Investigation of the Therapeutic Effect of Total Alkaloids of Corydalis saxicola Bunting on CCl4-Induced Liver Fibrosis in Rats by LC/MS-Based Metabolomics Analysis and Network Pharmacology
Abstract
:1. Introduction
2. Material and Methods
2.1. Drugs and Chemicals
2.2. Preparation of TACS
2.3. Animals and Treatments
2.4. Sample Collections and Preparation
2.5. Biochemical Analysis
2.6. Histopathological Assessment
2.7. Immunohistochemistry (IHC)
2.8. Metabolites and Metabolic Pathway Analysis
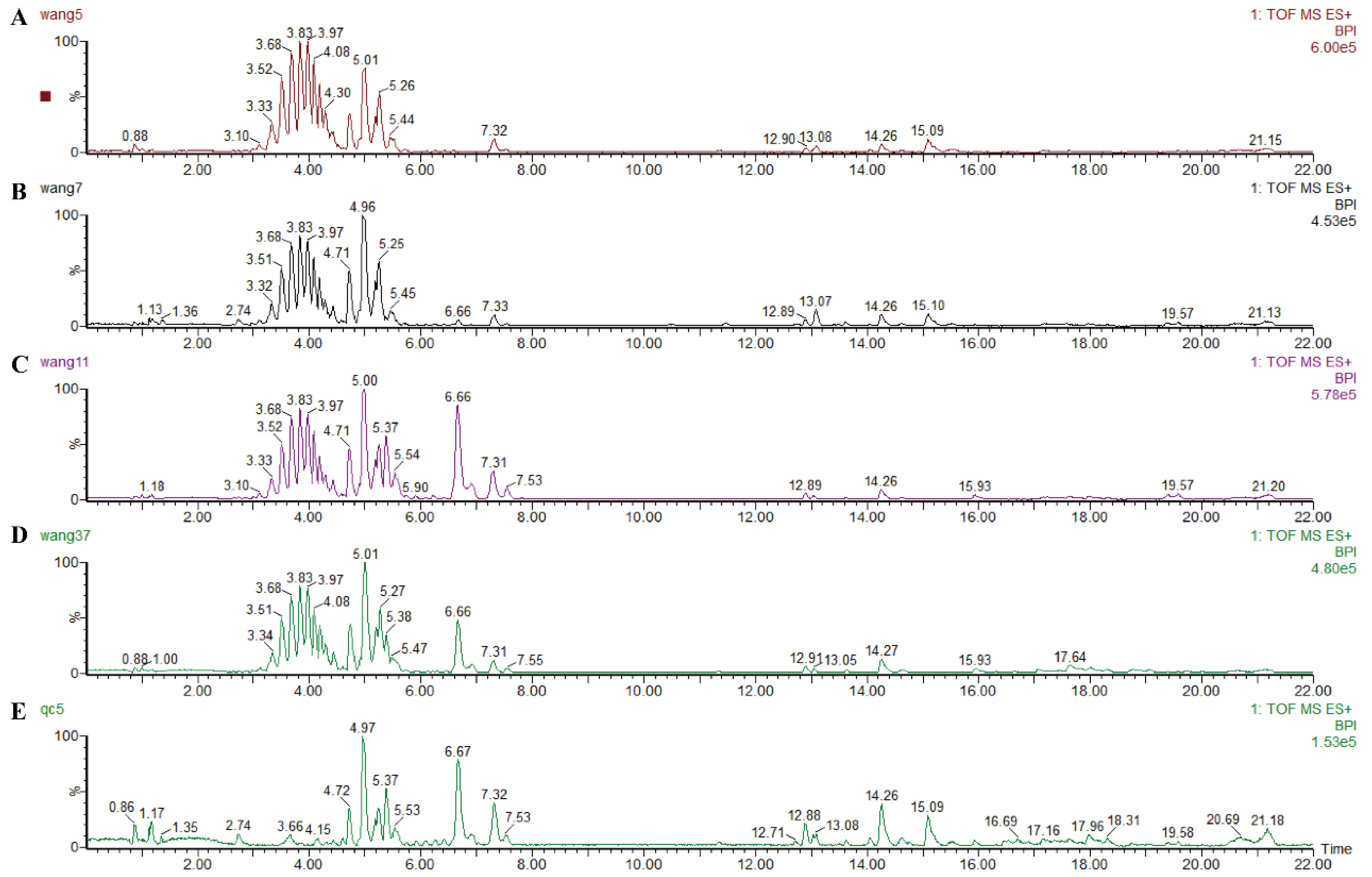
2.8.1. UPLC-Q-TOF/MS Analysis
2.8.2. Method Validation
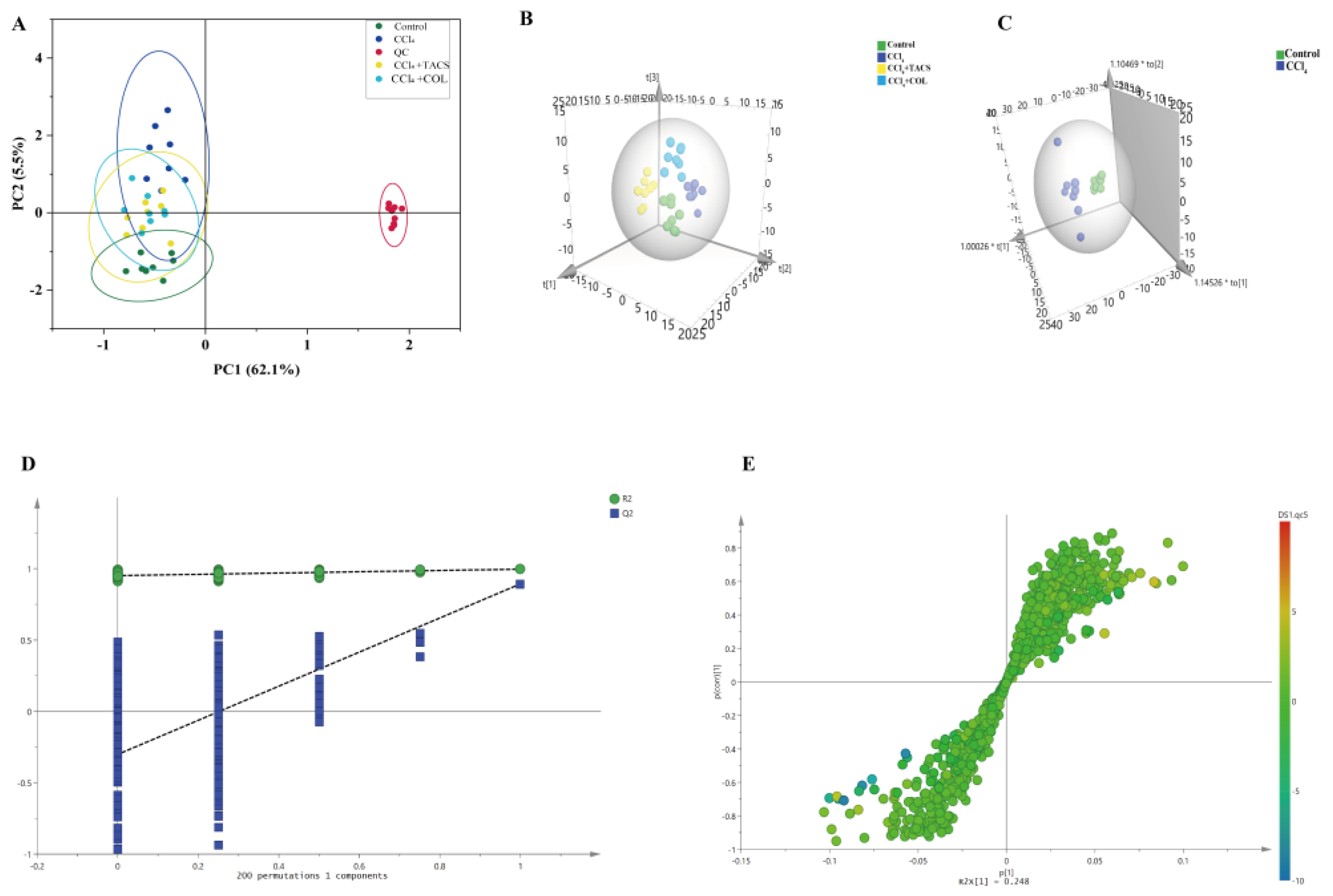
2.8.3. Data Processing and Multivariate Analysis
2.8.4. Statistical Analysis
2.9. Network Pharmacology Analysis
2.10. Molecular Docking
3. Results
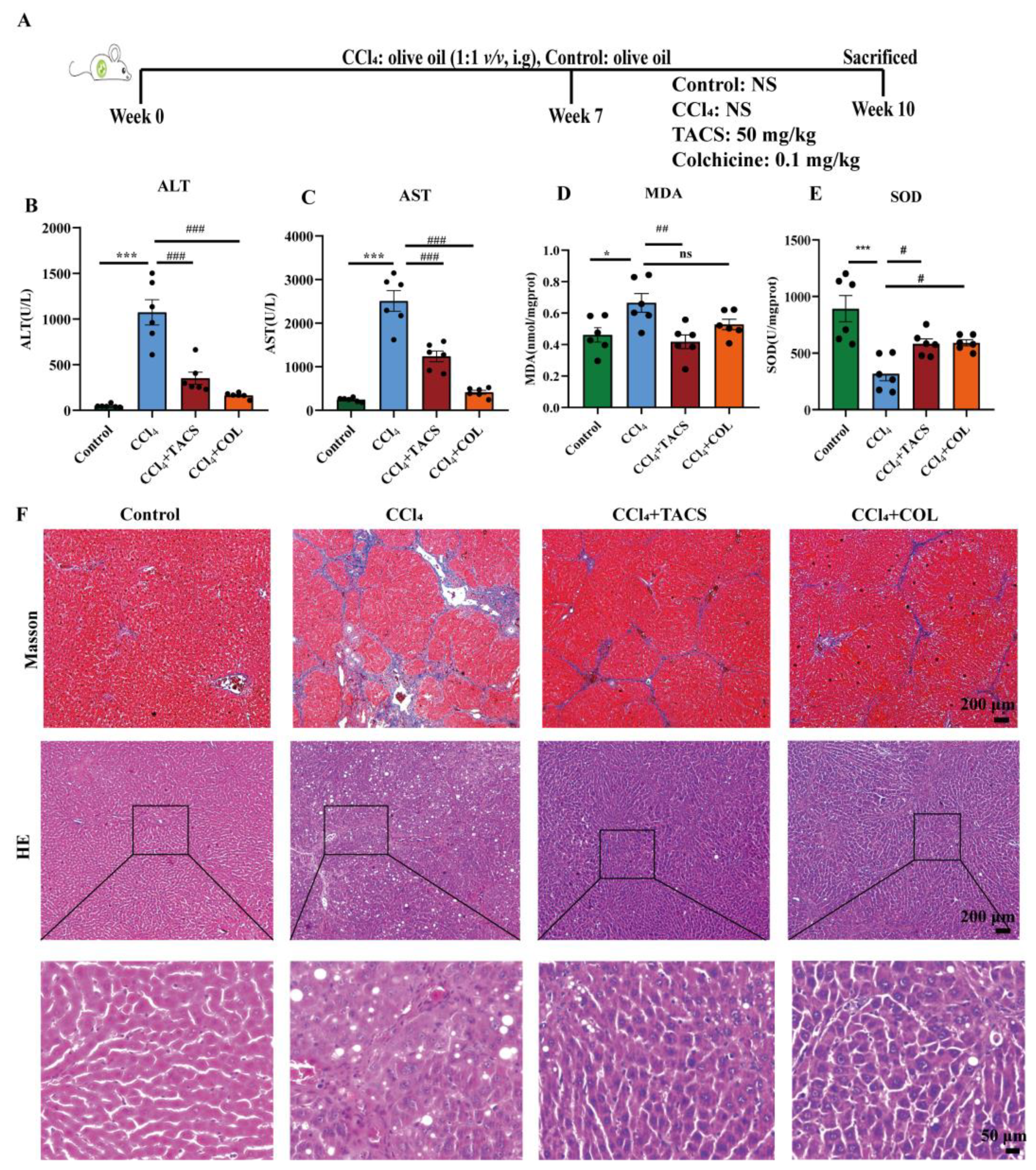
3.1. Effects of TACS on Liver Fibrosis in CCl4-Induced Rats
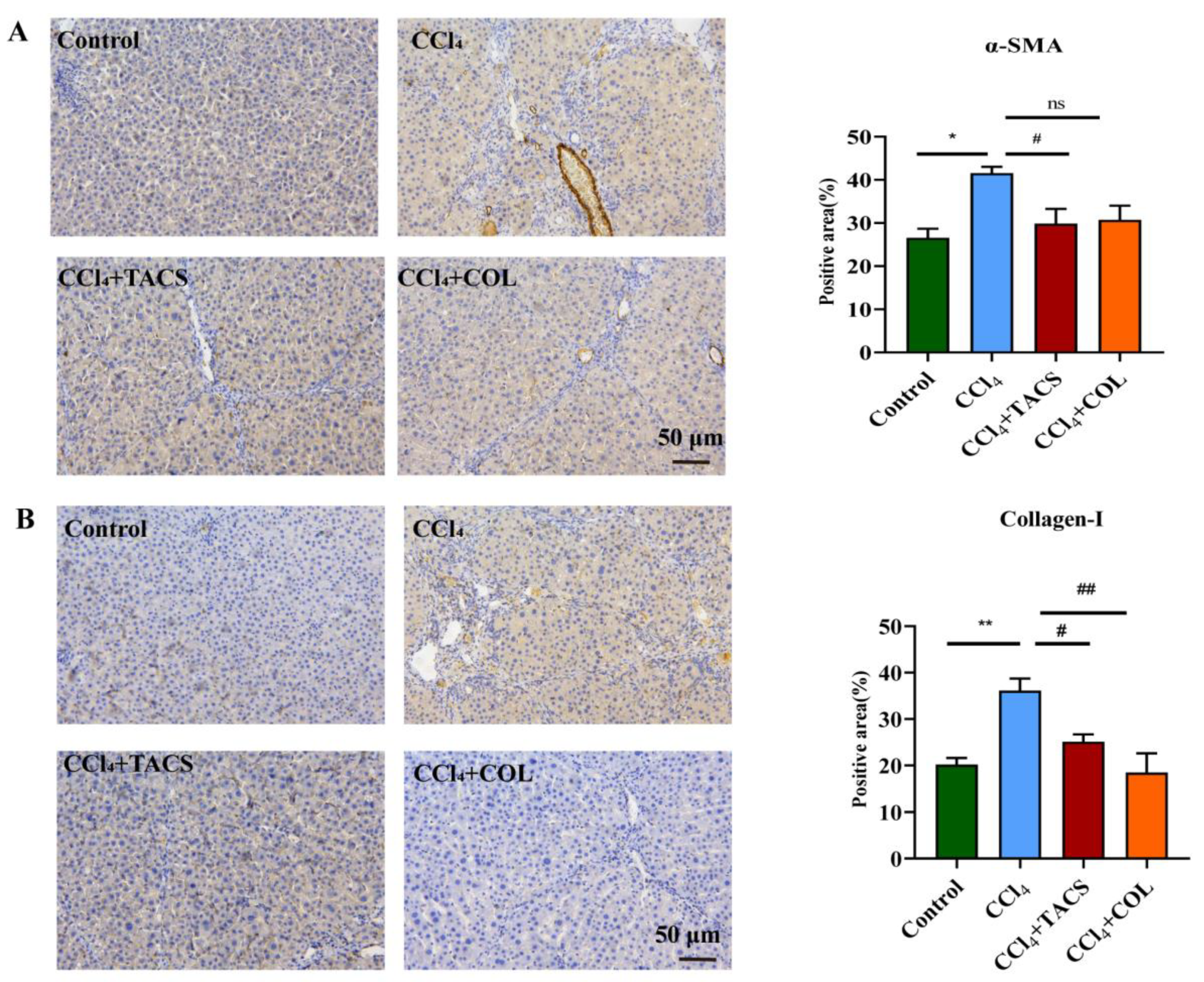
3.2. TACS Inhibited Fibrotic Protein Expression in CCl4-Induced Rats
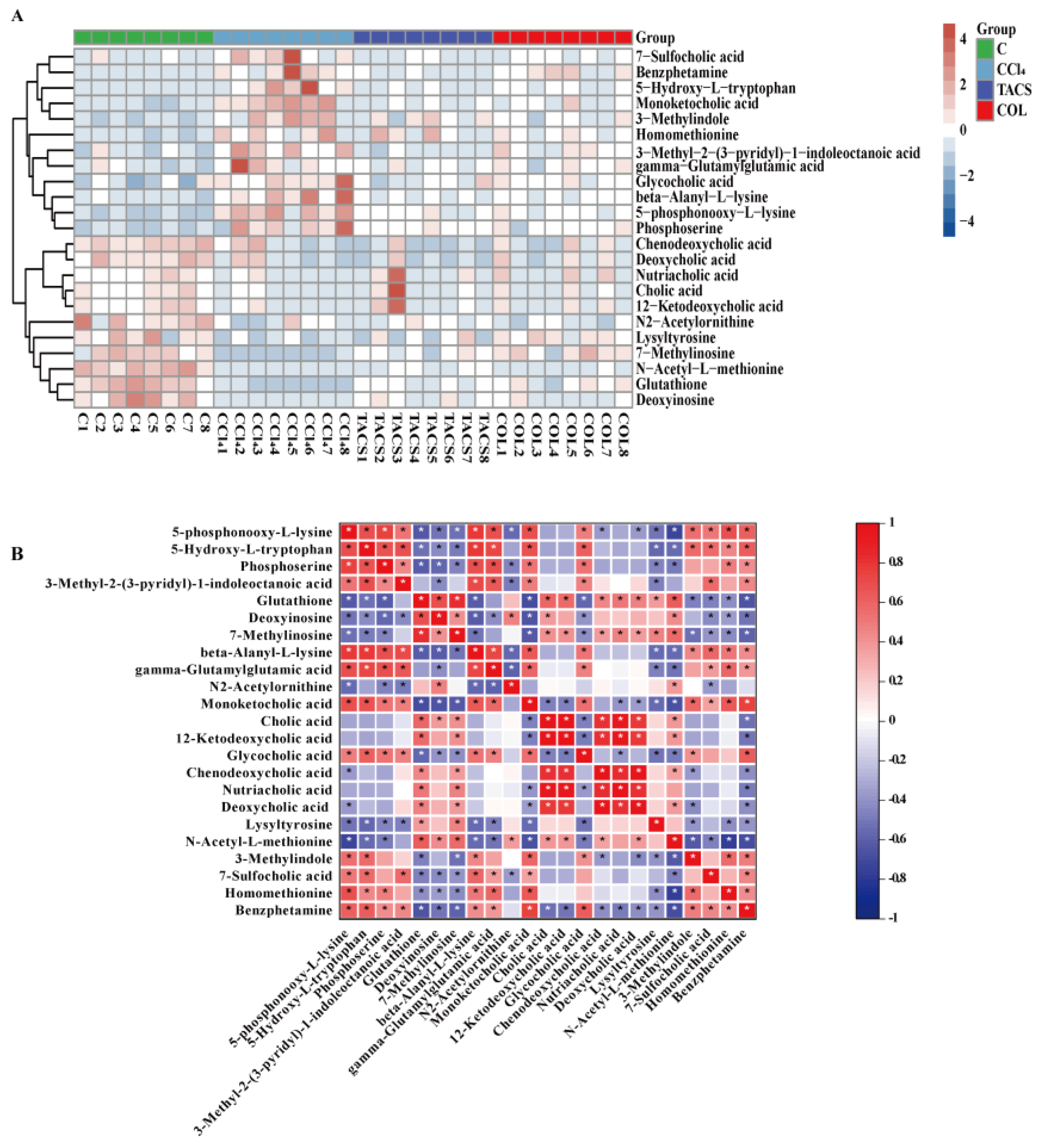
3.3. Metabolic Profiling of Feces Metabolites Induced by Liver Fibrosis
3.4. Identification of Potential Biomarkers for Liver Fibrosis
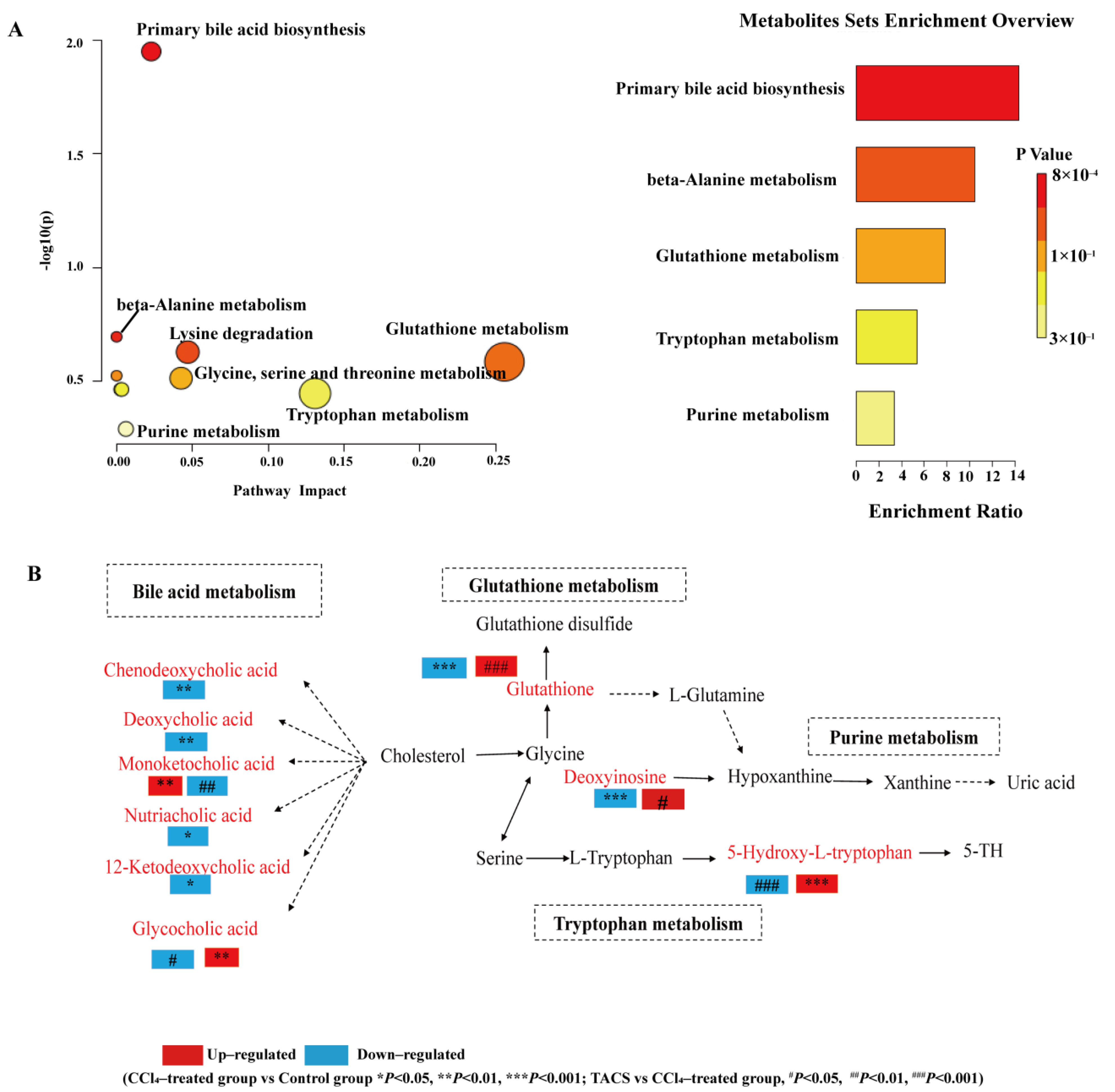
3.5. Metabolic Pathway Analysis
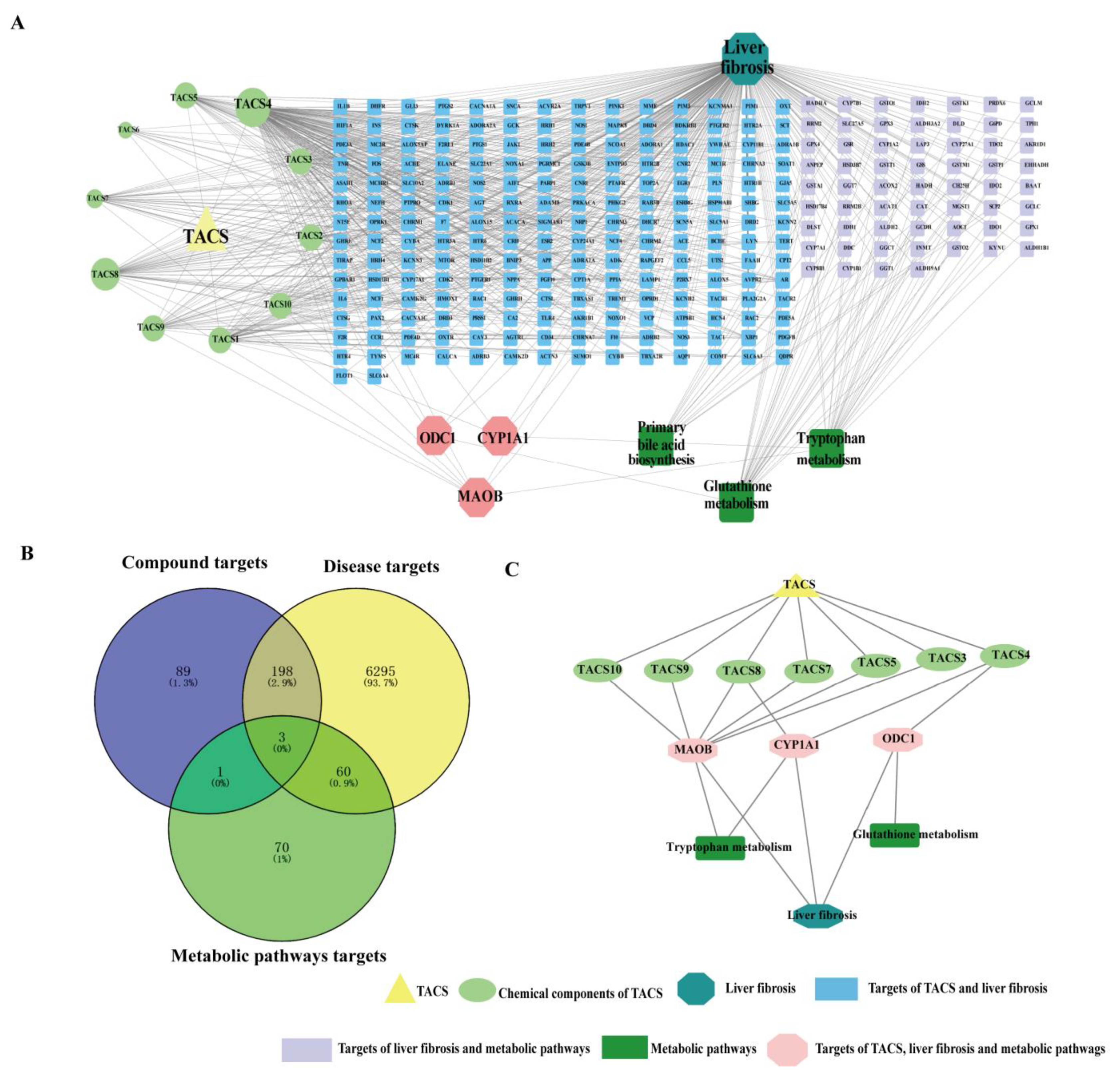
3.6. Network Pharmacology Analysis
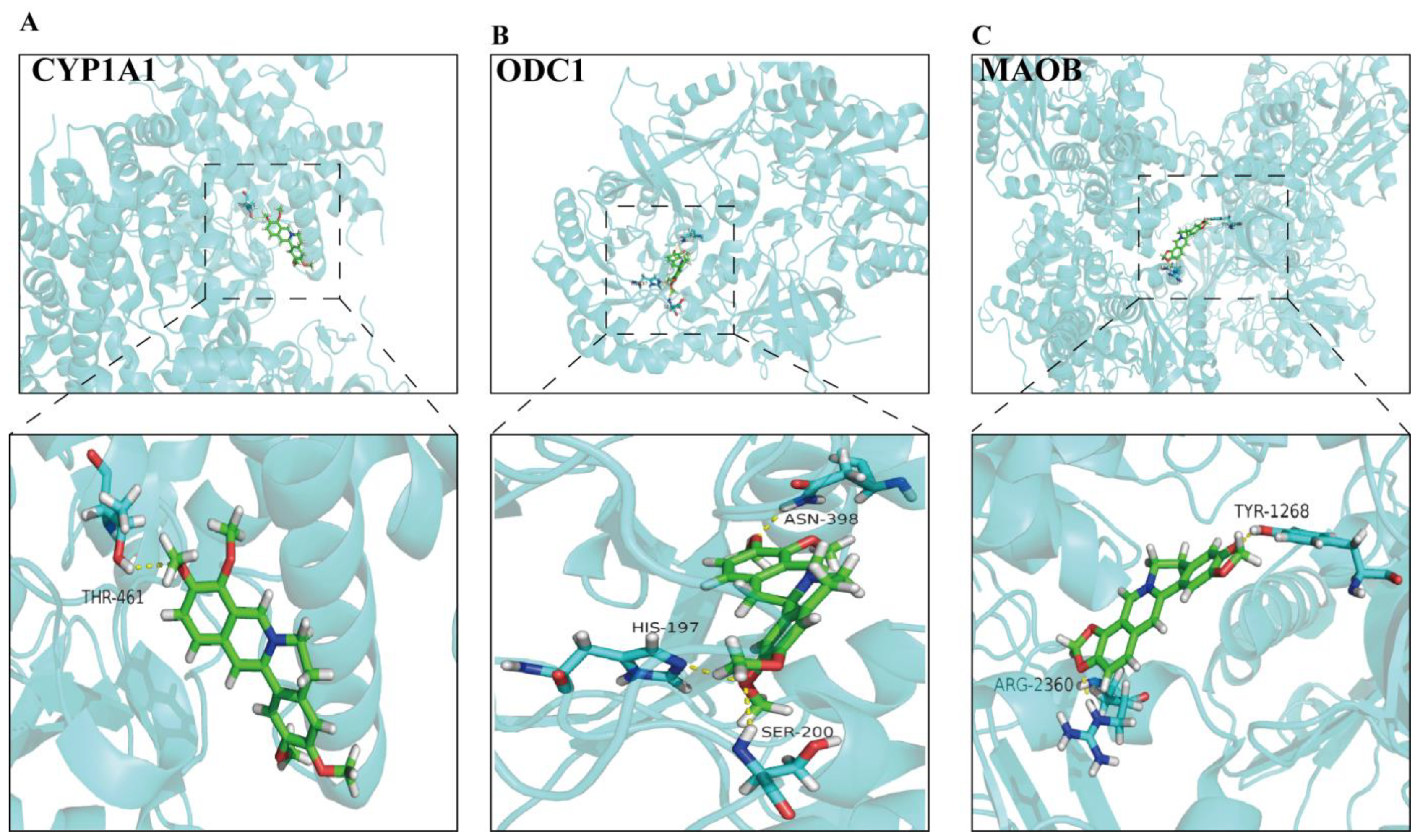
3.7. Molecular Docking
4. Discussion
4.1. Bile Acid Metabolism
4.2. Glutathione Metabolism
4.3. Tryptophan Metabolism
4.4. Purine Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, H.; Yang, Z.T.; Cheng, B.; Wu, J.X.; Liu, X.W.; Tang, C.L.; Lu, S.Y.; Chen, Z.N.; Song, F.M.; et al. Urinary metabonomics study of the hepatoprotective effects of total alkaloids from Corydalis saxicola Bunting on carbon tetrachloride-induced chronic hepatotoxicity in rats using 1H NMR analysis. J. Pharm. Biomed. Anal. 2017, 140, 199–209. [Google Scholar] [CrossRef]
- Liu, X.W.; Tang, C.L.; Zheng, H.; Wu, J.X.; Wu, F.; Mo, Y.Y.; Liu, X.; Zhu, H.J.; Yin, C.L.; Cheng, B.; et al. Investigation of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced liver fibrosis in rats by 1H-NMR-based metabonomics and network pharmacology approaches. J. Pharm. Biomed. Anal. 2018, 159, 252–261. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, Q.; Ao, Q.; Luo, C.; Wang, B.; Bai, C.; Ge, X.; Wang, Y.; Wang, J.; et al. On the Core Prescriptions and Their Mechanisms of Traditional Chinese Medicine in Hepatitis B, Liver Cirrhosis, and Liver Cancer Treatment. J. Oncol. 2022, 2022, 5300523. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Chang, B.; Yu, J.; Bao, J.; Yao, Q.; Luo, J. The Traditional Uses, Phytochemistry, Pharmacokinetics, Pharmacology, Toxicity, and Applications of Corydalis saxicola Bunting: A Review. Front Pharmacol. 2022, 13, 822792. [Google Scholar] [CrossRef]
