α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Silico Metabolite Prediction
2.2. Chemicals and Reagents
2.3. Hepatocyte Incubations
2.4. Authentic Samples
2.5. Sample Preparation
2.5.1. Incubates
2.5.2. Urine and Blood
2.6. Instrumental
2.6.1. Liquid Chromatography
2.6.2. High-Resolution Tandem Mass Spectrometry
2.7. Data Mining
3. Results
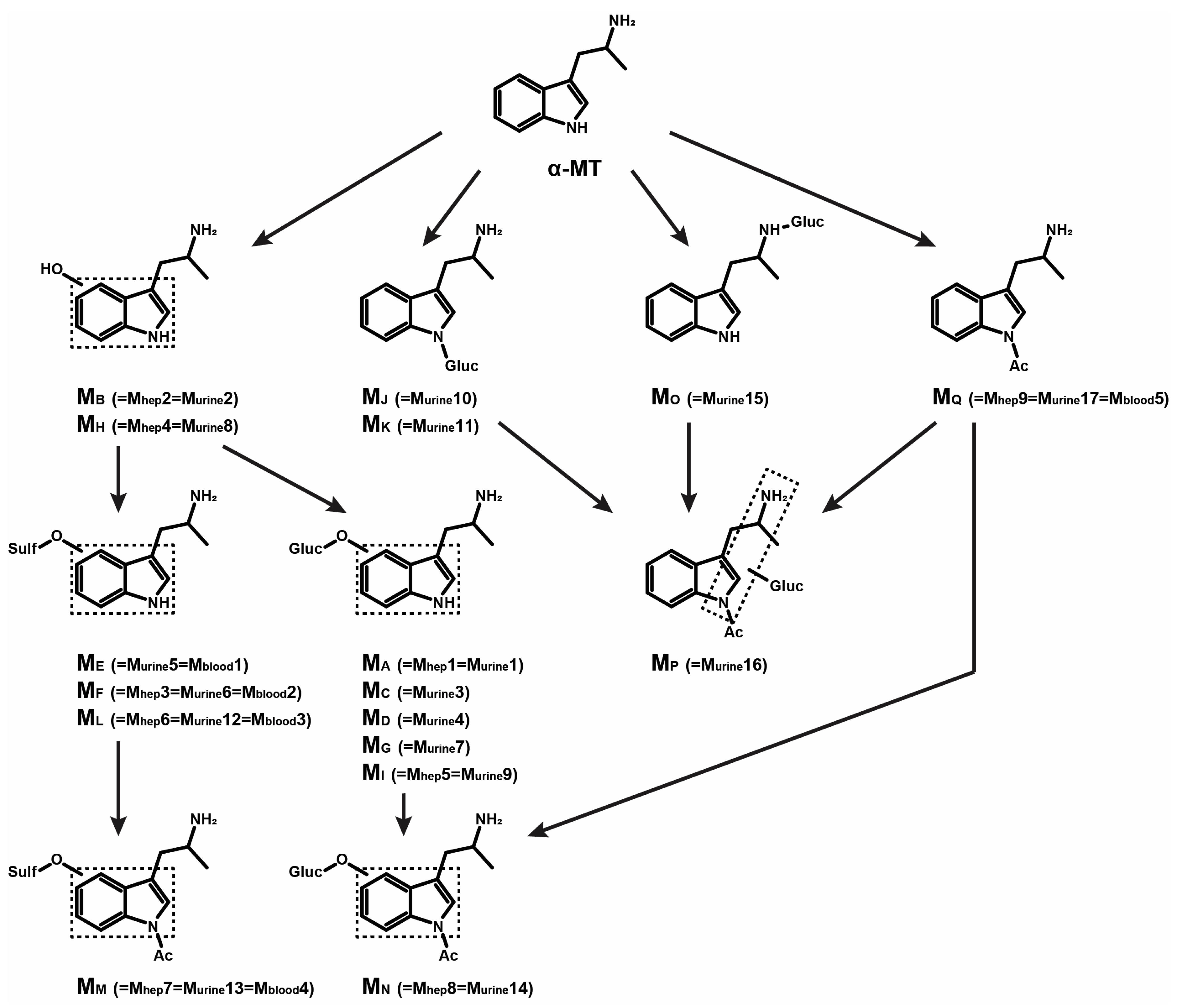
3.1. In Silico Metabolite Predictions
3.2. α-MT HRMS/MS Fragmentation Pattern
3.3. α-MT Metabolites in Human Hepatocyte Incubations
3.3.1. α-MT Hydroxylation and Further O-Sulfation or O-Glucuronidation
3.3.2. α-MT N-Acetylation and Combinations
3.4. α-MT Metabolites in Postmortem Urine
3.4.1. α-MT Hydroxylation and O-Sulfation or O-Glucuronidation
3.4.2. α-MT N-Glucuronidation and Combination
3.5. α-MT Metabolites in Postmortem Blood
4. Discussion
4.1. General Analytical Considerations
4.2. In Vitro Versus Postmortem Metabolites
4.3. Comparison to Analogues
4.4. Comparison to α-MT Metabolism in Rats
4.5. Recommended Biomarkers of Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malaca, S.; Lo Faro, A.F.; Tamborra, A.; Pichini, S.; Busardò, F.P.; Huestis, M.A. Toxicology and Analysis of Psychoactive Tryptamines. Int. J. Mol. Sci. 2020, 21, 9279. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, M. Introduction: Evidence for Entheogen Use in Prehistory and World Religions. J. Psychedelic Stud. 2019, 3, 43–62. [Google Scholar] [CrossRef]
- United Nations (UN) World Drug Report 2019—Cannabis and Hallucinogens. Available online: https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_5_CANNABIS_HALLUCINOGENS.pdf (accessed on 1 January 2023).
- United Nations (UN) Convention on Psychotropic Substances. 1971. Available online: https://www.unodc.org/pdf/convention_1971_en.pdf (accessed on 1 January 2023).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report—Trends and Developments. Available online: https://www.emcdda.europa.eu/system/files/publications/14644/TDAT22001ENN.pdf (accessed on 1 January 2023).
