The Role of Advanced Glycation End Products on Dyslipidemia
Abstract
:1. Introduction
2. The Role of AGEs: Relationship with Dyslipidemia
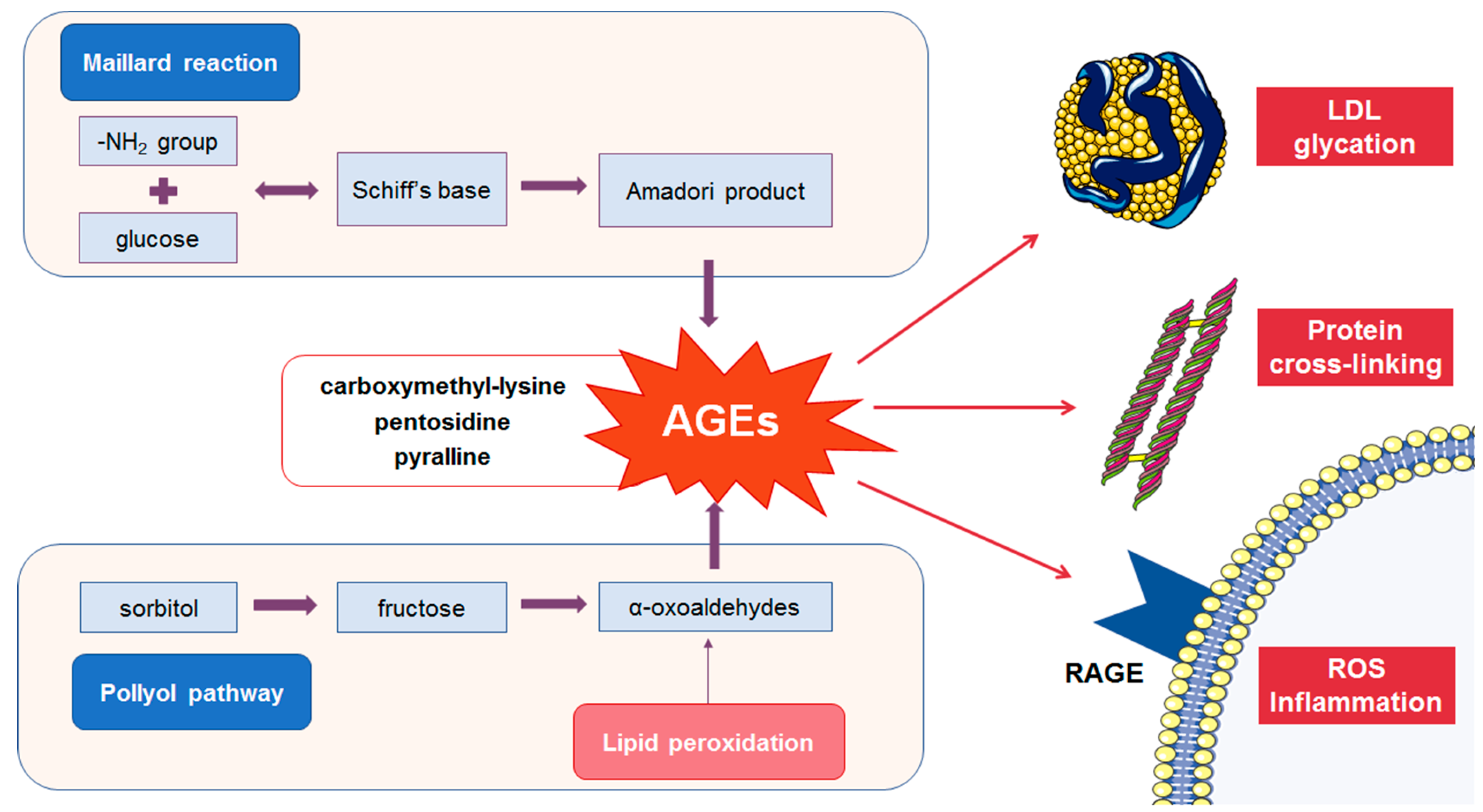
2.1. Formation of AGEs
2.2. Detrimental Effects of AGEs
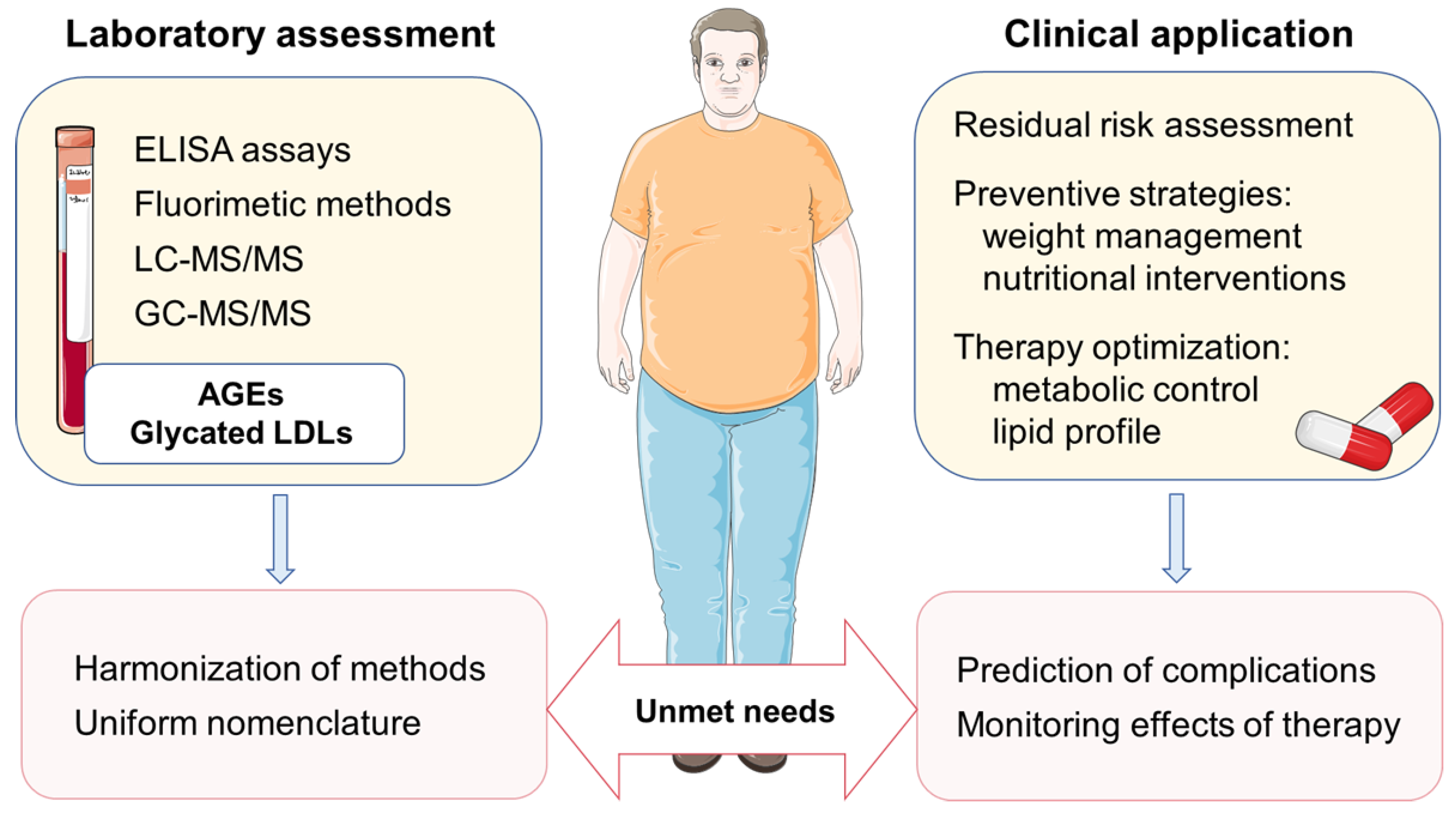
2.3. Assessment of AGEs in Biological Samples
2.4. Relationship between AGEs and Dyslipidemia in Patients with DM
3. The Role of Glycated LDL: Relationships with Inflammation
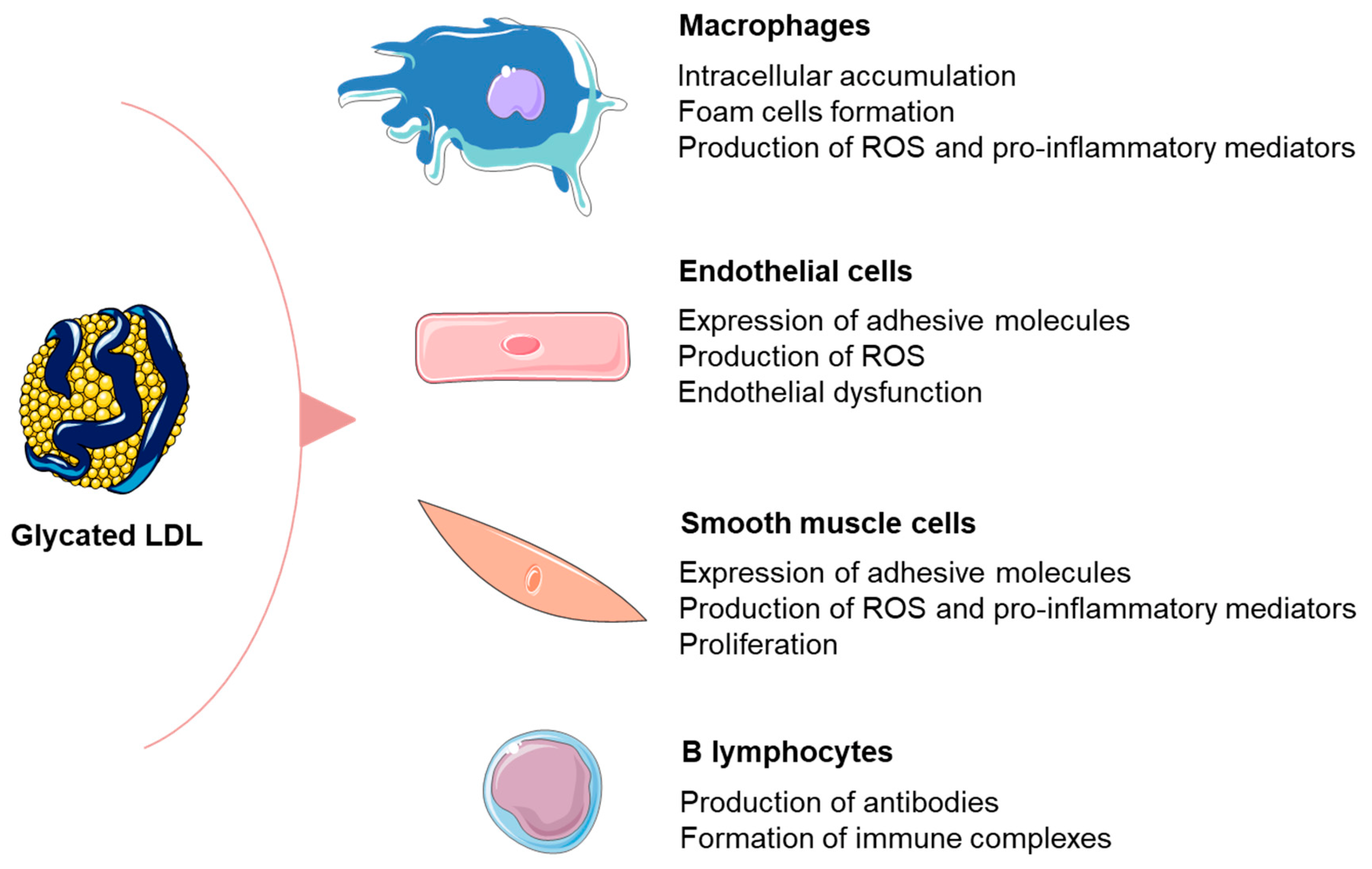
3.1. Formation and Detrimental Effects of Glycated LDL Particles
3.2. Relationship between Glycated LDL and Inflammation in Patients with DM
4. Implications for Cardiovascular Prevention and Future Directions
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes Silva, L.; Vangipurapu, J.; Laakso, M. The “Common Soil Hypothesis” Revisited-Risk Factors for Type 2 Diabetes and Cardiovascular Disease. Metabolites 2021, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Lorenzatti, A.J.; Toth, P.P. New Perspectives on Atherogenic Dyslipidaemia and Cardiovascular Disease. Eur. Cardiol. 2020, 15, e04. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Al Rasadi, K.; Cesur, M.; Silva-Nunes, J.; Stoian, A.P.; Rizzo, M. A New Look at Novel Cardiovascular Risk Biomarkers: The Role of Atherogenic Lipoproteins and Innovative Antidiabetic Therapies. Metabolites 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [Green Version]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Giglio, R.V.; Ciaccio, M.; Rizzo, M. Diabetes and Colorectal Cancer Risk: A New Look at Molecular Mechanisms and Potential Role of Novel Antidiabetic Agents. Int. J. Mol. Sci. 2021, 22, 12409. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Cicero, A.F.G.; Janez, A.; Stoian, A.P.; Sonmez, A.; Rizzo, M. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Younis, N.N.; Soran, H.; Sharma, R.; Charlton-Menys, V.; Greenstein, A.; Elseweidy, M.M.; Durrington, P.N. Small-dense LDL and LDL glycation in metabolic syndrome and in statin-treated and non-statin-treated type 2 diabetes. Diab. Vasc. Dis. Res. 2010, 7, 289–295. [Google Scholar] [CrossRef]
- Younis, N.; Charlton-Menys, V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef]
- Gautieri, A.; Passini, F.S.; Silvan, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef]
- Chuyen, N.V. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 2006, 50, 1140–1149. [Google Scholar] [CrossRef]
- Wu, B.; Yu, L.; Hu, P.; Lu, Y.; Li, J.; Wei, Y.; He, R. GRP78 protects CHO cells from ribosylation. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 629–637. [Google Scholar] [CrossRef]
- Federico, G.; Gori, M.; Randazzo, E.; Vierucci, F. Skin advanced glycation end-products evaluation in infants according to the type of feeding and mother’s smoking habits. SAGE Open Med. 2016, 4, 2050312116682126. [Google Scholar] [CrossRef] [Green Version]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef]
