Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cascino, G.D. Temporal Lobe Epilepsy: More than Hippocampal Pathology. Epilepsy Curr. 2005, 5, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Sànchez, J.; Centanaro, M.; Solís, J.; Delgado, F.; Yépez, L. Factors predicting the outcome following medical treatment of mesial temporal epilepsy with hippocampal sclerosis. Seizure 2014, 23, 448–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, S.E.; Shin, K.J.; Ha, S.Y.; Park, J.; Kim, T.H.; Mun, C.W.; Lee, B.I. Effective connectivity in temporal lobe epilepsy with hippocampal sclerosis. Acta Neurol. Scand. 2017, 135, 670–676. [Google Scholar] [CrossRef]
- Tai, X.Y.; Bernhardt, B.; Thom, M.; Thompson, P.; Baxendale, S.; Koepp, M.; Bernasconi, N. Review: Neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathol. Appl. Neurobiol. 2018, 44, 70–90. [Google Scholar] [CrossRef]
- Duncan, J.S.; Winston, G.P.; Koepp, M.J.; Ourselin, S. Brain imaging in the assessment for epilepsy surgery. Lancet Neurol. 2016, 15, 420–433. [Google Scholar] [CrossRef]
- Weidner, L.D.; Kannan, P.; Mitsios, N.; Kang, S.J.; Hall, M.D.; Theodore, W.H.; Innis, R.B.; Mulder, J. The expression of inflammatory markers and their potential influence on efflux transporters in drug-resistant mesial temporal lobe epilepsy tissue. Epilepsia 2018, 59, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.; Orozco-Suárez, S.; Rosetti, M.; Maldonado, L.; Bautista, S.I.; Flores, X.; Arellano, A.; Moreno, S.; Alonso, M.; Martínez-Juárez, I.E.; et al. Temporal lobe epilepsy: Evaluation of central and systemic immune-inflammatory features associated with drug resistance. Seizure 2021, 91, 447–455. [Google Scholar] [CrossRef]
- Langenbruch, L.; Bleß, L.; Schulte-Mecklenbeck, A.; Sundermann, B.; Brix, T.; Elger, C.E.; Melzer, N.; Wiendl, H.; Meuth, S.G.; Gross, C.C.; et al. Blood and cerebrospinal fluid immune cell profiles in patients with temporal lobe epilepsy of different etiologies. Epilepsia 2020, 61, e153–e158. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2020, 15, 459–472. [Google Scholar] [CrossRef]