- Howland, R.H. Antidepressant, Antipsychotic, and Hallucinogen Drugs for the Treatment of Psychiatric Disorders: A Convergence at the Serotonin-2A Receptor. J. Psychosoc. Nurs. Ment. Health Serv. 2016, 54, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased Global Integration in the Brain after Psilocybin Therapy for Depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef]
- Dipartimento per le Politiche Antidroga. Nuove Sostanze Psicoattive—Triptamine. 2022. Available online: https://www.politicheantidroga.gov.it/media/1286/36_triptamine.pdf (accessed on 1 January 2023).
- Long, H.; Nelson, L.S.; Hoffman, R.S. Alpha-Methyltryptamine Revisited via Easy Internet Access. Vet. Hum. Toxicol. 2003, 45, 149. [Google Scholar]
- Wilcox, J. Psychoactive Properties of Alpha-Methyltryptamine: Analysis from Self Reports of Users. J. Psychoactive Drugs 2012, 44, 274–276. [Google Scholar] [CrossRef]
- Holstege, C.; Baer, A.; Kirk, M. Abstracts of the 2003 North American Congress of Clinical Toxicology Annual Meeting—Abstract 251. Prolonged Hallucinations Following Ingestion of Alpha-Methyl-Tryptamine. J. Toxicol. Clin. Toxicol. 2003, 41, 746. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Critical Review Report: Alpha-Methyltryptamine (AMT). Available online: https://legal-high-inhaltsstoffe.de/sites/default/files/uploads/amt.pdf (accessed on 1 January 2023).
- Boland, D.M.; Andollo, W.; Hime, G.W.; Hearn, W.L. Fatality Due to Acute Alpha-Methyltryptamine Intoxication. J. Anal. Toxicol. 2005, 29, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Drug Enforcement Agency (DEA). Scheduling Actions. 2022. Available online: https://www.deadiversion.usdoj.gov/schedules/orangebook/a_sched_alpha.pdf (accessed on 1 January 2023).