- Boyanova, M.; Huppertz, B. Cytotoxic effect of advanced glycation end products. Biotechnol. Biotechnol. Equip. 2009, 23, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Leung, S.S.; Forbes, J.M.; Borg, D.J. Receptor for Advanced Glycation End Products (RAGE) in Type 1 Diabetes Pathogenesis. Curr. Diab. Rep. 2016, 16, 100. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- Hudson, B.I.; Carter, A.M.; Harja, E.; Kalea, A.Z.; Arriero, M.; Yang, H.; Grant, P.J.; Schmidt, A.M. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008, 22, 1572–1580. [Google Scholar] [CrossRef]
- Ninic, A.; Bojanin, D.; Sopic, M.; Mihajlovic, M.; Munjas, J.; Milenkovic, T.; Stefanovic, A.; Vekic, J.; Spasojevic-Kalimanovska, V. Transforming Growth Factor-beta1 and Receptor for Advanced Glycation End Products Gene Expression and Protein Levels in Adolescents with Type 1 iabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol. Cell Biochem. 2019, 451, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Piras, S.; Furfaro, A.L.; Domenicotti, C.; Traverso, N.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. RAGE Expression and ROS Generation in Neurons: Differentiation versus Damage. Oxid. Med. Cell Longev. 2016, 2016, 9348651. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. The RAGE connection to diabetes and atherosclerosis: An intertwined web of advanced glycation and inflammation. Future Lipidol. 2007, 11, 239–250. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Advanced glycation endproducts: From precursors to RAGE: Round and round we go. Amino Acids 2012, 42, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Yozgatli, K.; Lefrandt, J.D.; Noordzij, M.J.; Oomen, P.H.N.; Brouwer, T.; Jager, J.; Castro Cabezas, M.; Smit, A.J. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in Type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1242–1248. [Google Scholar] [CrossRef]
- Monami, M.; Lamanna, C.; Gori, F.; Bartalucci, F.; Marchionni, N.; Mannucci, E. Skin autofluorescence in type 2 diabetes: Beyond blood glucose. Diabetes Res. Clin. Pract. 2008, 79, 56–60. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Lutgers, H.L.; Kleefstra, N.; Graaff, R.; Groenier, K.H.; Smit, A.J.; Gans, R.O.; Bilo, H.J. Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008, 31, 517–521. [Google Scholar] [CrossRef]
- Sternberg, M.; M’Bemba, J.; Urios, P.; Borsos, A.M.; Selam, J.L.; Peyroux, J.; Slama, G. Skin collagen pentosidine and fluorescence in diabetes were predictors of retinopathy progression and creatininemia increase already 6years after punch-biopsy. Clin. Biochem. 2016, 49, 225–231. [Google Scholar] [CrossRef]