- Ravizza, T.; Noé, F.; Zardoni, D.; Vaghi, V.; Sifringer, M.; Vezzani, A. Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol. Dis. 2008, 31, 327–333. [Google Scholar] [CrossRef]
- Vezzani, A.; Granata, T. Brain Inflammation in Epilepsy: Experimental and Clinical Evidence. Epilepsia 2005, 46, 1724–1743. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Wolinski, P.; Ksiazek-Winiarek, D.; Glabinski, A. Cytokines and Neurodegeneration in Epileptogenesis. Brain Sci. 2022, 12, 380. [Google Scholar] [CrossRef]
- Borger, V.; Hamed, M.; Taube, J.; Aydin, G.; Ilic, I.; Schneider, M.; Schuss, P.; Güresir, E.; Becker, A.; Helmstaedter, C.; et al. Resective temporal lobe surgery in refractory temporal lobe epilepsy: Prognostic factors of postoperative seizure outcome. J. Neurosurg. 2021, 135, 3. [Google Scholar] [CrossRef]
- Lipatova, L.V.; Serebryanaya, N.B.; Sivakova, N.A. The role of neuroinflammation in the pathogenesis of epilepsy. Neurol. Neuropsychiatry Psychosom. 2018, 10, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Pitkänen, A.; Engel, J., Jr. Past and Present Definitions of Epileptogenesis and Its Biomarkers. Neurotherapeutics 2014, 11, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Halász, P. The medial temporal lobe epilepsy is a bilateral disease—novel aspects. J. Epileptol. 2016, 24, 141–155. [Google Scholar] [CrossRef]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.-M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar] [CrossRef]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2019, 167, 107742. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhou, D.; Stefan, H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: Uncontrolled inflammation drives disease progression? J. Neurol. Sci. 2010, 296, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Ndode-Ekane, X.E.; Lapinlampi, N.; Puhakka, N. Epilepsy biomarkers—Toward etiology