- Szara, S. 6-Hydroxylation: An Important Metabolic Route for Alpha-Methyltryptamine. Experientia 1961, 17, 77. [Google Scholar] [CrossRef]
- Kanamori, T.; Kuwayama, K.; Tsujikawa, K.; Miyaguchi, H.; Iwata, Y.T.; Inoue, H. In Vivo Metabolism of Alpha-Methyltryptamine in Rats: Identification of Urinary Metabolites. Xenobiotica 2008, 38, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Huestis, M.A. Approaches, Challenges, and Advances in Metabolism of New Synthetic Cannabinoids and Identification of Optimal Urinary Marker Metabolites. Clin. Pharmacol. Ther. 2017, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Diao, X.; Sempio, C.; Huestis, M.A. Identification of New Synthetic Cannabinoid ADB-CHMINACA (MAB-CHMINACA) Metabolites in Human Hepatocytes. AAPS J. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, K.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Identification and Quantification of Predominant Metabolites of Synthetic Cannabinoid MAB-CHMINACA in an Authentic Human Urine Specimen. Drug Test. Anal. 2018, 10, 365–371. [Google Scholar] [CrossRef]
- Carlier, J.; Diao, X.; Huestis, M.A. Synthetic Cannabinoid BB-22 (QUCHIC): Human Hepatocytes Metabolism with Liquid Chromatography-High Resolution Mass Spectrometry Detection. J. Pharm. Biomed. Anal. 2018, 157, 27–35. [Google Scholar] [CrossRef]
- Minakata, K.; Hasegawa, K.; Nozawa, H.; Yamagishi, I.; Saitoh, T.; Yoshino, A.; Suzuki, M.; Kitamoto, T.; Suzuki, O.; Watanabe, K. Sensitive Quantification of BB-22 and Its Metabolite BB-22 3-Carboxyindole, and Characterization of New Metabolites in Authentic Urine and/or Serum Specimens Obtained from Three Individuals by LC-QTRAP-MS/MS and High-Resolution LC-Orbitrap-MS/MS. Forensic Toxicol. 2019, 37, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Carlier, J.; Diao, X.; Giorgetti, R.; Busardò, F.P.; Huestis, M.A. Pyrrolidinyl Synthetic Cathinones α-PHP and 4F-α-PVP Metabolite Profiling Using Human Hepatocyte Incubations. Int. J. Mol. Sci. 2021, 22, 230. [Google Scholar] [CrossRef]
- Grapp, M.; Kaufmann, C.; Schwelm, H.M.; Neukamm, M.A. Toxicological Investigation of a Case Series Involving the Synthetic Cathinone α-Pyrrolidinohexiophenone (α-PHP) and Identification of Phase I and II Metabolites in Human Urine. J. Anal. Toxicol. 2022; in print. [Google Scholar] [CrossRef]
- Busardò, F.P.; Lo Faro, A.F.; Sirignano, A.; Giorgetti, R.; Carlier, J. In Silico, in Vitro, and in Vivo Human Metabolism of Acetazolamide, a Carbonic Anhydrase Inhibitor and Common “Diuretic and Masking Agent” in Doping. Arch. Toxicol. 2022, 96, 1989–2001. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A Comprehensive Computational Tool for Small Molecule Metabolism Prediction and Metabolite Identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Carlier, J.; Berardinelli, D.; Montanari, E.; Sirignano, A.; Di Trana, A.; Busardò, F.P. 3F-α-Pyrrolydinovalerophenone (3F-α-PVP) in Vitro Human Metabolism: Multiple in Silico Predictions to Assist in LC-HRMS/MS Analysis and Targeted/Untargeted Data Mining. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1193, 123162. [Google Scholar] [CrossRef]