- Papachristou, S.; Rizzo, M.; Papanas, N. Advanced Glycation End Products: Do They Impair Bone Health in Diabetes? Exp. Clin. Endocrinol. Diabetes 2022, 130, 636–637. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Lutgers, H.L.; Links, T.P.; Graaff, R.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007, 30, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Kilhovd, B.K.; Berg, T.J.; Birkeland, K.I.; Thorsby, P.; Hanssen, K.F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999, 22, 1543–1548. [Google Scholar] [CrossRef]
- Lobo, J.P.J.; Brescansin, C.P.; Santos-Weiss, I.C.R.; Welter, M.; Souza, E.M.; Rego, F.G.M.; Picheth, G.; Alberton, D. Serum Fluorescent Advanced Glycation End (F-AGE) products in gestational diabetes patients. Arch. Endocrinol. Metab. 2017, 61, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Scheijen, J.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Lee, J.S.; Chung, Y.S.; Chang, S.Y.; Jung, Y.S.; Kim, S.H. Simple Quantification of Pentosidine in Human Urine and Plasma by High-Performance Liquid Chromatography. Int. J. Anal. Chem. 2017, 2017, 1389807. [Google Scholar] [CrossRef] [Green Version]
- Munch, G.; Keis, R.; Wessels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.D.; Schinzel, R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–677. [Google Scholar] [CrossRef]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef] [Green Version]
- Koito, W.; Araki, T.; Horiuchi, S.; Nagai, R. Conventional antibody against Nepsilon-(carboxymethyl)lysine (CML) shows cross-reaction to Nepsilon-(carboxyethyl)lysine (CEL): Immunochemical quantification of CML with a specific antibody. J. Biochem. 2004, 136, 831–837. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple noninvasive measurement of skin autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.B.; Chu, N.F.; Syu, J.T.; Hsieh, A.T.; Hung, Y.R. Advanced glycation end products (AGEs) in relation to atherosclerotic lipid profiles in middle-aged and elderly diabetic patients. Lipids Health Dis. 2011, 10, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, M.; Rabizadeh, S.; Mirahmad, M.; Hajmiri, M.S.; Nakhjavani, M.; Hemmatabadi, M.; Shirzad, N. The association between advanced glycation end products (AGEs) and ABC (hemoglobin A1C, blood pressure, and low-density lipoprotein cholesterol) control parameters among patients with type 2 diabetes mellitus. Diabetol. Metab Syndr. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Indyk, D.; Bronowicka-Szydelko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.; Dai, F.; Liu, C.; Hu, H.; Zhang, Q. Advanced glycation end products and diabetes and other metabolic indicators. Diabetol. Metab Syndr. 2022, 14, 104. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Berneis, K. Should we measure routinely the LDL peak particle size? Int. J. Cardiol. 2006, 107, 166–170. [Google Scholar] [CrossRef]