and pathology specificity. Neurobiol. Dis. 2019, 123, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.C.; Cawston, E.E.; Chen, G.; Brooks, C.; Douwes, J.; McLean, D.; Graham, E.S.; Dragunow, M.; Scotter, E.L. Serum biomarkers of neuroinflammation and blood-brain barrier leakage in amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Sharma, A.; Kumar, D.; Asthana, M.K.; Lalhlenmawia, H.; Kumar, A.; Bhattacharyya, S. Promissing protein biomarkers in the early diagnosis of Alzheimer’s disease. Metab. Brain Dis. 2022, 34, 1727–1744. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, K.D.; Dmitrenko, D.V.; Panina, I.S.; Usoltseva, A.A.; Gazenkampf, K.A.; Konovalenko, O.V.; Kantimirova, E.A.; Novitsky, M.A.; Nasyrova, R.F.; Shnayder, N.A. Expression Profile of miRs in Mesial Temporal Lobe Epilepsy: Systematic Review. Int. J. Mol. Sci. 2022, 23, 951. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Liu, X.; Feng, Y.; Pang, Z.; Lin, Y. HMGB1/CXCL12-Mediated Immunity and Th17 Cells Might Underlie Highly Suspected Autoimmune Epilepsy in Elderly Individuals. Neuropsychiatr. Dis. Treat. 2020, 19, 1285–1293. [Google Scholar] [CrossRef]
- Walker, L.; Tse, K.; Ricci, E.; Thippeswamy, T.; Sills, G.J.; White, S.H.; Antoine, D.J.; Marson, A.; Pirmohamed, M. High mobility group box 1 in the inflammatory pathogenesis of epilepsy: Profiling circulating levels after experimental and clinical seizures. Lancet 2014, 383, 105. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Shang, Y.; Zhao, L.; Wang, M.; Shi, J.; Li, S. HMGB1-TLR4 Axis Plays a Regulatory Role in the Pathogenesis of Mesial Temporal Lobe Epilepsy in Immature Rat Model and Children via the p38MAPK Signaling Pathway. Neurochem. Res. 2017, 42, 1179–1190. [Google Scholar] [CrossRef]