- Di Trana, A.; Brunetti, P.; Giorgetti, R.; Marinelli, E.; Zaami, S.; Busardò, F.P.; Carlier, J. In Silico Prediction, LC-HRMS/MS Analysis, and Targeted/Untargeted Data-Mining Workflow for the Profiling of Phenylfentanyl in Vitro Metabolites. Talanta 2021, 235, 122740. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.O.; Sui, J.; Young, A.B.; Whittal, R.M. Interferences and Contaminants Encountered in Modern Mass Spectrometry. Anal. Chim. Acta 2008, 627, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Katagi, M.; Kamata, H.T.; Miki, A.; Shima, N.; Zaitsu, K.; Nishikawa, M.; Tanaka, E.; Honda, K.; Tsuchihashi, H. Metabolism of the Psychotomimetic Tryptamine Derivative 5-Methoxy-N,N-Diisopropyltryptamine in Humans: Identification and Quantification of Its Urinary Metabolites. Drug Metab. Dispos. 2006, 34, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolato, M.; Chen, K.; Shih, J.C. The Degradation of Serotonin: Role of MAO. Handb. Behav. Neurosci. 2010, 21, 203–218. [Google Scholar] [CrossRef]
- Michely, J.A.; Helfer, A.G.; Brandt, S.D.; Meyer, M.R.; Maurer, H.H. Metabolism of the New Psychoactive Substances N,N-Diallyltryptamine (DALT) and 5-Methoxy-DALT and Their Detectability in Urine by GC-MS, LC-MSn, and LC-HR-MS-MS. Anal. Bioanal. Chem. 2015, 407, 7831–7842. [Google Scholar] [CrossRef] [Green Version]
- Michely, J.A.; Brandt, S.D.; Meyer, M.R.; Maurer, H.H. Biotransformation and Detectability of the New Psychoactive Substances N,N-Diallyltryptamine (DALT) Derivatives 5-Fluoro-DALT, 7-Methyl-DALT, and 5,6-Methylenedioxy-DALT in Urine Using GC-MS, LC-MS n, and LC-HR-MS/MS. Anal. Bioanal. Chem. 2017, 409, 1681–1695. [Google Scholar] [CrossRef] [Green Version]
- Dinis-Oliveira, R.J. Metabolism of Psilocybin and Psilocin: Clinical and Forensic Toxicological Relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef]
- Manier, S.K.; Felske, C.; Zapp, J.; Eckstein, N.; Meyer, M.R. Studies on the in Vitro and in Vivo Metabolic Fate of the New Psychoactive Substance N-Ethyl-N-Propyltryptamine for Analytical Purposes. J. Anal. Toxicol. 2021, 45, 195–202. [Google Scholar] [CrossRef]
- Wagmann, L.; Manier, S.K.; Meyer, M.R. Can the Intake of a Synthetic Tryptamine Be Detected Only by Blood Plasma Analysis? A Clinical Toxicology Case Involving 4-HO-MET. J. Anal. Toxicol. 2022, 46, 567–572. [Google Scholar] [CrossRef]
- Malaca, S.; Huestis, M.A.; Lattanzio, L.; Marsella, L.T.; Tagliabracci, A.; Carlier, J.; Busardò, F.P. Human Hepatocyte 4-Acetoxy-N,N-Diisopropyltryptamine Metabolite Profiling by Reversed-Phase Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry. Metabolites 2022, 12, 705. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Technische Universität Braunschweig. BRENDA—The Comprehensive Enzyme Information System. Available online: https://www.brenda-enzymes.org/index.php (accessed on 1 January 2023).

| ID | Metabolic Transformation | Elemental Composition | RT, min | m/z (Δppm): [M+H]+ [M−H]− | Peak Area [M+H]+ [M−H]− |
|---|---|---|---|---|---|
| Mhep1 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 3.06 | 367.1501 (0.39) 365.1353 (−0.34) | 5.2 × 105 3.6 × 105 |
| Mhep2 | Hydroxylation (indole) | C11H14N2O | 4.65 | 191.1180 (0.42) ND | 4.5 × 106 ND |
| Mhep3 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 5.40 | 271.0747 (−0.2) 269.0599 (−0.93) | 4.1 × 105 1.6 × 106 |
| Mhep4 | Hydroxylation (indole) | C11H14N2O | 6.15 | 191.1180 (0.63) ND | 1.2 × 105 ND |
| Mhep5 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 6.35 | 367.1502 (0.69) 365.1353 (−0.34) | 1.6 × 105 1.3 × 105 |
| Mhep6 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 7.48 | 271.0748 (0.28) 269.0600 (−0.56) | 9.1 × 105 3.1 × 106 |