- Kuai, C.P.; Ju, L.J.; Hu, P.P.; Huang, F. Corydalis saxicola Alkaloids Attenuate Cisplatin-Induced Neuropathic Pain by Reducing Loss of IENF and Blocking TRPV1 Activation. Am. J. Chin. Med. 2020, 48, 407–428. [Google Scholar] [CrossRef]
- Zeng, F.L.; Xiang, Y.F.; Liang, Z.R.; Wang, X.; Huang, D.E.; Zhu, S.N.; Li, M.M.; Yang, D.P.; Wang, D.M.; Wang, Y.F. Anti-hepatitis B virus effects of dehydrocheilanthifoline from Corydalis saxicola. Am. J. Chin. Med. 2013, 41, 119–130. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, L.J.; Li, P.; Jiang, H.; Lu, G.C.; Zhang, W.D.; Li, H.L.; Yuan, B.J. Hepatoprotective effects and mechanisms of dehydrocavidine in rats with carbon tetrachloride-induced hepatic fibrosis. J. Ethnopharmacol. 2011, 138, 76–84. [Google Scholar] [CrossRef]
- Liang, Y.H.; Tang, C.L.; Lu, S.Y.; Cheng, B.; Wu, F.; Chen, Z.N.; Song, F.; Ruan, J.X.; Zhang, H.Y.; Song, H.; et al. Serum metabonomics study of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced acute hepatotoxicity in rats by 1H NMR analysis. J. Pharm. Biomed. Anal. 2016, 129, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Wang, X.; Cao, X.; Zhang, K.; Sun, J.; Li, Y.; Sui, J.; Liu, Y. Integration of transcriptomics and metabolomics confirmed hepatoprotective effects of steamed shoot extracts of ginseng (Panax ginseng C.A. Meyer) on toxicity caused by overdosed acetaminophen. Biomed. Pharmacother. 2021, 143, 112177. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, B.; Pan, Y.; Gu, J.; Tan, R.; Gong, P. Phyllanthus emblica aqueous extract retards hepatic steatosis and fibrosis in NAFLD mice in association with the reshaping of intestinal microecology. Front. Pharmacol. 2022, 13, 893561. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Li, X.; Luo, H.; Huang, T.; Xu, L.; Shi, X.; Hu, K. Statistically correlating NMR spectra and LC-MS data to facilitate the identification of individual metabolites in metabolomics mixtures. Anal. Bioanal. Chem. 2019, 411, 1301–1309. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.J.; He, L.; Yan, X.L.; Li, Q.R.; Zhang, X.; He, M.H.; Chang, S.; Tu, B.; Long, Q.D.; et al. Systematic elucidation of the bioactive alkaloids and potential mechanism from Sophora flavescens for the treatment of eczema via network pharmacology. J. Ethnopharmacol. 2023, 301, 115799. [Google Scholar] [CrossRef]
