Unlocking Potentially Therapeutic Phytochemicals in Capadulla (Doliocarpus dentatus) from Guyana Using Untargeted Mass Spectrometry-Based Metabolomics
Abstract
:1. Introduction
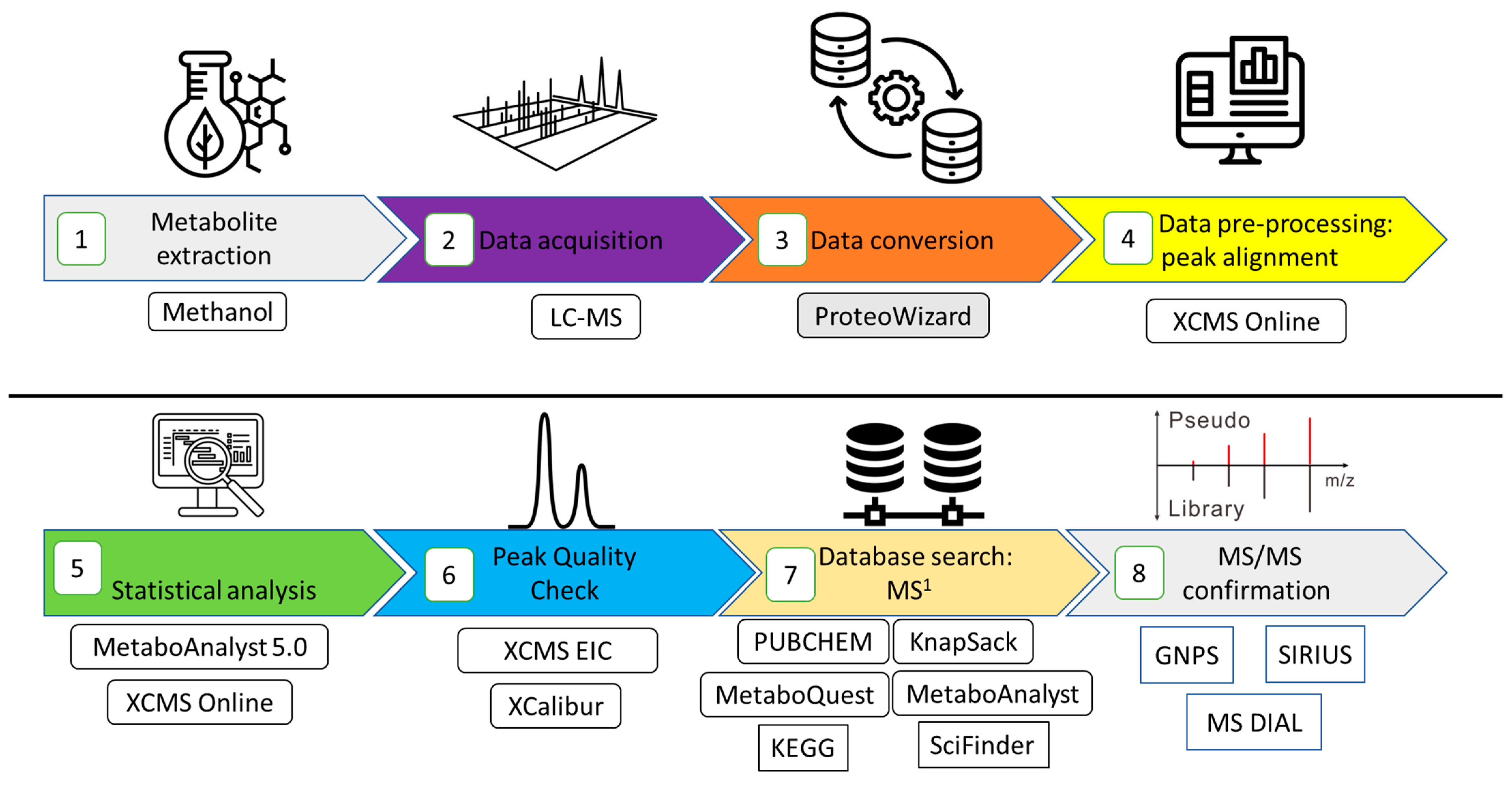
2. Materials and Methods
2.1. Chemicals Used for Metabolite Extraction and Liquid Chromatography–Mass Spectrometry
2.2. Study Site and Plots–Eagle Mountain Forest Potaro–Siparuni, Guyana
2.3. Biological Sample Preparation and Metabolite Extraction
2.4. Data Acquisition via Liquid Chromatography–Mass Spectrometry (LC-MS)
2.5. Data Processing of Untargeted Mass Spectrometry Data
2.6. Statistical Analysis of Metabolomics Datasets
2.7. Tandem Mass Spectrometry-Data Analysis for Metabolite Annotation
2.7.1. Metabolite Annotation Using MS-DIAL
2.7.2. Metabolite Annotation Using GNPS via Classical Molecular Networking
2.7.3. Metabolite Annotation Using GNPS via Feature-Based Molecular Networking
2.7.4. Using SIRIUS as an In Silico Fragmentation Tool for Metabolite Annotation
2.7.5. Cheminformatics Using ClassyFire for Compound Class Groupings
3. Results
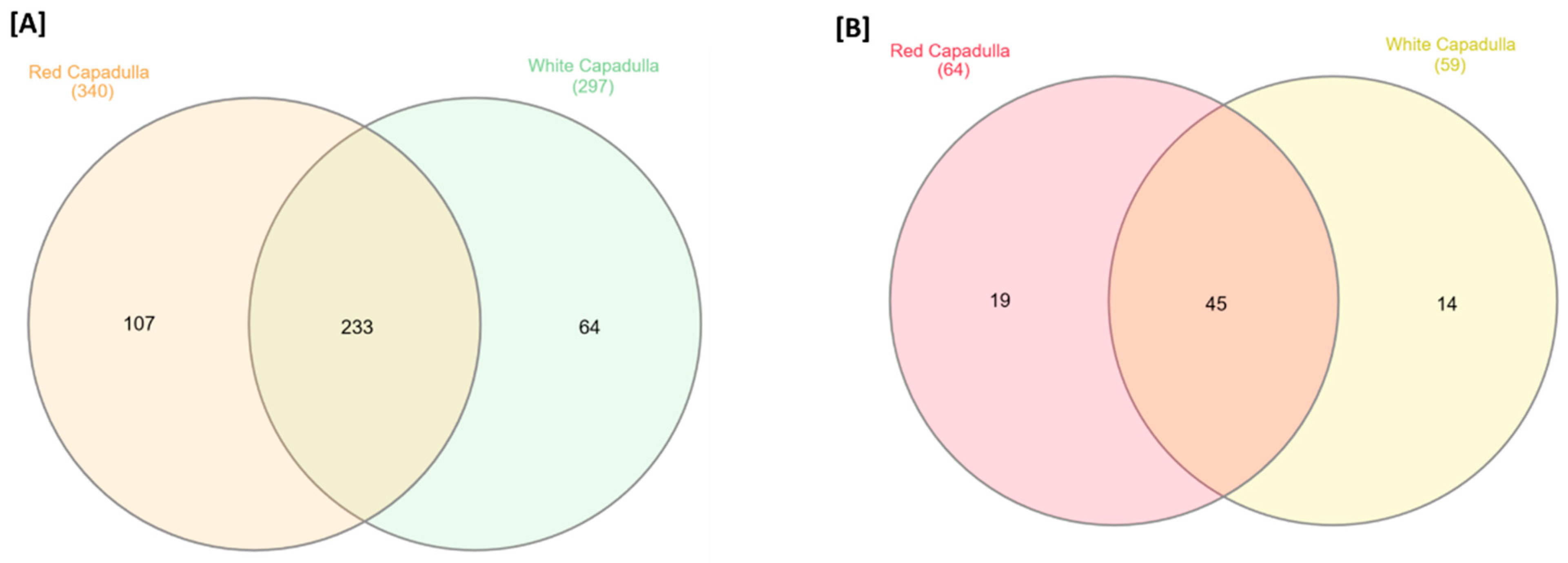
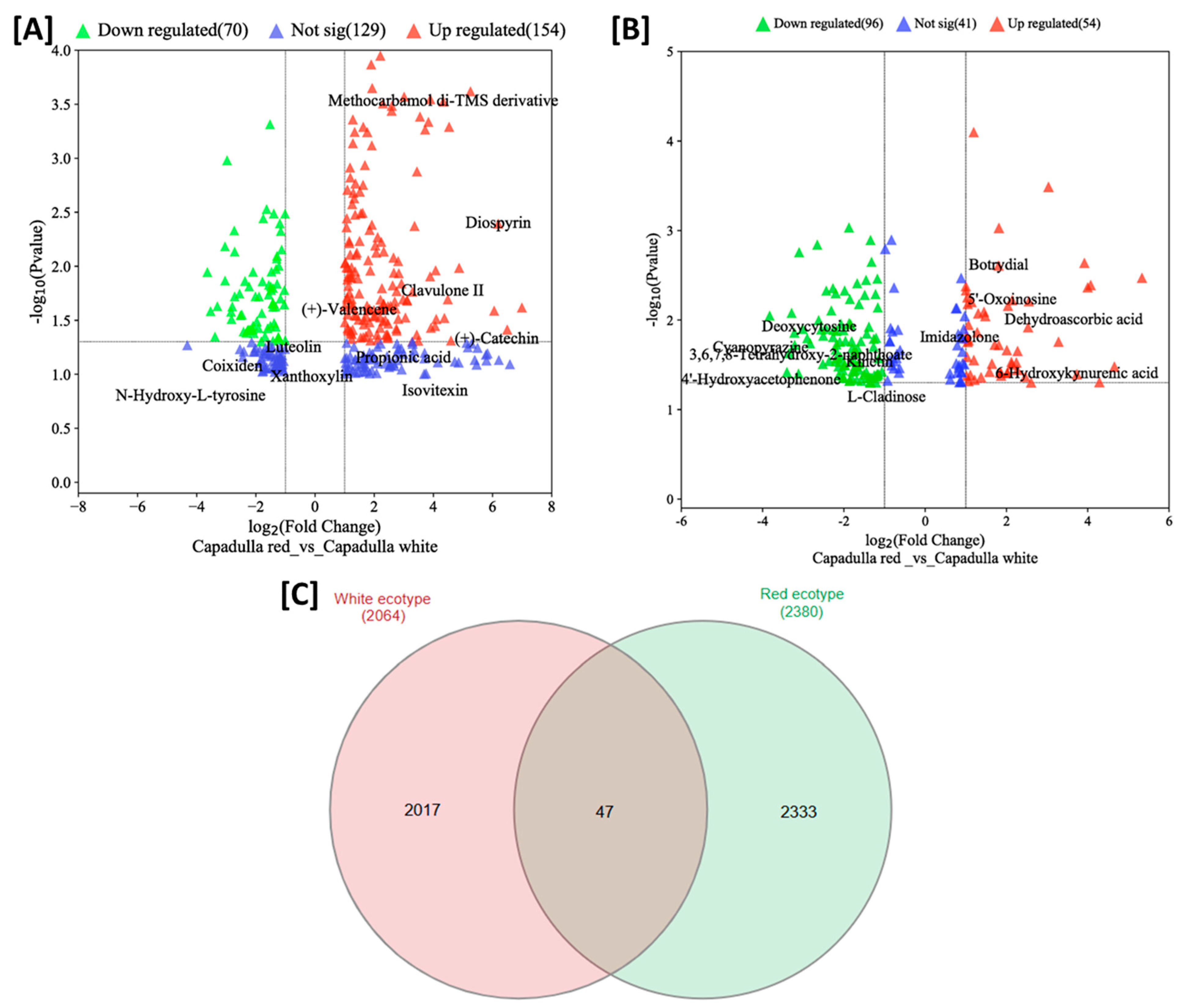
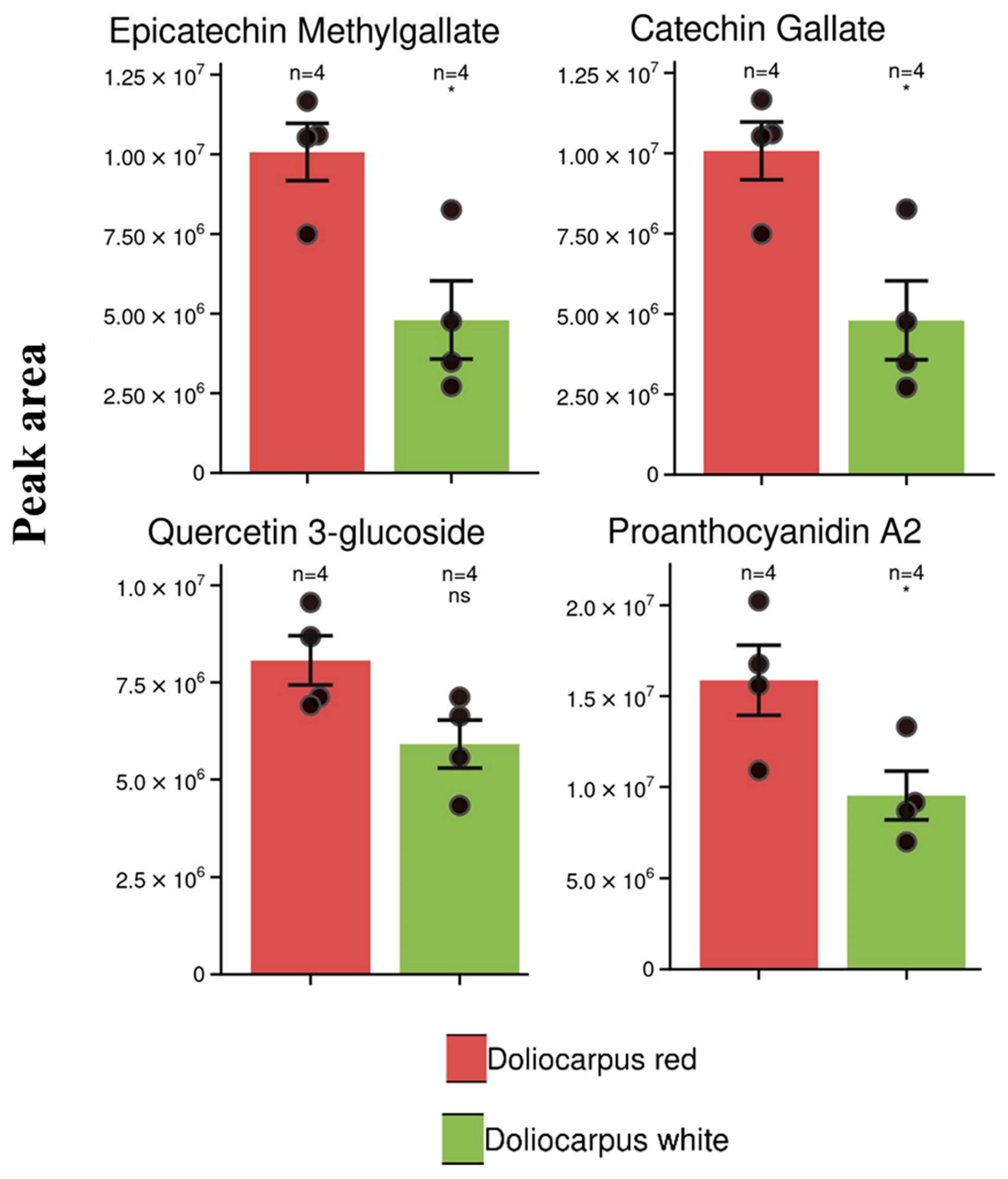
3.1. Untargeted Metabolomics: Analysis of D. dentatus Red and White Ecotypes
3.2. Tentative Identification of the Significant Metabolites
3.3. Pathway Analysis of D. dentatus Ecotypes
3.4. Principal Component Analyses of D. dentatus Red and White Ecotypes
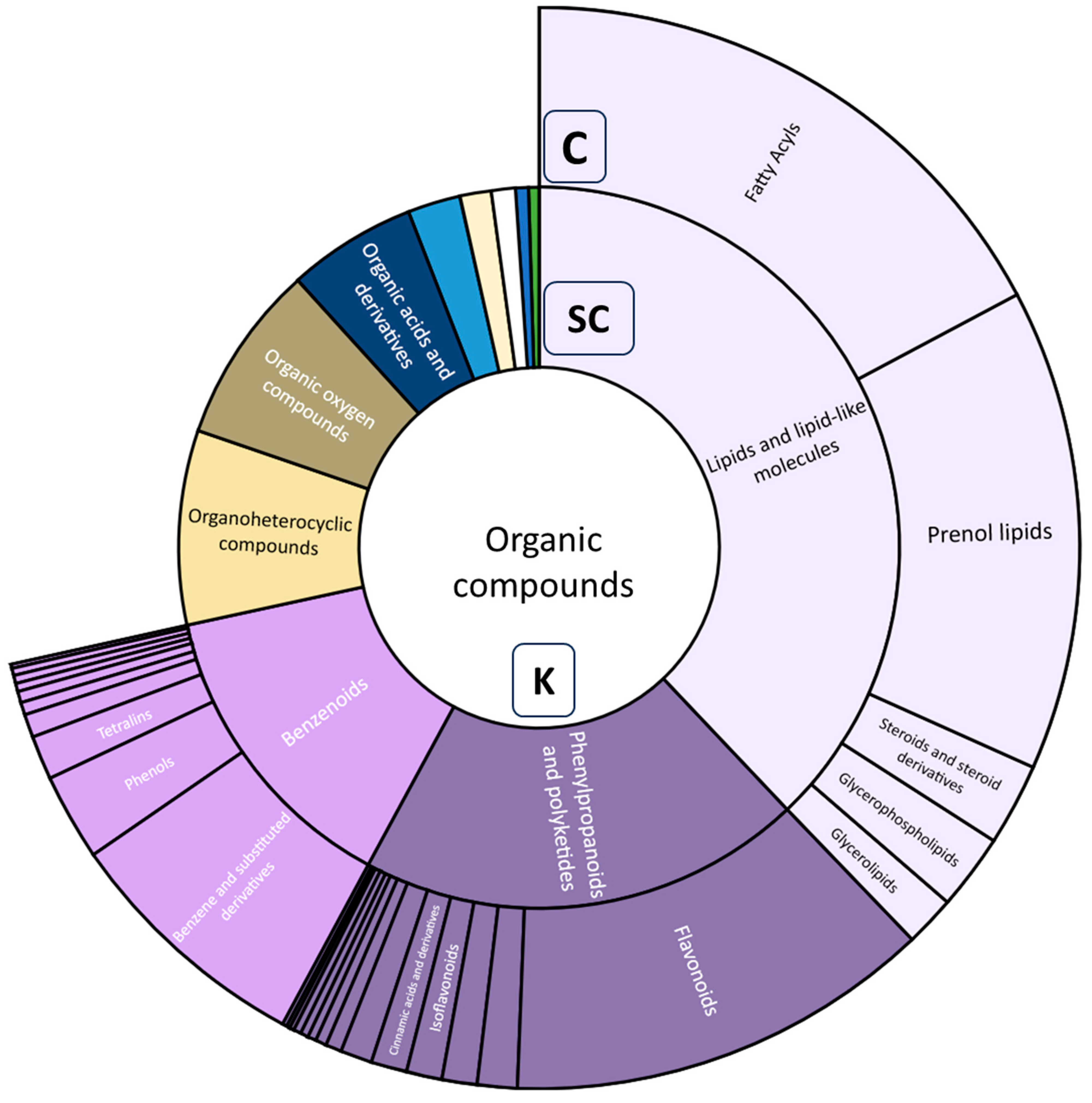
3.5. Metabolite Annotation and Compound Class Contribution Using Tandem Mass Spectrometry Data
4. Discussion
4.1. Untargeted Metabolomics Analysis Revealed Metabolites Present in Different Ecotypes of Doliocarpus Dentatus
4.1.1. Flavonoids
4.1.2. Alkaloids
4.1.3. Terpenoids
4.1.4. Therapeutic Compounds and Possible Synergistic Effects
4.2. Several Metabolites Are Ecotype Specific
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurni, A.A.; König, W.A.; Kubitzki, K. Flavonoid glycosides and sulphates from the Dilleniaceae. Phytochemistry 1981, 20, 1057–1059. [Google Scholar] [CrossRef]
- Lima, C.C.; Lemos, R.P.L.; Conserva, L.M. Dilleniaceae family: An overview of its ethnomedicinal uses, biological and phytochemical profile. J. Pharmacogn. Phytochem. 2014, 3, 181–204. [Google Scholar]