| α-MT (Parent) | No transformation | C11H14N2 | 9.12 | 175.1231 (0.54) ND | 4.5 × 107 ND |
| Mhep7 | Hydroxylation (indole) + O-Sulfation + N-Acetylation (indole) | C13H16N2O5S | 9.48 | 313.0854 (0.58) 311.0707 (−0.05) | 1.2 × 105 6.7 × 105 |
| Mhep8 | Hydroxylation (indole) + O-Glucuronidation + N-Acetylation (indole) | C19H24N2O8 | 9.62 | 409.1609 (0.85) 407.1460 (0.02) | 2.9 × 105 5.2 × 105 |
| Mhep9 | N-Acetylation (indole) | C13H16N2O | 14.07 | 217.1336 (0.46) ND | 2.7 × 106 ND |
| ID | Metabolic Transformation | Elemental Composition | RT, min | m/z (Δppm): [M+H]+ [M−H]− | Peak Area [M+H]+ [M−H]− | |
|---|---|---|---|---|---|---|
| Non-Hydrolyzed | Hydrolyzed | |||||
| Murine1 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 2.86 | 367.1500 (0.06) 365.1357 (0.75) | 2.4 × 108 4.5 × 107 | ND |
| Murine2 | Hydroxylation (indole) | C11H14N2O | 4.62 | 191.1179 (0.05) ND | 2.5 × 106 ND | 8.4 × 107 ND |
| Murine3 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 4.81 | 367.1499 (−0.21) ND | 2.7 × 106 ND | ND |
| Murine4 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 4.88 | 367.1498 (−0.48) ND | 2.1 × 106 ND | ND |
| Murine5 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 5.08 | 271.0746 (−0.38) 269.0604 (1.00) | 4.4 × 107 9.7 × 107 | 3.9 × 107 1.1 × 108 |
| Murine6 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 5.36 | 271.0746 (−0.38) 269.0604 (0.92) | 3.4 × 108 5.3 × 108 | 3.8 × 108 5.6 × 108 |
| Murine7 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 5.98 | 367.1502 (0.60) 365.1361 (1.80) | 1.2 × 107 5.2 × 106 | 2.6 × 106 1.0 × 106 |
| Murine8 | Hydroxylation (indole) | C11H14N2O | 6.11 | 191.1181 (1.10) ND | 8.5 × 105 ND | 2.1 × 107 ND |
| Murine9 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 6.28 | 367.1501 (0.33) 365.1357 (−0.75) | 3.4 × 107 1.0 × 107 | ND |
| Murine10 | N-Glucuronidation (indole) | C17H22N2O6 | 7.06 | 351.1548 (−0.75) 349.1409 (1.03) | 5.2 × 106 1.6 × 106 | 5.2 × 106 1.6 × 106 |
| Murine11 | N-Glucuronidation (indole) | C17H22N2O6 | 7.15 | 351.1550 (−0.18) 349.1412 (1.20) | 6.6 × 106 2.0 × 106 | 7.1 × 106 1.9 × 106 |
| Murine12 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 7.44 | 271.0747 (−0.02) 269.0605 (1.29) | 8.5 × 107 2.3×108 | 8.0 × 107 2.3 × 108 |
| α-MT (Parent) | No transformation | C11H14N2 | 8.56 | 175.1231 (−0.02) ND | 3.5 × 109 ND | 3.6 × 109 ND |
| Murine13 | Hydroxylation (indole) + O-Sulfation + N-Acetylation (indole) | C13H16N2O5S | 9.60 | 313.0853 (0.71) 311.0711 (1.24) | 1.8 × 107 1.0 × 108 | 1.6 × 107 9.3 × 107 |
| Murine14 | Hydroxylation (indole) + O-Glucuronidation + N-Acetylation (indole) | C19H24N2O8 | 9.61 | 409.1609 (0.88) 407.1464 (1.00) | 4.2 × 106 4.0 × 106 | ND |
| Murine15 | N-Glucuronidation (alkyl) | C17H22N2O6 | 10.55 | 351.1551 (0.16) 349.1412 (1.46) | 1.5 × 107 7.3 × 106 | 1.5 × 107 9.0 × 106 |
| Murine16 | N-Acetylation (indole) +N-Glucuronidation | C19H24N2O7 | 12.98 | 393.1661 (1.15) 391.1517 (1.68) | 3.0 × 107 4.4 × 107 | 3.2 × 107 4.4 × 107 |
| Murine17 | N-Acetylation (indole) | C13H16N2O | 14.08 | 217.1336 (0.28) ND | 5.8 × 106 ND | 5.8 × 106 ND |
| ID | Metabolic Transformation | Elemental Composition | RT, min | m/z (Δppm): [M+H]+ [M−H]− | Peak Area [M+H]+ [M−H]− |
|---|---|---|---|---|---|
| Mblood1 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 5.11 | 271.0750 (1.09) 269.0602 (0.18) | 8.5 × 105 2.3 × 106 |
| Mblood2 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 5.38 | 271.0750 (1.09) 269.0603 (0.55) | 9.2 × 106 3.1 × 107 |