- Diffenderfer, M.R.; Schaefer, E.J. The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 2014, 25, 221–226. [Google Scholar] [CrossRef]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Therond, P.; Meikle, P.J. LDL subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense LDL. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.P.; Barylski, M.; Nikolic, D.; Rizzo, M.; Montalto, G.; Banach, M. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 353–368. [Google Scholar] [CrossRef]
- Vavlukis, M.; Vavlukis, A.; Krsteva, K.; Topuzovska, S. Paraoxonase 1 gene polymorphisms in lipid oxidation and atherosclerosis development. Front. Genet. 2022, 13, 966413. [Google Scholar] [CrossRef]
- Younis, N.; Sharma, R.; Soran, H.; Charlton-Menys, V.; Elseweidy, M.; Durrington, P.N. Glycation as an atherogenic modification of LDL. Curr. Opin. Lipidol. 2008, 19, 378–384. [Google Scholar] [CrossRef]
- Soran, H.; Liu, Y.; Adam, S.; Siahmansur, T.; Ho, J.H.; Schofield, J.D.; Kwok, S.; Gittins, M.; France, M.; Younis, N.; et al. A comparison of the effects of low- and high-dose atorvastatin on lipoprotein metabolism and inflammatory cytokines in type 2 diabetes: Results from the Protection Against Nephropathy in Diabetes with Atorvastatin (PANDA) randomized trial. J. Clin. Lipidol. 2018, 12, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Chittari, M.V.; Bodmer, C.W.; Zehnder, D.; Ceriello, A.; Thornalley, P.J. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010, 59, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Atherogenic Lipoproteins for the Statin Residual Cardiovascular Disease Risk. Int. J. Mol. Sci. 2022, 23, 13499. [Google Scholar] [CrossRef]
- Superko, H.; Garrett, B. Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels. Biomedicines 2022, 10, 829. [Google Scholar] [CrossRef]
- Sanchez-Quesada, J.L.; Vinagre, I.; De Juan-Franco, E.; Sanchez-Hernandez, J.; Bonet-Marques, R.; Blanco-Vaca, F.; Ordonez-Llanos, J.; Perez, A. Impact of the LDL subfraction phenotype on Lp-PLA2 distribution, LDL modification and HDL composition in type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef]
- Brown, B.E.; Rashid, I.; van Reyk, D.M.; Davies, M.J. Glycation of low-density lipoprotein results in the time-dependent accumulation of cholesteryl esters and apolipoprotein B-100 protein in primary human monocyte-derived macrophages. FEBS J. 2007, 274, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Stancu, C.S.; Botez, G.M.; Sima, A.V.; Simionescu, M. Irreversibly glycated LDL induce oxidative and inflammatory state in human endothelial cells; added effect of high glucose. Biochem. Biophys. Res. Commun. 