- Branquinho, L.S.; Verdan, M.H.; dos Santos, E.; Macorini, L.F.B.; Maris, R.S.; Kuraoka-Oliveira, A.M.; Bacha, F.B.; Cardoso, C.A.L.; Arena, A.C.; Silva-Filho, S.E.; et al. Toxicological evaluation of ethanolic extract of leaves from Doliocarpus dentatus in Swiss mice. Drug Chem. Toxicol. 2021, 45, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jayaveera, K.; Kumar, C.; Sanjay, U.P.; Swamy, B.; Kumar, D. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop. J. Pharm. Res. 2007, 6, 717–723. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnopharmacol. 2014, 151, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D.-M. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2021, 9, 12–39. [Google Scholar] [CrossRef]
- Andel, T.V.; Banki, O. Commercial Non-Timber Forest Products of the Guiana Shield: An Inventory of Commercial NTFP Extration and Possibilities for Sustainable Harvesting; Netherlands Committee for IUCN: Amsterdam, The Netherlands, 2003; p. 128. [Google Scholar]
- Van Andel, T.R. Floristic composition and diversity of three swamp forests in northwest Guyana. Plant Ecol. 2003, 167, 293–317. [Google Scholar] [CrossRef]
- Van Andel, T. Floristic composition and diversity of mixed primary and secondary forests in northwest Guyana. Biodivers. Conserv. 2001, 10, 1645–1682. [Google Scholar] [CrossRef]
- Singh, R.; Ali, A.; Jeyabalan, G.; Semwal, A. An overview of the current methodologies used for evaluation of aphrodisiac agents. J. Acute Dis. 2013, 2, 85–91. [Google Scholar] [CrossRef]
- Van Andel, T.R. Non-Timber Forest Products of the North-West District of Guyana; Utrecht University: Utrecht, The Netherlands, 2000. [Google Scholar]
- Ishikawa, R.B.; Leitão, M.M.; Kassuya, R.M.; Macorini, L.F.; Moreira, F.M.F.; Cardoso, C.A.L.; Coelho, R.G.; Pott, A.; Gelfuso, G.M.; Croda, J.; et al. Anti-inflammatory, antimycobacterial and genotoxic evaluation of Doliocarpus dentatus. J. Ethnopharmacol. 2017, 204, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Jagessar, R.C.; Hoolas, G.; Maxwell, A.R. Phytochemical screening, isolation of betulinic acid, trigonelline and evaluation of heavy metals ion content of Doliocarpus dentatus. J. Nat. Prod. 2013, 6, 5–16. [Google Scholar]
- Branquinho, L.S.; Verdan, M.H.; Santos, E.d.; Neves, S.C.d.; Oliveira, R.J.; Cardoso, C.A.L.; Kassuya, C.A.L. Aqueous extract from leaves of Doliocarpus dentatus (Aubl.) Standl. relieves pain without genotoxicity activity. J. Ethnopharmacol. 2021, 266, 113440. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.B.; Vani, J.M.; das Neves, S.C.; Rabacow, A.P.M.; Kassuya, C.A.L.; Croda, J.; Cardoso, C.A.L.; Monreal, A.; Antoniolli, A.; Cunha-Laura, A.L.; et al. The safe use of Doliocarpus dentatus in the gestational period: Absence of changes in maternal reproductive performance, embryo-fetal development and DNA integrity. J. Ethnopharmacol. 2018, 217, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sauvain, M.; Kunesch, N.; Poisson, J.; Gantier, J.C.; Gayral, P.; Dedet, J.P. Isolation of leishmanicidal triterpenes and lignans from the Amazonian liana Doliocarpus dentatus (Dilleniaceae). Phytother. Res. 1996, 10, 1–4. [Google Scholar] [CrossRef]