- Zhang, W.-N.; Li, A.-P.; Qi, Y.-S.; Qin, X.-M.; Li, Z.-Y. Metabolomics coupled with system pharmacology reveal the protective effect of total flavonoids of Astragali Radix against adriamycin-induced rat nephropathy model. J. Pharm. Biomed. Anal. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H.; Lu, R.; Huang, H.; Zhu, H.; Yin, C.; Mo, Y.; Wu, J.; Liu, X.; Deng, M.; et al. Intervening Effects of Total Alkaloids of Corydalis saxicola Bunting on Rats With Antibiotic-Induced Gut Microbiota Dysbiosis Based on 16S rRNA Gene Sequencing and Untargeted Metabolomics Analyses. Front. Microbiol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Iezzoni, J.C. Diagnostic histochemistry in hepatic pathology. Semin. Diagn. Pathol. 2018, 35, 381–389. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, R.; Kumar, A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Li, H.; Cao, W.; Wang, F.; Zhang, T.; Wang, S.W. Protective Effects of Amarogentin against Carbon Tetrachloride-Induced Liver Fibrosis in Mice. Molecules 2017, 22, 754. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Che, Q.M.; Zhao, X.; Pu, X.P. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur. J. Pharmacol. 2010, 631, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Y.; Sun, Y.; Yang, X. Protective effects of Ziyang tea polysaccharides on CCl4-induced oxidative liver damage in mice. Food Chem. 2014, 143, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Covelli, C.; Sacchi, D.; Sarcognato, S.; Cazzagon, N.; Grillo, F.; Baciorri, F.; Fanni, D.; Cacciatore, M.; Maffeis, V.; Guido, M. Pathology of autoimmune hepatitis. Pathologica 2021, 113, 185–193. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Liu, X.; Wu, G.; Zhong, J.; Zhao, T.; Li, J.; Lin, Y.; Zhou, Y.; Wei, Y. Plumbagin ameliorates liver fibrosis via a ROS-mediated NF-small ka, CyrillicB signaling pathway in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 108923. [Google Scholar] [CrossRef]