2009, 390, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.V.; Botez, G.M.; Stancu, C.S.; Manea, A.; Raicu, M.; Simionescu, M. Effect of irreversibly glycated LDL in human vascular smooth muscle cells: Lipid loading, oxidative and inflammatory stress. J. Cell Mol. Med. 2010, 14, 2790–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.Y.; Alouffi, S.; Khan, M.S.; Husain, F.M.; Akhter, F.; Ahmad, S. The neoepitopes on methylglyoxal (MG) glycated LDL create autoimmune response; autoimmunity detection in T2DM patients with varying disease duration. Cell Immunol. 2020, 351, 104062. [Google Scholar] [CrossRef] [PubMed]
- Virella, G.; Lopes-Virella, M.F. The Pathogenic Role of the Adaptive Immune Response to Modified LDL in Diabetes. Front. Endocrinol. 2012, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Virella, M.F.; Virella, G. Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis. J. Atheroscler. Thromb. 2013, 20, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Akhter, F.; Khan, M.S.; Alatar, A.A.; Faisal, M.; Ahmad, S. Antigenic role of the adaptive immune response to d-ribose glycated LDL in diabetes, atherosclerosis and diabetes atherosclerotic patients. Life Sci. 2016, 151, 139–146. [Google Scholar] [CrossRef]
- Mironova, M.A.; Klein, R.L.; Virella, G.T.; Lopes-Virella, M.F. Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes 2000, 49, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Virella, G.; Thorpe, S.R.; Alderson, N.L.; Stephan, E.M.; Atchley, D.; Wagner, F.; Lopes-Virella, M.F.; Group, D.E.R. Autoimmune response to advanced glycosylation end-products of human LDL. J. Lipid Res. 2003, 44, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low density lipoprotein-containing circulating immune complexes: Role in atherosclerosis and diagnostic value. Biomed. Res. Int. 2014, 2014, 205697. [Google Scholar] [CrossRef]
- Virella, G.; Atchley, D.; Koskinen, S.; Zheng, D.; Lopes-Virella, M.F.; Group, D.E.R. Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin. Immunol. 2002, 105, 81–92. [Google Scholar] [CrossRef]
- Oksjoki, R.; Kovanen, P.T.; Lindstedt, K.A.; Jansson, B.; Pentikainen, M.O. OxLDL-IgG immune complexes induce survival of human monocytes. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Tertov, V.V.; Orekhov, A.N.; Kacharava, A.G.; Sobenin, I.A.; Perova, N.V.; Smirnov, V.N. Low density lipoprotein-containing circulating immune complexes and coronary atherosclerosis. Exp. Mol. Pathol. 1990, 52, 300–308. [Google Scholar] [CrossRef]
- Cohen, M.P.; Jin, Y.; Lautenslager, G.T. Increased plasma glycated low-density lipoprotein concentrations in diabetes: A marker of atherogenic risk. Diabetes Technol. Ther. 2004, 6, 348–356. [Google Scholar] [CrossRef]
