.001 | 0.138 | 0.075 | Down |
| 4-Aminobutyraldehyde | Organooxygen compounds | 0.0143 | 0.0123 | 0.0316 | 0.0347 | 0.0058 | 0.0049 | 0.0021 | <0.001 | 0.957 | 0.834 | Down |
| n-Acetylleucine | Carboxylic acids and derivatives | 0.0300 | 0.0339 | 0.0378 | 0.0405 | 0.0218 | 0.0243 | 0.0015 | <0.001 | 0.239 | 0.868 | Down |
| dl-2-Aminooctanoic acid | Carboxylic acids and derivatives | 0.0063 | 0.0075 | 0.0042 | 0.0056 | 0.0028 | 0.0026 | 0.0004 | <0.001 | 0.444 | 0.267 | Down |
| Pipecolic acid | Carboxylic acids and derivatives | 0.0105 | 0.0126 | 0.0133 | 0.0148 | 0.0070 | 0.0074 | 0.0006 | <0.001 | 0.174 | 0.373 | Down |
| pc(20:1(11z)/15:0) | Glycerophospholipids | 0.0458 | 0.0527 | 0.0314 | 0.0380 | 0.0242 | 0.0245 | 0.0020 | <0.001 | 0.264 | 0.305 | Down |
| Metabolites | Class 1 | Relative Area (Yolk) | SEM | p-Value | Trend 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fenghua Teal | Captive Mallard | Shaoxing Duck | ||||||||||
| Wild | Wild | Domestic | ||||||||||
| Raw | Boiled | Raw | Boiled | Raw | Boiled | Boiling | Domestication | Boiling × Domestication | ||||
| Cystine | Carboxylic acids and derivatives | 0.0039 | 0.0069 | 0.0035 | 0.0062 | 0.0047 | 0.0098 | 0.0004 | <0.001 | <0.001 | 0.044 | Up |
| Leucyl-phenylalanine | Carboxylic acids and derivatives | 0.0017 | 0.0032 | 0.0016 | 0.0028 | 0.0018 | 0.0037 | 0.0002 | <0.001 | 0.023 | 0.203 | Up |
| Isoleucyl-leucine | Carboxylic acids and derivatives | 0.0017 | 0.0029 | 0.0017 | 0.0030 | 0.0018 | 0.0035 | 0.0002 | <0.001 | 0.254 | 0.333 | Up |
| 5′-Methylthioadenosine | 5′-deoxyribonucleosides | 0.0095 | 0.0156 | 0.0063 | 0.0124 | 0.0043 | 0.0099 | 0.0008 | <0.001 | 0.001 | 0.846 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, G.; Du, X.; Zeng, T.; Chen, L.; Xu, W.; Gu, T.; Tao, Z.; Lu, L. Integration of LC-MS-Based and GC-MS-Based Metabolic Profiling to Reveal the Effects of Domestication and Boiling on the Composition of Duck Egg Yolks. Metabolites 2023, 13, 135. https://doi.org/10.3390/metabo13010135
Tian Y, Li G, Du X, Zeng T, Chen L, Xu W, Gu T, Tao Z, Lu L. Integration of LC-MS-Based and GC-MS-Based Metabolic Profiling to Reveal the Effects of Domestication and Boiling on the Composition of Duck Egg Yolks. Metabolites. 2023; 13(1):135. https://doi.org/10.3390/metabo13010135
Chicago/Turabian StyleTian, Yong, Guoqin Li, Xizhong Du, Tao Zeng, Li Chen, Wenwu Xu, Tiantian Gu, Zhengrong Tao, and Lizhi Lu. 2023. "Integration of LC-MS-Based and GC-MS-Based Metabolic Profiling to Reveal the Effects of Domestication and Boiling on the Composition of Duck Egg Yolks" Metabolites 13, no. 1: 135. https://doi.org/10.3390/metabo13010135
APA StyleTian, Y., Li, G., Du, X., Zeng, T., Chen, L., Xu, W., Gu, T., Tao, Z., & Lu, L. (2023). Integration of LC-MS-Based and GC-MS-Based Metabolic Profiling to Reveal the Effects of Domestication and Boiling on the Composition of Duck Egg Yolks. Metabolites, 13(1), 135. https://doi.org/10.3390/metabo13010135