- Aponte, J.C.; Vaisberg, A.J.; Rojas, R.; Caviedes, L.; Lewis, W.H.; Lamas, G.; Sarasara, C.; Gilman, R.H.; Hammond, G.B. Isolation of cytotoxic metabolites from targeted peruvian amazonian medicinal plants. J. Nat. Prod. 2008, 71, 102–105. [Google Scholar] [CrossRef]
- Rodrigues, V.E.G.; de Carvalho, D.A. Florística de plantas medicinais nativas de remanescentes de floresta estacional semidecidual na região do Alto Rio Grande-Minas Gerais. Cerne 2008, 14, 93–112. [Google Scholar]
- Mari, A.; Montoro, P.; D’Urso, G.; Macchia, M.; Pizza, C.; Piacente, S. Metabolic profiling of Vitex agnus castus leaves, fruits and sprouts: Analysis by LC/ESI/(QqQ) MS and (HR) LC/ESI/(Orbitrap)/MSn. J. Pharm. Biomed. Anal. 2015, 102, 215–221. [Google Scholar] [CrossRef]
- Prabakaran, M.; Chung, I.-M.; Son, N.-Y.; Chi, H.-Y.; Kim, S.-Y.; Yang, Y.-J.; Kwon, C.; An, Y.-J.; Ahmad, A.; Kim, S.-H. Analysis of selected phenolic compounds in organic, pesticide-free, conventional rice (Oryza sativa L.) using LC-ESI-MS/MS. Molecules 2018, 24, 67. [Google Scholar] [CrossRef]
- Shimizu, T.; Watanabe, M.; Fernie, A.R.; Tohge, T. Targeted LC-MS analysis for plant secondary metabolites. Plant Metabolom. Methods Protoc. 2018, 1778, 171–181. [Google Scholar]
- Feussner, K.; Feussner, I. Comprehensive LC-MS-based metabolite fingerprinting approach for plant and fungal-derived samples. High-Throughput Metabolom. Methods Protoc. 2019, 1978, 167–185. [Google Scholar]
- Gorrochategui, E.; Jaumot, J.; Lacorte, S.; Tauler, R. Data analysis strategies for targeted and untargeted LC-MS metabolomic studies: Overview and workflow. TrAC Trends Anal. Chem. 2016, 82, 425–442. [Google Scholar] [CrossRef]
- Wang, L.; Xing, X.; Chen, L.; Yang, L.; Su, X.; Rabitz, H.; Lu, W.; Rabinowitz, J.D. Peak annotation and verification engine for untargeted LC–MS metabolomics. Anal. Chem. 2018, 91, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Aime, M.C.; Henkel, T.W. New species of Inocybe from Dicymbe forests of Guyana. Mycol. Res. 2003, 107, 495–505. [Google Scholar] [CrossRef]
- Hammond, D.S. Tropical Forests of the Guiana Shield: Ancient Forests in a Modern World; CABI: Wallingford, UK, 2005. [Google Scholar]