- Shi, Y.S.; Li, X.X.; Li, H.T.; Zhang, Y. Pelargonidin ameliorates CCl4-induced liver fibrosis by suppressing the ROS-NLRP3-IL-1β axis via activating the Nrf2 pathway. Food Funct. 2020, 11, 5156–5165. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Liu, Y.; Liu, T.; Zhao, C.; Wang, M. Investigation of the therapeutic effect of Yinchen Wuling Powder on CCl4-induced hepatic fibrosis in rats by 1H NMR and MS-based metabolomics analysis. J. Pharm. Biomed. Anal. 2021, 200, 114073. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R. Bile acids and the metabolic syndrome. Ann. Clin. Biochem. 2019, 56, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jiang, R.; Wang, X.; Liu, P.; Zhao, A.; Wu, Y.; Huang, F.; Liu, Z.; Rajani, C.; Zheng, X.; et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine 2021, 66, 103290. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2021, 66, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Yang, F.; Lin, Z.; Zhuo, J.; Liu, P.; Cen, B.; Lian, Z.; Xie, H.; Zheng, S.; Xu, X. A prognostic fingerprint in liver transplantation for hepatocellular carcinoma based on plasma metabolomics profiling. Eur. J. Surg. Oncol. 2019, 45, 2347–2352. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Zhang, H.; Chai, Y.; Xu, Y.; Miao, Y.; Yuan, Z.; Zhang, L.; Jiang, Z.; Yu, Q. Glycocholic acid aggravates liver fibrosis by promoting the up-regulation of connective tissue growth factor in hepatocytes. Cell. Signal. 2022, 101, 110508. [Google Scholar] [CrossRef]
- Kwan, S.Y.; Jiao, J.; Qi, J.; Wang, Y.; Wei, P.; McCormick, J.B.; Fisher-Hoch, S.P.; Beretta, L. Bile Acid Changes Associated with Liver Fibrosis and Steatosis in the Mexican-American Population of South Texas. Hepatol. Commun. 2020, 4, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Kong, Y.; Zhang, T.; Gong, Z.; Xiao, W. L-Theanine regulates lipid metabolism by modulating gut microbiota and bile acid metabolism. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef]
- Geenen, S.; du Preez, F.B.; Snoep, J.L.; Foster, A.J.; Sarda, S.; Kenna, J.G.; Wilson, I.D.; Westerhoff, H.V. Glutathione metabolism modeling: A mechanism for liver drug-robustness and a new biomarker strategy. Biochim. Biophys. Acta 2013, 1830, 4943–4959. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Kitade, M.; Nishimura, N.; Kaji, K.; Asada, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Okura, Y.; Takaya, H.; et al. Oral administration of fructose exacerbates liver fibrosis and hepatocarcinogenesis via increased intestinal permeability in a rat steatohepatitis model. Oncotarget 2018, 9, 28638–28651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltruskeviciene, E.; Kazbariene, B.; Badaras, R.; Bagdonaite, L.; Krikstaponiene, A.; Zdanavicius, L.; Aleknavicius, E.; Didziapetriene, J. Glutathione and glutathione S-transferase levels in patients with liver metastases of colorectal cancer and other hepatic disorders. Turk. J. Gastroenterol. 