- Basnyat, P.; Pesu, M.; Söderqvist, M.; Grönholm, A.; Liimatainen, S.; Peltola, M.; Raitanen, J.; Peltola, J. Chronically reduced IL-10 plasma levels are associated with hippocampal sclerosis in temporal lobe epilepsy patients. BMC Neurol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Yang, F.; Hu, Y.; Liu, J.; Hu, H.; Su, W. Sodium valproate combined with levetiracetam in pediatric epilepsy and its influence on NSE, IL-6, hs-CRP and electroencephalogram improvement. Exp. Ther. Med. 2020, 20, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Cepok, S.; Todorova-Rudolph, A.; Nowak, M.; Köller, M.; Lorenz, R.; Oertel, W.H.; Rosenow, F.; Hemmer, B.; Hamer, H.M. Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res. 2009, 86, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lipatova, L.V.; Serebryanaya, N.B.; Sivakova, N.A.; Vasilenko, V.; Kapustina, T.V. Immune disorders in patients with epilepsy and the possibility of immunomodulation with recombinant human interleukin IL-2. Epilepsy Paroxysmal Cond. 2014, 6, 6–12. [Google Scholar]
- Walker, L.E.; Frigerio, F.; Ravizza, T.; Ricci, E.; Tse, K.; Jenkins, R.E.; Sills, G.; Jorgensen, A.; Porcu, L.; Thippeswamy, T.; et al. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J. Clin. Investig. 2017, 127, 2118–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaşak, T.; Dilber, B.; Yaman, S.; Durgut, B.D.; Kurt, T.; Çoban, E.; Arslan, E.A.; Şahin, S.; Karahan, S.C.; Cansu, A. HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers? Epileptic Disord. 2020, 22, 183–193. [Google Scholar]
- Lehtimäki, K.A.; Keränen, T.; Palmio, J.; Peltola, J. Levels of IL-1beta and IL-1RA in cerebrospinal fluid of human patients after single and prolonged. Neuroimmunomodulation 2010, 17, 19–22. [Google Scholar] [CrossRef]
- Lagarde, S.; Villeneuve, N.; Trébuchon, A.; Kaphan, E.; Lepine, A.; McGonigal, A.; Roubertie, A.; Barthez, M.-A.J.; Trommsdorff, V.; Lefranc, J.; et al. Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussen’s encephalitis: An open pilot study. Epilepsia 2016, 57, 956–966. [Google Scholar] [CrossRef] [Green Version]