- Castilla, G.; Hall, R.J.; Skakun, R.; Filiatrault, M.; Beaudoin, A.; Gartrell, M.; Smith, L.; Groenewegen, K.; Hopkinson, C.; van der Sluijs, J. The Multisource Vegetation Inventory (MVI): A Satellite-Based Forest Inventory for the Northwest Territories Taiga Plains. Remote Sens. 2022, 14, 1108. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Butler, C.; Palberg, D.; Kisiala, A.; Emery, R.J.N. The tRNA-degradation pathway impacts the phenotype and metabolome of Arabidopsis thaliana: Evidence from atipt2 and atipt9 knockout mutants. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Shinbo, Y.; Nakamura, Y.; Altaf-Ul-Amin, M.; Asahi, H.; Kurokawa, K.; Arita, M.; Saito, K.; Ohta, D.; Shibata, D.; Kanaya, S. KNApSAcK: A comprehensive species-metabolite relationship database. In Plant Metabolomics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 165–181. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Hawkins, C.; Ginzburg, D.; Zhao, K.; Dwyer, W.; Xue, B.; Xu, A.; Rice, S.; Cole, B.; Paley, S.; Karp, P. Plant Metabolic Network 15: A resource of genome-wide metabolism databases for 126 plants and algae. J. Integr. Plant Biol. 2021, 63, 1888–1905. [Google Scholar] [CrossRef]
- Fan, Z.; Alley, A.; Ghaffari, K.; Ressom, H.W. MetFID: Artificial neural network-based compound fingerprint prediction for metabolite annotation. Metabolomics 2020, 16, 1–11. [Google Scholar]
- Hounguè, U.M.; Tokoudagba, M.; Gbaguidi, A.; Schini-Kerth, B. Carissa edulis phytochemical and pharmacological studies: A review article. Int. J. Sci. Acad. Res. 2022, 3, 3701–3706. [Google Scholar]
- Kim, S.; Cheng, T.; He, S.; Thiessen, P.A.; Li, Q.; Gindulyte, A.; Bolton, E.E. PubChem Protein, Gene, Pathway, and Taxonomy Data Collections: Bridging Biology and Chemistry through Target-Centric Views of PubChem Data. J. Mol. Biol. 2022, 434, 167514. [Google Scholar] [CrossRef] [PubMed]
- Pietzke, M.; Vazquez, A. Metabolite AutoPlotter—an application to process and visualise metabolite data in the web browser. Cancer Metab. 2020, 8, 15. [Google Scholar] [CrossRef]
- Pezzatti, J.; Boccard, J.; Codesido, S.; Gagnebin, Y.; Joshi, A.; Picard, D.; González-Ruiz, V.; Rudaz, S. Implementation of liquid chromatography–high resolution mass spectrometry methods for untargeted metabolomic analyses of biological samples: A tutorial. Anal. Chim. Acta 2020, 1105, 28–44. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; Li, H.; Yang, J.; Huang, J.; Zhang, P. Functional characterization of dihydroflavonol-4-reductase in anthocyanin biosynthesis of purple sweet potato underlies the direct evidence of anthocyanins function against abiotic stresses. PLoS ONE 2013, 8, e78484. [Google Scholar] [CrossRef]