2016, 27, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, A.L.; De Salvatore, G.; De Salvia, M.A.; Mitolo, C.I.; Siro-Brigiani, G.; Marzullo, A.; Grattagliano, I.; Mitolo-Chieppa, D.; Palasciano, G.; Portincasa, P. Indomethacin-induced ileitis is associated with tensiometric, vascular and oxidative changes in the experimental rat model. Eur. J. Clin. Investig. 2005, 35, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J. Pineal Res. 2022, 73, e12812. [Google Scholar] [CrossRef]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef]
- Afzali, A.; Weiss, N.S.; Boyko, E.J.; Ioannou, G.N. Association between serum uric acid level and chronic liver disease in the United States. Hepatology 2010, 52, 578–589. [Google Scholar] [CrossRef]
- Khazoom, F.; L’Ecuyer, S.; Gilbert, K.; Gagne, M.A.; Bouchard, C.; Rose, C.F.; Rousseau, G.; Charbonney, E. Impact of uric acid on liver injury and intestinal permeability following resuscitated hemorrhagic shock in rats. J. Trauma Acute Care Surg. 2020, 89, 1076–1084. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cao, S.; Li, C.; Zhang, J.; Liu, C.; Qiu, F.; Kang, N. Berberrubine, a Main Metabolite of Berberine, Alleviates Non-Alcoholic Fatty Liver Disease via Modulating Glucose and Lipid Metabolism and Restoring Gut Microbiota. Front. Pharmacol. 2022, 13, 913378. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Chen, S.; Ding, X.; Wang, L.; Li, S.; Yang, G.; Lan, T. Tetrahydropalmatine attenuates liver fibrosis by suppressing endoplasmic reticulum stress in hepatic stellate cells. Chin. Med. J. 2021, 135, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Lassila, V.; Njimi, T.; Mengata, D.E. Natural protoberberine alkaloids from Enantia chlorantha, palmatine, columbamine and jatrorrhizine for thioacetamide-traumatized rat liver. Cells Tissues Organs 1988, 131, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.N.; Zhang, J.; Xiang, H.M.; Xu, H.S.; Li, Y.F.; Ma, W.Y.; Li, X.G.; Ye, X.L. Protective effect of Coptisine from Rhizoma Coptidis on LPS/D-GalN-induced acute liver failure in mice through up-regulating expression of miR-122. Biomed. Pharmacother. 2018, 98, 180–190. [Google Scholar] [CrossRef]
- Lin, G.S.; Zhao, M.M.; Fu, Q.C.; Zhao, S.Y.; Ba, T.T.; Yu, H.X. Palmatine attenuates hepatocyte injury by promoting autophagy via the AMPK/mTOR pathway after alcoholic liver disease. Drug Dev. Res. 2022, 83, 1613–1622. [Google Scholar] [CrossRef]