| Mblood3 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 7.47 | 271.0750 (1.09) 269.0603 (0.55) | 3.5 × 106 1.3 × 107 |
| α-MT (Parent) | No transformation | C11H14N2 | 9.04 | 175.1233 (2.08) ND | 1.0 × 108 ND |
| Mblood4 | Hydroxylation (indole) + O-Sulfation + N-Acetylation (indole) | C13H16N2O5S | 9.52 | 313.0857 (1.38) 311.0711 (1.24) | 5.9 × 105 3.6 × 106 |
| Mblood5 | N-Acetylation (indole) | C13H16N2O | 14.07 | 217.1338 (1.20) ND | 2.1 × 106 ND |
| ID | ID in Samples | Metabolic Transformation | Elemental Composition | Theoretical m/z: [M+H]+ [M−H]− | RT, min |
|---|---|---|---|---|---|
| MA | =Mhep1 =Murine1 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 367.1500 365.1354 | 2.86 |
| MB | =Mhep2 =Murine2 | Hydroxylation (indole) | C11H14N2O | 191.1179 189.1033 | 4.62 |
| MC | =Murine3 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 367.1500 365.1354 | 4.81 |
| MD | =Murine4 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 367.1500 365.1354 | 4.88 |
| ME | =Murine5 =Mblood1 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 271.0747 269.0602 | 5.08 |
| MF | =Mhep3 =Murine6 =Mblood2 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 271.0747 269.0602 | 5.36 |
| MG | =Murine7 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 367.1500 365.1354 | 5.98 |
| MH | =Mhep4 =Murine8 | Hydroxylation (indole) | C11H14N2O | 191.1179 189.1033 | 6.11 |
| MI | =Mhep5 =Murine9 | Hydroxylation (indole) + O-Glucuronidation | C17H22N2O7 | 367.1500 365.1354 | 6.28 |
| MJ | =Murine10 | N-Glucuronidation (indole) | C17H22N2O6 | 351.1551 349.1405 | 7.06 |
| MK | =Murine11 | N-Glucuronidation (indole) | C17H22N2O6 | 351.1551 349.1405 | 7.15 |
| ML | =Mhep6 =Murine12 =Mblood3 | Hydroxylation (indole) + O-Sulfation | C11H14N2O4S | 271.0747 269.0602 | 7.44 |
| α-MT (Parent) | NA | No transformation | C11H14N2 | 175.1230 173.1084 | 8.56 |
| MM | =Mhep7 =Murine13 =Mblood4 | Hydroxylation (indole) + O-Sulfation + N-Acetylation (indole) | C13H16N2O5S | 313.0853 311.0707 | 9.60 |
| MN | =Mhep8 =Murine14 | Hydroxylation (indole) + O-Glucuronidation + N-Acetylation (indole) | C19H24N2O8 | 409.1605 407.1460 | 9.61 |
| MO | =Murine15 | N-Glucuronidation (alkyl) | C17H22N2O6 | 351.1551 349.1405 | 10.55 |
| MP | =Murine16 | N-Acetylation (indole) +N-Glucuronidation | C19H24N2O7 | 393.1656 391.1511 | 12.98 |
| MQ | =Mhep9 =Murine17 =Mblood5 | N-Acetylation (indole) | C13H16N2O | 217.1335 215.1190 | 14.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaca, S.; Bottinelli, C.; Fanton, L.; Cartiser, N.; Carlier, J.; Busardò, F.P. α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood. Metabolites 2023, 13, 92. https://doi.org/10.3390/metabo13010092
Malaca S, Bottinelli C, Fanton L, Cartiser N, Carlier J, Busardò FP. α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood. Metabolites. 2023; 13(1):92. https://doi.org/10.3390/metabo13010092
Chicago/Turabian StyleMalaca, Sara, Charline Bottinelli, Laurent Fanton, Nathalie Cartiser, Jeremy Carlier, and Francesco Paolo Busardò. 2023. "α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood" Metabolites 13, no. 1: 92. https://doi.org/10.3390/metabo13010092
APA StyleMalaca, S., Bottinelli, C., Fanton, L., Cartiser, N., Carlier, J., & Busardò, F. P. (2023). α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood. Metabolites, 13(1), 92. https://doi.org/10.3390/metabo13010092