- Houriet, J.; Vidar, W.S.; Manwill, P.K.; Todd, D.A.; Cech, N.B. How Low Can You Go? Selecting Intensity Thresholds for Untargeted Metabolomics Data Preprocessing. Anal. Chem. 2022, 94, 17964–17971. [Google Scholar] [CrossRef]
- Jiang, L.; Sullivan, H.; Wang, B. Principal component analysis (PCA) loading and statistical tests for nuclear magnetic resonance (NMR) metabolomics involving multiple study groups. Anal. Lett. 2022, 55, 1648–1662. [Google Scholar] [CrossRef]
- Fonville, J.M.; Richards, S.E.; Barton, R.H.; Boulange, C.L.; Ebbels, T.M.; Nicholson, J.K.; Holmes, E.; Dumas, M.E. The evolution of partial least squares models and related chemometric approaches in metabonomics and metabolic phenotyping. J. Chemom. 2010, 24, 636–649. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Bartel, J.; Krumsiek, J.; Theis, F.J. Statistical methods for the analysis of high-throughput metabolomics data. Comput. Struct. Biotechnol. J. 2013, 4, e201301009. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Dias, D.A. Metabolomics Tools for Natural Product Discovery; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of metabolomic data: Tools, current strategies and future challenges for omics data integration. Brief. Bioinform. 2017, 18, 498–510. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Sweeney, S.R.; Rodak, O.; Sentandreu, E.; Tiziani, S. R-metabolist 2: A flexible tool for metabolite annotation from high-resolution data-independent acquisition mass spectrometry analysis. Metabolites 2019, 9, 187. [Google Scholar] [CrossRef]
- Blaise, B.J.; Correia, G.D.; Haggart, G.A.; Surowiec, I.; Sands, C.; Lewis, M.R.; Pearce, J.T.; Trygg, J.; Nicholson, J.K.; Holmes, E. Statistical analysis in metabolic phenotyping. Nat. Protoc. 2021, 16, 4299–4326. [Google Scholar] [CrossRef]
- Böckel, A.; Hörisch, J.; Tenner, I. A systematic literature review of crowdfunding and sustainability: Highlighting what really matters. Manag. Rev. Q. 2021, 71, 433–453. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojärvi, J.; Voigt, A.Y. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, B.; Huan, T. Enhancing Metabolome Coverage in Data-Dependent LC-MS/MS Analysis through an Integrated Feature Extraction Strategy. Anal. Chem. 2019, 91, 14433–14441. [Google Scholar] [CrossRef] [PubMed]
- Perez de Souza, L.; Alseekh, S.; Naake, T.; Fernie, A. Mass Spectrometry-Based Untargeted Plant Metabolomics. Curr. Protoc. Plant Biol. 2019, 4, e20100. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.; Wang, M.; Nothias, L.F.; van der Hooft, J.J.J.; Caraballo-Rodriguez, A.M.; Fox, E.; Balunas, M.J.; Klassen, J.L.; Lopes, N.P.; Dorrestein, P.C. Propagating annotations of molecular networks using in silico fragmentation. PLoS Comput. Biol. 2018, 14, e1006089. [Google Scholar] [CrossRef]