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The pharmacological activity of berberine, a review for liver protection. Eur. J. Pharmacol. 2021, 890, 173655. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, J.K.; Kang, J.W.; Oh, W.Y.; Jung, J.Y.; Kim, Y.S.; Jung, H.A.; Choi, J.S.; Lee, S.M. Palmatine attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Food Chem. Toxicol. 2010, 48, 222–228. [Google Scholar] [CrossRef]
- Huang, B.; Bao, J.; Cao, Y.R.; Gao, H.F.; Jin, Y. Cytochrome P450 1A1 (CYP1A1) Catalyzes Lipid Peroxidation of Oleic Acid-Induced HepG2 Cells. Biochemistry 2018, 83, 595–602. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, X.; Zhang, X.; Jiang, H.; Li, B.; Wang, Z.; Li, D.; Jin, Y. Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice. Biomed. Pharmacother. 2019, 118, 109287. [Google Scholar] [CrossRef]
- Ye, Z.; Zeng, Z.; Shen, Y.; Yang, Q.; Chen, D.; Chen, Z.; Shen, S. ODC1 promotes proliferation and mobility via the AKT/GSK3beta/beta-catenin pathway and modulation of acidotic microenvironment in human hepatocellular carcinoma. OncoTargets Ther. 2019, 12, 4081–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Oh, S.T.; Won, M.A.; Choi, K.M.; Ko, M.J.; Seo, D.; Jeon, T.W.; Baik, I.H.; Ye, S.K.; Park, K.U.; et al. Targeting ODC1 inhibits tumor growth through reduction of lipid metabolism in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2016, 478, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Jarcuska, P.; Janicko, M.; Veseliny, E.; Jarcuska, P.; Skladany, L. Circulating markers of liver fibrosis progression. Clin. Chim. Acta 2010, 411, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nekagawa, J.; Minakuchi, C.; Fukase, M. A clinical evaluation of serum monoamine oxidase, with special reference to hepatic fibrosis. Digestion 1971, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Wu, C.; Zhou, Y.; Wang, X.; Li, P.; Liu, Z.; Tang, B. Rapid Two-Photon Fluorescence Imaging of Monoamine Oxidase B for Diagnosis of Early-Stage Liver Fibrosis in Mice. Anal. Chem. 2021, 93, 7110–7117. [Google Scholar] [CrossRef] [PubMed]
| N0 | RT | m/z | VIP | PPM | MS/MS | Metabolites | Formula | Adduct Ion | Pathway | CCl4/C | TACS/CCl4 | COL/CCl4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.82 | 207.0543 | 1.49829 | 1 | 128.0755, 99.0988 | 5-phosphonooxy-L-lysine | C6H15N2O6P | [M+H-2H2O]+ | Lysine degradation | ↑ ** | ↓ # | ↓ # |