- De Michele, G.; Correale, M.; De Michele, O.; Guerra, V.; Mazzarelli, R.; Misciagna, G. Evaluation of serum biomarkers in nutritional disorders: Glycated apolipoprotein B, fasting serum glucose, fructosamine, stable and labile glycated hemoglobin in diabetic and non-diabetic subjects. Immunopharmacol. Immunotoxicol. 2008, 30, 925–936. [Google Scholar] [CrossRef]
- Misciagna, G.; Logroscino, G.; De Michele, G.; Guerra, V.; Cisternino, A.M.; Caruso, M.G.; Trevisan, M. Glycated apolipoprotein B and myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 6–12. [Google Scholar] [CrossRef]
- Siddiqui, K.; George, T.P.; Nawaz, S.S.; Yaslam, M.; Almogbel, E.; Al-Rubeaan, K. Significance of glycated LDL in different stages of diabetic nephropathy. Diabetes Metab. Syndr. 2019, 13, 548–552. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. The clinical relevance of low-density-lipoproteins size modulation by statins. Cardiovasc. Drugs Ther. 2006, 20, 205–217. [Google Scholar] [CrossRef]
- Kheniser, K.G.; Kashyap, S.R.; Kasumov, T. A systematic review: The appraisal of the effects of metformin on lipoprotein modification and function. Obes. Sci. Pract. 2019, 5, 36–45. [Google Scholar] [CrossRef]
- Burchardt, P.; Zawada, A.; Tabaczewski, P.; Naskret, D.; Kaczmarek, J.; Marcinkaniec, J.; Wierusz-Wysocka, B.; Wysocki, H. Metformin added to intensive insulin therapy reduces plasma levels of glycated but not oxidized lowdensity lipoprotein in young patients with type 1 diabetes and obesity in comparison with insulin alone: A pilot study. Pol. Arch. Med. Wewn. 2013, 123, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmah, S.; Roy, A.S. A review on prevention of glycation of proteins: Potential therapeutic substances to mitigate the severity of diabetes complications. Int. J. Biol. Macromol. 2022, 195, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Cardelo, M.P.; de la Cruz, S.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Guler, I.; Vals-Delgado, C.; Lopez-Moreno, A.; Luque, R.M.; Delgado-Lista, J.; et al. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol. Nutr. Food Res. 2021, 65, e1901290. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Betriu, A.; Salas-Salvado, J.; Pamplona, R.; Barbe, F.; Purroy, F.; Farras, C.; Fernandez, E.; Lopez-Cano, C.; Mizab, C.; et al. Mediterranean diet, physical activity and subcutaneous advanced glycation end-products’ accumulation: A cross-sectional analysis in the ILERVAS project. Eur. J. Nutr. 2020, 59, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Ganmore, I.; Shelly, S.; Zacharia, M.; Uribarri, J.; Beisswenger, P.; Cai, W.; Troen, A.M.; Schnaider Beeri, M. Long Term Dietary Restriction of Advanced Glycation End-Products (AGEs) in Older Adults with Type 2 Diabetes Is Feasible and Efficacious-Results from a Pilot RCT. Nutrients 2020, 12, 3143. [Google Scholar] [CrossRef]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; Smit, A.J.; van der Schans, C.; Hobbelen, H. Advanced Glycation End Products Are Associated With Physical Activity and Physical Functioning in the Older Population. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1545–1551. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Borges, J.P.; Lopes, G.O.; Pereira, E.; Mediano, M.F.F.; Farinatti, P.; Tibirica, E.; Daliry, A. Influence of Physical Exercise on Advanced Glycation End Products Levels in Patients Living with the Human Immunodeficiency Virus. Front. Physiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [Green Version]
- Kochli, S.; Endes, K.; Trinkler, M.; Mondoux, M.; Zahner, L.; Hanssen, H. Association of physical fitness with skin autofluorescence-derived advanced glycation end products in children. Pediatr. Res. 2020, 87, 1106–1111. [Google Scholar] [CrossRef]
- van de Zande, S.C.; de Vries, J.K.; van den Akker-Scheek, I.; Zwerver, J.; Smit, A.J. A physically active lifestyle is related to a lower level of skin autofluorescence in a large population with chronic-disease (LifeLines cohort). J. Sport Health Sci. 2022, 11, 260–265. [Google Scholar] [CrossRef]
- Gogas Yavuz, D.; Apaydin, T.; Imre, E.; Uygur, M.M.; Yazici, D. Skin Autofluorescence and Carotid Intima-Media Thickness Evaluation Following Bariatric Surgery in Patients with Severe Obesity. Obes. Surg. 2021, 31, 1055–1061. [Google Scholar] [CrossRef]
- Chao, P.C.; Huang, C.N.; Hsu, C.C.; Yin, M.C.; Guo, Y.R. Association of dietary AGEs with circulating AGEs, glycated LDL, IL-1alpha and MCP-1 levels in type 2 diabetic patients. Eur. J. Nutr. 2010, 49, 429–434. [Google Scholar] [CrossRef]
- Deo, P.; Keogh, J.B.; Price, N.J.; Clifton, P.M. Effects of Weight Loss on Advanced Glycation End Products in Subjects with and without Diabetes: A Preliminary Report. Int. J. Environ. Res. Public Health 2017, 14, 1553. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Bashir, B.; Adam, S.; Ho, J.H.; Dhage, S.; Azmi, S.; Ferdousi, M.; Yusuf, Z.; Donn, R.; Malik, R.A.; et al. Glycated apolipoprotein B decreases after bariatric surgery in people with and without diabetes: A potential contribution to reduction in cardiovascular risk. Atherosclerosis 2022, 346, 10–17. [Google Scholar] [CrossRef]
- Lamprea-Montealegre, J.A.; Arnold, A.M.; Mc, C.R.L.; Mukamal, K.J.; Djousse, L.; Biggs, M.L.; Siscovick, D.S.; Tracy, R.P.; Beisswenger, P.J.; Psaty, B.M.; et al. Plasma Levels of Advanced Glycation Endproducts and Risk of Cardiovascular Events: Findings From 2 Prospective Cohorts. J. Am. Heart Assoc. 2022, 11, e024012. [Google Scholar] [CrossRef]