- Ernst, M.; Kang, K.B.; Caraballo-Rodriguez, A.M.; Nothias, L.F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, J.; Kim, J.; Lee, J.; Yang, H. Investigation of Chemical Profiles of Different Parts of Morus alba Using a Combination of Molecular Networking Methods with Mass Spectral Data from Two Ionization Modes of LC/MS. Plants 2021, 10, 1711. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 Data-Preprocessing To Enhance Molecular Networking Reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef]
- Reveglia, P.; Raimondo, M.L.; Masi, M.; Cimmino, A.; Nuzzo, G.; Corso, G.; Fontana, A.; Carlucci, A.; Evidente, A. Untargeted and Targeted LC-MS/MS Based Metabolomics Study on In Vitro Culture of Phaeoacremonium Species. J. Fungi 2022, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Petras, D.; Schmid, R.; Duhrkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Duhrkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Bocker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Duhrkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef]
- Duhrkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Bocker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Dorrestein, P.C.; Duhrkop, K.; Bocker, S. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- Blatt-Janmaat, K.; Neumann, S.; Schmidt, F.; Ziegler, J.; Qu, Y.; Peters, K. Impact of in vitro phytohormone treatments on the metabolome of the leafy liverwort Radula complanata (L.) Dumort. Metabolomics 2023, 19, 17. [Google Scholar] [CrossRef]
- Blatt-Janmaat, K.L.; Neumann, S.; Ziegler, J.; Peters, K. Host Tree and Geography Induce Metabolic Shifts in the Epiphytic Liverwort Radula complanata. Plants 2023, 12, 571. [Google Scholar] [CrossRef]
- Peters, K.; Blatt-Janmaat, K.L.; Tkach, N.; van Dam, N.M.; Neumann, S. Untargeted Metabolomics for Integrative Taxonomy: Metabolomics, DNA Marker-Based Sequencing, and Phenotype Bioimaging. Plants 2023, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, S.; Jehan, P.; Olivier-Jimenez, D.; Lambert, F.; Boustie, J.; Lohezic-Le Devehat, F.; Le Yondre, N. New insights into the Van Krevelen diagram: Automated molecular formula determination from HRMS for a large chemical profiling of lichen extracts. Phytochem. Anal. 2022, 33, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. VENNY. An Interactive Tool for Comparing Lists with Venn Diagrams. 2007. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 10 June 2023).