| 2 | 0.83 | 185.0733 | 1.68887 | 7 | 173.0751, 158.0673, 74.0245 | 5-Hydroxy-L-tryptophan | C11H12N2O3 | [M+H-2H2O]+ | Tryptophan metabolism | ↑ ** | ↓ ## | ↓ # |
| 3 | 0.86 | 149.9975 | 1.11901 | 9 | 74.0280, 125.0015 | Phosphoserine | C3H8NO6P | [M+H-2H2O]+ | Cysteine and methionine metabolism, Glycine, serine and threonine metabolism | ↑ * | ↓ # | − |
| 4 | 1 | 176.1076 | 1.3219 | 3 | 95.0980, 97.1038 | 3-Methyl-2-(3-pyridyl)-1-indoleoctanoic acid | C22H26N2 | [M+2H]+ | − | ↑ ** | ↓ ### | ↓ |
| 5 | 1 | 325.1163 | 1.5421 | 4 | 290.0865, 274.1086, 264.1042, 233.0599, 216.0840, 205.0615, 86.0639 | Glutathione | C10H17N3O6S | [M+NH4]+ | Glutathione metabolism | ↓ *** | ↑ ## | ↑ ## |
| 6 | 1.01 | 522.2034 | 1.80521 | 4 | 253.0923, 226.0811, 191.0567, 137.0474, 110.0374 | Deoxyinosine | C10H12N4O4 | [2M+NH4]+ | Purine metabolism | ↓ *** | ↑ # | − |
| 7 | 1.02 | 300.1328 | 1.5686 | 7 | 96.0595, 108.0514, 123.0606, 153.0701 | 7-Methylinosine | C11H15N4O5 | [M+NH4]+ | − | ↑ *** | ↓ ### | ↓ ### |
| 8 | 1.13 | 118.0914 | 3.1785 | 4 | 58.0672, 129.1021, 155.1189 | beta-Alanyl-L-lysine | C9H19N3O3 | [M+H+NH4]+ | beta-Alanine metabolism | ↑ *** | ↓ ## | ↓ # |
| 9 | 1.17 | 139.0553 | 1.10885 | 1 | 102.0579, 86.0640, 84.0492 | gamma-Glutamylglutamic acid | C10H16N2O7 | [M+2H]+ | − | ↓ ** | ↑ # | − |
| 10 | 1.17 | 174.1215 | 1.0145 | 9 | 158.0818, 140.0777, 129.1059, 116.0757, 112.0769, 85.0762, 60.0808 | N2-Acetylornithine | C7H14N2O3 | [M+NH4-H2O]+ | − | ↓ ** | ↑ | − |
| 11 | 3.51 | 327.205 | 1.16328 | 7 | 310.1781, 182.0842, 136.0775, 129.1010, 95.0645, 84.0857 | Lysyltyrosine | C15H23N3O4 | [M+NH4]+ | − | ↓ ** | ↑ # | ↑ ## |
| 12 | 4.23 | 174.0599 | 1.60695 | 6 | 156.0491, 146.0647, 132.0499, 126.0599, 104.0569 | N-Acetyl-L-methionine | C7H13NO3S | [M+H-H2O]+ | − | ↓ ** | − | − |
| 13 | 4.35 | 245.143 | 1.64268 | 3 | 106.0655, 79.0510, 132.0871 | 3-Methylindole | C9H9N | [2M+H-H2O]+ | − | ↑ * | − | ↓ # |
| 14 | 4.36 | 506.2784 | 1.11455 | 0 | 489.2525, 471.2425, 367.1596, 85.0687, 55.0588 | 7-Sulfocholic acid | C24H40O8S | [M+NH4]+ | − | ↑ * | ↓ # | ↓ |
| 15 | 4.5 | 164.0756 | 1.55243 | 10 | 70.0697, 160.0727 | Homomethionine | C6H13NO2S | [M+H]+ | − | ↑ ** | − | − |
| 16 | 4.57 | 303.1839 | 1.888 | 2 | 122.0970, 120.0836, 93.0645 | Benzphetamine | C17H21N | [M+ACN+Na]+ | − | ↑ ** | ↓ ## | ↓ |
| 17 | 12.26 | 423.2771 | 1.6011 | 7 | 421.2584, 405.2632, 345.2471, 127.0787 | Monoketocholic acid | C24H38O6 | [M+H]+ | Bile Acid Metabolism | ↑ ** | ↓ ## | ↓ # |
| 18 | 14.06 | 391.2866 | 1.79775 | 6 | 391.2873, 373.2771, 365.2665, 289.2195 | Cholic acid | C24H40O5 | [M+NH4-H2O]+ | Bile Acid Metabolism | ↓ * | ↑ | − |