- Kan, A.A.; de Jager, W.; de Wit, M.; Heijnen, C.; van Zuiden, M.; Ferrier, C.; van Rijen, P.; Gosselaar, P.; Hessel, E.; van Nieuwenhuizen, O.; et al. Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. J. Neuroinflammation 2012, 30, 207. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, M.S.; Blake, B.L.; McCown, T.J. Opposing actions of hippocampus TNFα receptors on limbic seizure susceptibility. Exp. Neurol. 2013, 247, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Koschmieder, S.; Chatain, N. Role of inflammation in the biology of myeloproliferative neoplasms. Blood Rev. 2020, 42, 100711. [Google Scholar] [CrossRef]
- Viviani, B.; Gardoni, F.; Marinovich, M. Cytokines and Neuronal Ion Channels in Health and Disease. Int. Rev. Neurobiol. 2007, 82, 247–263. [Google Scholar] [PubMed]
- Koyama, R.; Ikegaya, Y. To BDNF or Not to BDNF: That Is the Epileptic Hippocampus. Neuroscientist 2005, 11, 282–287. [Google Scholar] [CrossRef] [PubMed]
- LaFrance, W.C.; Leaver, K.; Stopa, E.G.; Papandonatos, G.D.; Blum, A.S. Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 2010, 75, 1285–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittigau, P.; Sifringer, M.; Genz, K.; Reith, E.; Pospischil, D.; Govindarajalu, S.; Dzietko, M.; Pesditschek, S.; Mai, I.; Dikranian, K.; et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15089–15094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minichiello, L.; Calella, A.M.; Medina, D.L.; Bonhoeffer, T.; Klein, R.; Korte, M. Mechanism of TrkB-Mediated Hippocampal Long-Term Potentiation. Neuron 2002, 36, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Michalski, B.; Racine, R.; Fahnestock, M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, Trk expression and seizure-related morphological changes. Neuroscience 2004, 126, 521–531. [Google Scholar] [CrossRef]
- Heinrich, C.; Lähteinen, S.; Suzuki, F.; Anne-Marie, L.; Huber, S.; Häussler, U.; Haas, C.; Larmet, Y.; Castren, E.; Depaulis, A. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2011, 42, 35–47. [Google Scholar] [CrossRef]