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Kim, J.; Hong, S. Plant-derived polyphenol-based nanomaterials for drug delivery and theranostics. In Bioinspired and Biomimetic Materials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–54. [Google Scholar]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Kharazian, N.; Mohammadi, M. Flavonoid patterns and their diversity in ten Stachys L.(Lamiaceae) species from Iran. Prog. Biol. Sci. 2014, 4, 2. [Google Scholar]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea Catechins Protect against Lead-Induced Cytotoxicity, Lipid Peroxidation, and Membrane Fluidity in HepG2 Cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef]
- Zhu, W.; Deng, X.; Peng, J.; Zou, B.; Li, C. A-Type ECG and EGCG Dimers Inhibit 3T3-L1 Differentiation by Binding to Cholesterol in Lipid Rafts. J. Nutr. Biochem. 2017, 48, 62–73. [Google Scholar] [CrossRef]
- Natarajan, T.D.; Ramasamy, J.R.; Palanisamy, K. Nutraceutical Potentials of Synergic Foods: A Systematic Review. J. Ethn. Foods 2019, 6, 27. [Google Scholar] [CrossRef]
- Matysik, S.; Liebisch, G. Quantification of steroid hormones in human serum by liquid chromatography-high resolution tandem mass spectrometry. J. Chromatogr. A 2017, 1526, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Ling, Y.; Lin, Y.; Chang, J.; Chu, X. Analysis of additives in dairy products by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2014, 1336, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lanekoff, I.; Burnum-Johnson, K.; Thomas, M.; Cha, J.; Dey, S.K.; Yang, P.; Prieto Conaway, M.C.; Laskin, J. Three-dimensional imaging of lipids and metabolites in tissues by nanospray desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.C.; Edwards, A.V.; Verano-Braga, T.; Schwämmle, V.; Kjeldsen, F.; Jensen, O.N.; Larsen, M.R. High-performance hybrid Orbitrap mass spectrometers for quantitative proteome analysis: Observations and implications. Proteomics 2016, 16, 907–914. [Google Scholar] [CrossRef]
- Comtois-Marotte, S.; Chappuis, T.; Duy, S.V.; Gilbert, N.; Lajeunesse, A.; Taktek, S.; Desrosiers, M.; Veilleux, É.; Sauvé, S. Analysis of emerging contaminants in water and solid samples using high resolution mass spectrometry with a Q Exactive orbital ion trap and estrogenic activity with YES-assay. Chemosphere 2017, 166, 400–411. [Google Scholar] [CrossRef]
- Khammassi, M.; Mighri, H.; Mansour, M.B.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations wild-growing in Tunisia. South Afr. J. Bot. 2022, 148, 407–414. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Cavalcante, G.; da Silva Cabral, A.; Silva, C. Leishmanicidal Activity of Flavonoids Natural and Synthetic: A Minireview. Mintage J. Pharm. Med. Sci 2018, 7, 25–34. [Google Scholar]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Shan, X.; Cheng, J.; Chen, K.; Liu, Y.; Juan, L. Comparison of lipoxygenase, cyclooxygenase, xanthine oxidase inhibitory effects and cytotoxic activities of selected flavonoids. DEStech Trans. Environ. Energy Earth Sci 2017. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Pritzel, S.; Heiss, C.; Rodriguez-Mateos, A. Flavonoid intake and cardiovascular disease risk. Curr. Opin. Food Sci. 2015, 2, 92–99. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Pallangyo, P.; Nicholaus, P.; Kisenge, P.; Mayala, H.; Swai, N.; Janabi, M. A community-based study on prevalence and correlates of erectile dysfunction among Kinondoni District Residents, Dar Es Salaam, Tanzania. Reprod. Health 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Selvin, E.; Burnett, A.L.; Platz, E.A. Prevalence and risk factors for erectile dysfunction in the US. Am. J. Med. 2007, 120, 151–157. [Google Scholar] [CrossRef]
- Gupta, B.P.; Murad, M.H.; Clifton, M.M.; Prokop, L.; Nehra, A.; Kopecky, S.L. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: A systematic review and meta-analysis. Arch. Intern. Med. 2011, 171, 1797–1803. [Google Scholar] [CrossRef]
- Feldman, H.A.; Johannes, C.B.; Derby, C.A.; Kleinman, K.P.; Mohr, B.A.; Araujo, A.B.; McKinlay, J.B. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts male aging study. Prev. Med. 2000, 30, 328–338. [Google Scholar] [CrossRef]
- Romeo, J.H.; Seftel, A.D.; Madhun, Z.T.; Aron, D.C. Sexual function in men with diabetes type 2: Association with glycemic control. J. Urol. 2000, 163, 788–791. [Google Scholar] [CrossRef]
- Sipski, M.; Alexander, C. Sexual Function in People with Chronic Illness: A Health Professional’s Guide; Aspen Press: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Wing, R.R.; Rosen, R.C.; Fava, J.L.; Bahnson, J.; Brancati, F.; Gendrano III, I.N.C.; Kitabchi, A.; Schneider, S.H.; Wadden, T.A. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J. Sex. Med. 2010, 7, 156–165. [Google Scholar] [CrossRef]
- Barassi, A.; Colpi, G.M.; Piediferro, G.; Dogliotti, G.; Melzi D’Eril, G.V.; Corsi, M.M. Oxidative stress and antioxidant status in patients with erectile dysfunction. J. Sex. Med. 2009, 6, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Forest, C.; Padma-Nathan, H.; Liker, H. Efficacy and safety of pomegranate juice on improvement of erectile dysfunction in male patients with mild to moderate erectile dysfunction: A randomized, placebo-controlled, double-blind, crossover study. Int. J. Impot. Res. 2007, 19, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.S.; Wang, R.; Tsilidis, K.K.; Zhu, H.; Daniel, C.R.; Sinha, A.; Canfield, S. Role of caffeine intake on erectile dysfunction in US men: Results from NHANES 2001–2004. PLoS ONE 2015, 10, e0123547. [Google Scholar] [CrossRef] [PubMed]