| 19 | 14.06 | 413.2683 | 1.24289 | 5 | 391.2873, 373.2771, 355.2648, 289.2195, 271.2043 | 12-Ketodeoxycholic acid | C24H38O4 | [M+Na]+ | Bile Acid Metabolism | ↓ * | − | − |
| 20 | 14.63 | 488.3015 | 1.23856 | 7 | 407.2729, 365.2633, 355.2450, 85.0699 | Glycocholic acid | C24H40O6 | [M+ACN+Na]+ | Bile Acid Metabolism | ↑ ** | ↓ # | ↓ # |
| 21 | 15.1 | 410.3289 | 2.75643 | 6 | 375.2928, 347.2920, 315.2688, 73.0295, 299.2342 | Chenodeoxycholic acid | C24H40O4 | [M+NH4]+ | Bile Acid Metabolism | ↓ * | ↓ | − |
| 22 | 15.19 | 408.3129 | 1.60232 | 5 | 373.2743, 355.2679, 277.2149, 257.1904, 175.1107 | Nutriacholic acid | C24H38O4 | [M+NH4]+ | Bile Acid Metabolism | ↓ * | ↑ | − |
| 23 | 15.53 | 410.3285 | 1.97283 | 5 | 349.2756, 301.2562, 271.2059, 243.1787, 165.1282, 147.1184, 85.0655 | Deoxycholic acid | C24H40O4 | [M+NH4]+ | Bile Acid Metabolism | ↓ * | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Luo, Z.; Li, D.; Qin, J.; Pan, Z.; Guo, B.; Deng, L.; Nong, Y.; Huang, Z.; He, Y.; et al. Investigation of the Therapeutic Effect of Total Alkaloids of Corydalis saxicola Bunting on CCl4-Induced Liver Fibrosis in Rats by LC/MS-Based Metabolomics Analysis and Network Pharmacology. Metabolites 2023, 13, 9. https://doi.org/10.3390/metabo13010009
Wang Q, Luo Z, Li D, Qin J, Pan Z, Guo B, Deng L, Nong Y, Huang Z, He Y, et al. Investigation of the Therapeutic Effect of Total Alkaloids of Corydalis saxicola Bunting on CCl4-Induced Liver Fibrosis in Rats by LC/MS-Based Metabolomics Analysis and Network Pharmacology. Metabolites. 2023; 13(1):9. https://doi.org/10.3390/metabo13010009
Chicago/Turabian StyleWang, Qianyi, Zhuo Luo, Danfeng Li, Jinghua Qin, Ziping Pan, Bingjian Guo, Lijun Deng, Yunyuan Nong, Zheng Huang, Ying He, and et al. 2023. "Investigation of the Therapeutic Effect of Total Alkaloids of Corydalis saxicola Bunting on CCl4-Induced Liver Fibrosis in Rats by LC/MS-Based Metabolomics Analysis and Network Pharmacology" Metabolites 13, no. 1: 9. https://doi.org/10.3390/metabo13010009
APA StyleWang, Q., Luo, Z., Li, D., Qin, J., Pan, Z., Guo, B., Deng, L., Nong, Y., Huang, Z., He, Y., Guo, H., Zhu, D., Liang, Y., & Su, Z. (2023). Investigation of the Therapeutic Effect of Total Alkaloids of Corydalis saxicola Bunting on CCl4-Induced Liver Fibrosis in Rats by LC/MS-Based Metabolomics Analysis and Network Pharmacology. Metabolites, 13(1), 9. https://doi.org/10.3390/metabo13010009