- Branco-Madeira, F.; Lambrecht, B.N. High mobility group box-1 recognition: The beginning of a RAGEless era? EMBO Mol. Med. 2010, 2, 193–195. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Teng, J.; Miao, W. HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol. Med. Rep. 2018, 17, 5125–5131. [Google Scholar] [CrossRef] [Green Version]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; A Manfredi, A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef]
- Huang, J.S.; Wu, Y.; Huang, Q.; Li, S.J.; Ye, X.; Wei, Q.-D.; Liu, Y.; Liu, M.-G. Expression level and distribution of HMGB1 in Sombati’s cell model and kainic acid-induced epilepsy model. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2928–2933. [Google Scholar]
| Characteristics | Drug-Resistant, n = 49 | Drug Responsible, n = 117 | P* |
|---|---|---|---|
| Sex | 19 males, 30 females | 50 males, 67 females | |
| Age at the time of observation, Me [LQ25; UQ75] | 33.0 [28.0; 43.0] | 31.0 [25.0; 41.0] | |
| Age of the onset, Me [Q25; Q75] | 17.0 [8.5; 21.25] | 20.0 [13.0; 31.0] | |
| Duration of the disease | |||
| Less than 5 years n = 32 | 3 | 29 | 0.375 |
| 5–10 years n = 45 | 10 | 35 | 0.023 |
| More than 10 years n = 89 | 36 | 53 | 0.4331 |
| Seizure severity on the NHS-3 scale, Me [LQ; UQ], score | 15.0 [14.0; 16.0] | 12.0 [11.0; 15.0] | |
| TLE+HS | 21 | 41 | |
| TLE-HS | 28 | 76 | |
| Monotherapy | 8 | 76 | |
| Polytherapy | 41 | 41 | |
| Plasma Marker Concentration | TLE Patients (Drug-Resistant) Me [LQ; UQ] | TLE Patients (HS+) Me [LQ; UQ] | TLE Patients (Drug Responsible) Me [LQ; UQ] | TLE Patients, Me [LQ; UQ] | Control Group, Me [LQ; UQ] | P* |
|---|---|---|---|---|---|---|
| BDNF (ng/mL) | 25.98 * [22.06; 31.29] | 26.28 * [22.73; 31.27] | 24.44 * [19.56; 32.62] | 25.87 * [20.81; 32.17] | 74.85 [45.11; 128.85] | <0.001 |
| TNFa (pg/mL) | 11.44 * [9.42; 16.45] | 11.27 * [8.41; 18.68] | 10.85 * [10.29; 18.35] | 12.30 * [10.27; 20.95] | 73.40 [56.42; 92.88] | <0.001 |
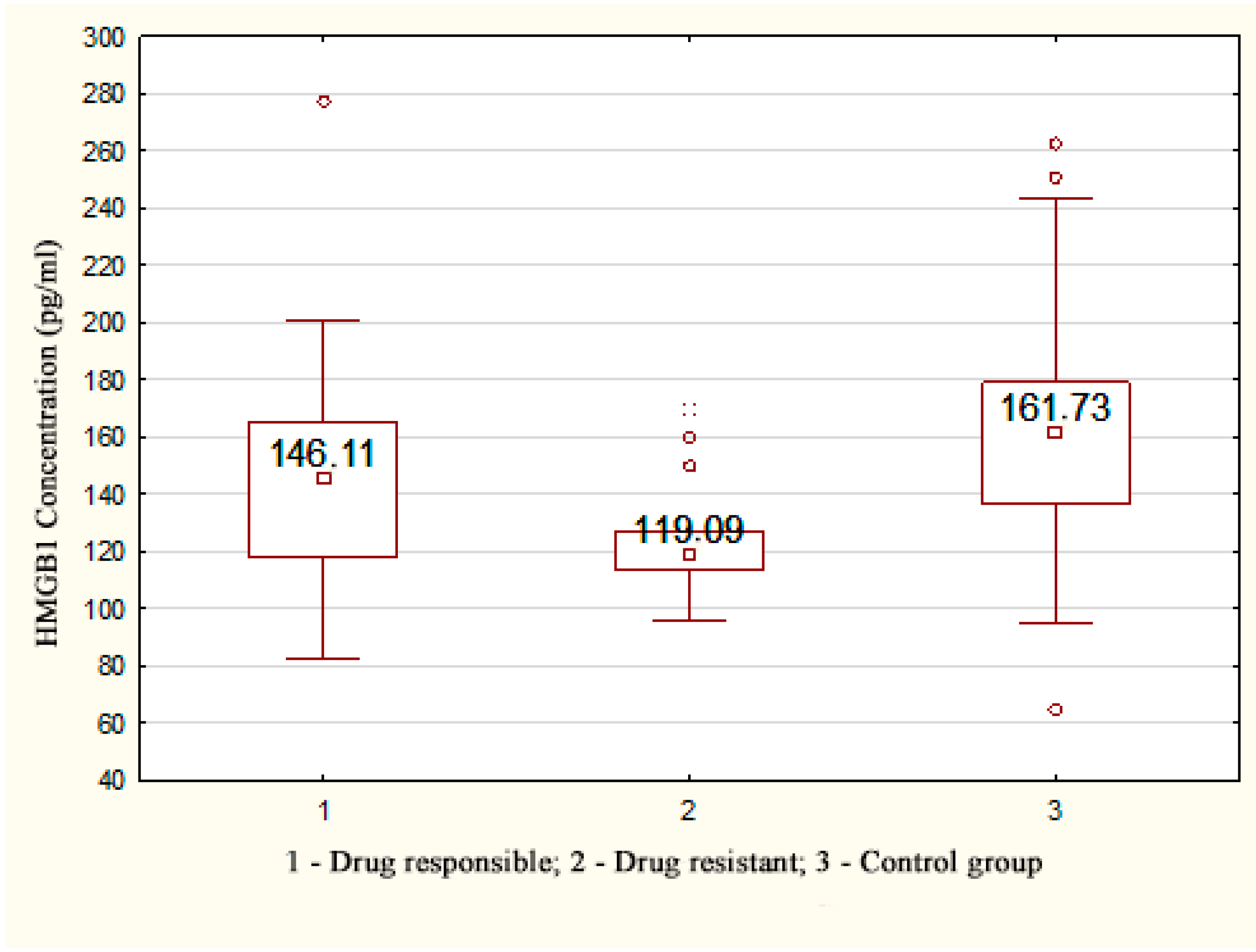