- Luo, W.; He, Y.; Ding, F.; Nie, X.; Li, X.L.; Song, H.L.; Li, G.X. Study on the levels of glycosylated lipoprotein in patients with coronary artery atherosclerosis. J. Clin. Lab. Anal. 2019, 33, e22650. [Google Scholar] [CrossRef] [Green Version]
- Al Saudi, R.M.; Kasabri, V.; Naffa, R.; Bulatova, N.; Bustanji, Y. Glycated LDL-C and glycated HDL-C in association with adiposity, blood and atherogenicity indices in metabolic syndrome patients with and without prediabetes. Ther. Adv. Endocrinol. Metab. 2018, 9, 311–323. [Google Scholar] [CrossRef]
- Langlois, M.R.; Chapman, M.J.; Cobbaert, C.; Mora, S.; Remaley, A.T.; Ros, E.; Watts, G.F.; Boren, J.; Baum, H.; Bruckert, E.; et al. Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin. Chem. 2018, 64, 1006–1033. [Google Scholar] [CrossRef] [Green Version]
- Giglio, R.V.; Pantea Stoian, A.; Al-Rasadi, K.; Banach, M.; Patti, A.M.; Ciaccio, M.; Rizvi, A.A.; Rizzo, M. Novel Therapeutical Approaches to Managing Atherosclerotic Risk. Int. J. Mol. Sci. 2021, 22, 4633. [Google Scholar] [CrossRef]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2814–2821. [Google Scholar] [CrossRef]
- Nikolic, D.; Giglio, R.V.; Rizvi, A.A.; Patti, A.M.; Montalto, G.; Maranta, F.; Cianflone, D.; Stoian, A.P.; Rizzo, M. Liraglutide Reduces Carotid Intima-Media Thickness by Reducing Small Dense Low-Density Lipoproteins in a Real-World Setting of Patients with Type 2 Diabetes: A Novel Anti-Atherogenic Effect. Diabetes Ther. 2021, 12, 261–274. [Google Scholar] [CrossRef]
| Method Principle | Benefits | Disadvantages |
|---|---|---|
| Fluorescence | Rapid Simple Non-expensive | Non-fluorescent AGEs are not measured Low sensitivity and specificityInterferences |
| Chromatography (HPLC-fluorescence; LC–MS/MS and GC–MS) | High sensitivity and specificity High accuracy Measurement of specific AGEs (CML, CEL, etc.) | Time-consuming High costs Qualified personnel required |
| Immunochemistry (ELISA, Western blot) | Simple Rapid Non-expensive | Only for measurement of protein–AGEs Low specificity and accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vekic, J.; Vujcic, S.; Bufan, B.; Bojanin, D.; Al-Hashmi, K.; Al-Rasadi, K.; Stoian, A.P.; Zeljkovic, A.; Rizzo, M. The Role of Advanced Glycation End Products on Dyslipidemia. Metabolites 2023, 13, 77. https://doi.org/10.3390/metabo13010077
Vekic J, Vujcic S, Bufan B, Bojanin D, Al-Hashmi K, Al-Rasadi K, Stoian AP, Zeljkovic A, Rizzo M. The Role of Advanced Glycation End Products on Dyslipidemia. Metabolites. 2023; 13(1):77. https://doi.org/10.3390/metabo13010077
Chicago/Turabian StyleVekic, Jelena, Sanja Vujcic, Biljana Bufan, Dragana Bojanin, Khamis Al-Hashmi, Khaild Al-Rasadi, Anca Pantea Stoian, Aleksandra Zeljkovic, and Manfredi Rizzo. 2023. "The Role of Advanced Glycation End Products on Dyslipidemia" Metabolites 13, no. 1: 77. https://doi.org/10.3390/metabo13010077