| HMGB1 (pg/mL) | 119.09 * [113.55; 126.93] | 134.65 * [113.19; 156.11] | 135.765 * [114.17; 159.53] | 135.765 * [114.17; 159.53] | 161.73 [136.34; 179.01] | 0.034 |
| NTRK-2 (pg/mL) | 4.40 * [2.60; 5.50] | 4.15 * [3.05; 4.65] | 3.80 * [3.075; 4.83] | 3.80 * [2.90; 4,70] | 3.00 [2.40; 4.80] | 0.365 |
| Plasma Marker Concentration | Duration Less Than 10 Years | Duration More Than 10 Years | Control Group | p1,2 | p1,3 p2,3 p Common |
|---|---|---|---|---|---|
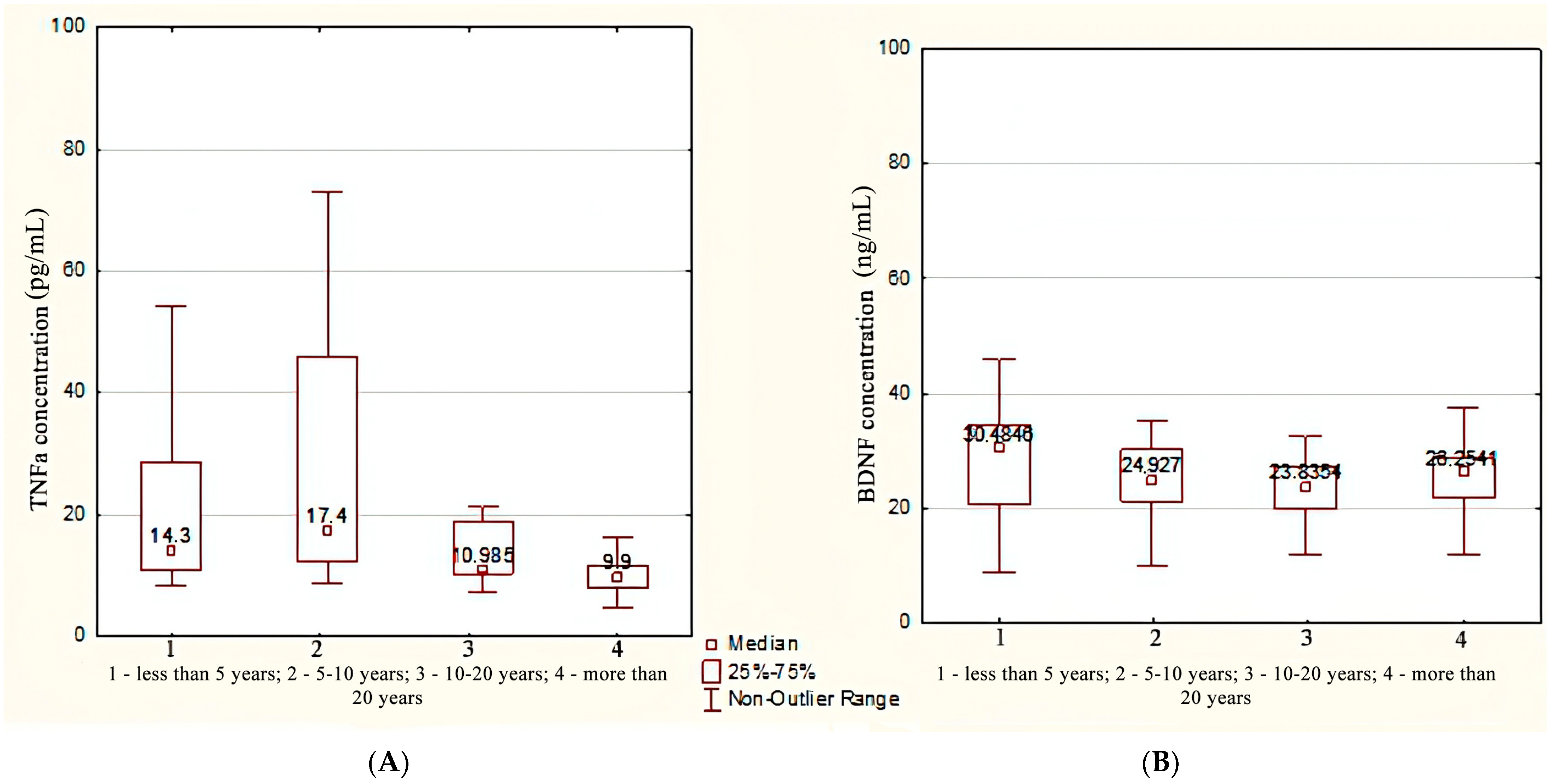
| BDNF (ng/mL) | 26.50 [21.07; 32.87] | 25.62 [19.83; 28.66] | 74.85 [45.11; 128.85] | 0.212 | <0.001 |
| TNFa (pg/mL) | 14.30 [11.22; 26.85] | 10.58 [8.70; 16.30] | 73.40 [56.42; 92.88] | 0.05 | <0.001 |
| Marker | Area under the Curve | Standard Error | Asymptomatic Significance | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||
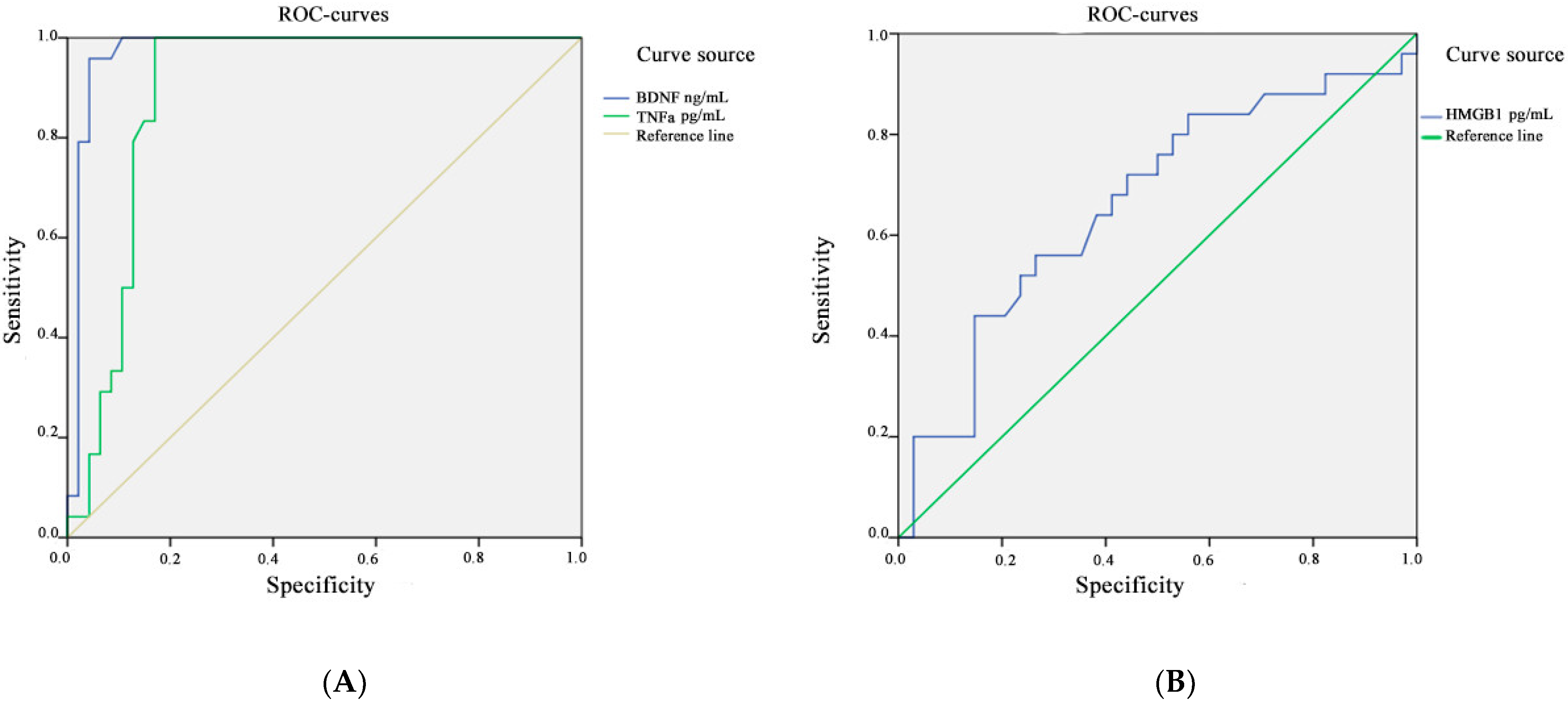
| HMGB1 | 0.664 | 0.073 | 0.033 | 0.520 | 0.807 |
| BDNF | 0.974 | 0.020 | 0.000 | 0.934 | 1.000 |
| TNFa | 0.894 | 0.040 | 0.000 | 0.816 | 0.972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panina, Y.S.; Timechko, E.E.; Usoltseva, A.A.; Yakovleva, K.D.; Kantimirova, E.A.; Dmitrenko, D.V. Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults. Metabolites 2023, 13, 83. https://doi.org/10.3390/metabo13010083
Panina YS, Timechko EE, Usoltseva AA, Yakovleva KD, Kantimirova EA, Dmitrenko DV. Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults. Metabolites. 2023; 13(1):83. https://doi.org/10.3390/metabo13010083
Chicago/Turabian StylePanina, Yulia S., Elena E. Timechko, Anna A. Usoltseva, Kristina D. Yakovleva, Elena A. Kantimirova, and Diana V. Dmitrenko. 2023. "Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults" Metabolites 13, no. 1: 83. https://doi.org/10.3390/metabo13010083
APA StylePanina, Y. S., Timechko, E. E., Usoltseva, A. A., Yakovleva, K. D., Kantimirova, E. A., & Dmitrenko, D. V. (2023). Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults. Metabolites, 13(1), 83. https://doi.org/10.3390/metabo13010083