- Dorrance, A.; Lewis, R.W.; Mills, T. Captopril treatment reverses erectile dysfunction in male stroke prone spontaneously hypertensive rats. Int. J. Impot. Res. 2002, 14, 494–497. [Google Scholar] [CrossRef]
- Ramírez, R.; Pedro-Botet, J.; García, M.; Corbella, E.; Merino, J.; Zambón, D.; Corbella, X.; Pintó, X.; the Xarxa de Unitats de Lípids i Arteriosclerosi (XULA) Investigators Group. Erectile dysfunction and cardiovascular risk factors in a Mediterranean diet cohort. Intern. Med. J. 2016, 46, 52–56. [Google Scholar] [CrossRef]
- Jang, D.J.; Lee, M.S.; Shin, B.C.; Lee, Y.C.; Ernst, E. Red ginseng for treating erectile dysfunction: A systematic review. Br. J. Clin. Pharmacol. 2008, 66, 444–450. [Google Scholar] [CrossRef]
- Wang, F.; Dai, S.; Wang, M.; Morrison, H. Erectile dysfunction and fruit/vegetable consumption among diabetic Canadian men. Urology 2013, 82, 1330–1335. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Di Francesco, S.; Tenaglia, R.L. Mediterranean diet and erectile dysfunction: A current perspective. Cent. Eur. J. Urol. 2017, 70, 185. [Google Scholar]
- Solomon, H.; Man, J.; Jackson, G. Erectile dysfunction and the cardiovascular patient: Endothelial dysfunction is the common denominator. Heart 2003, 89, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A review of various antioxidant compounds and their potential utility as complementary therapy in multiple sclerosis. Nutrients 2019, 11, 1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Q.; Gao, Y.; Granato, D. Effects of epigallocatechin gallate, epigallocatechin and epicatechin gallate on the chemical and cell-based antioxidant activity, sensory properties, and cytotoxicity of a catechin-free model beverage. Food Chem. 2021, 339, 128060. [Google Scholar] [CrossRef] [PubMed]
- Kim-Park, W.K.; Allam, E.S.; Palasuk, J.; Kowolik, M.; Park, K.K.; Windsor, L.J. Green tea catechin inhibits the activity and neutrophil release of Matrix Metalloproteinase-9. J. Tradit. Complement. Med. 2016, 6, 343–346. [Google Scholar] [CrossRef]
- Oz, H.S. Chronic inflammatory diseases and green tea polyphenols. Nutrients 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological actions and underlying mechanisms of catechin: A review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Kalender, S.; Kalender, Y.; Ates, A.; Yel, M.; Olcay, E.; Candan, S. Protective role of antioxidant vitamin E and catechin on idarubicin-induced cardiotoxicity in rats. Braz. J. Med. Biol. Res. 2002, 35, 1379–1387. [Google Scholar] [CrossRef]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2020; ISBN 9781839625763. [Google Scholar]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of grape seed extract and proanthocyanidin B2 on in vitro proliferation, viability, steroidogenesis, oxidative stress, and cell signaling in human granulosa cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef]
- Lee, K.E.; Bharadwaj, S.; Yadava, U.; Kang, S.G. Computational and in vitro investigation of (-)-epicatechin and proanthocyanidin B2 as inhibitors of human matrix metalloproteinase 1. Biomolecules 2020, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Q.; Chen, Y.; Duan, M.; Tian, G.; Deng, X.; Sun, Y.; Zhou, T.; Zhang, G.; Chen, W. Inhibition of proanthocyanidin A2 on porcine reproductive and respiratory syndrome virus replication in vitro. PLoS ONE 2018, 13, e0193309. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Plumb, G.W.; Uda, Y.; Price, K.R.; Rhodes, M.J.C. Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis 1996, 17, 2385–2387. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Acute effects of quercetin-3-O-glucoside on endothelial function and blood pressure: A randomized dose-response study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Choi, S.-J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-H.; Jang, H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, O.-H.; Sung, M.-K. Effects of phenol-depleted and phenol-rich diets on blood markers of oxidative stress, and urinary excretion of quercetin and kaempferol in healthy volunteers. J. Am. Coll. Nutr. 2003, 22, 217–223. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise. Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Kaur, P.; Shukla, S.; Gupta, S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: An in vitro and in vivo study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Negri, E.; Lagiou, P.; Talamini, R.; Dal Maso, L.; Montella, M.; Franceschi, S.; La Vecchia, C. Flavonoids and ovarian cancer risk: A case–control study in Italy. Int. J. Cancer 2008, 123, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Tahirovic, A.; Basic, N. Determination of phenolic content and antioxidant properties of methanolic extracts from Viscum album ssp. album Beck. Bull. Chem. Technol. Bosnia Herzeg. 2017, 49, 25–30. [Google Scholar]
- Augustine, C. Unravelling the competence of leucocyanidin in free radical scavenging: A theoretical approach based on electronic structure calculations. J. Struct. Chem. 2019, 60, 198–209. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A.; Butnariu, M. Screening of bioflavonoid and antioxidant activity of lens culinaris medikus. Dig. J. Nanomater. Biostruct. (DJNB) 2014, 9, 519–529. [Google Scholar]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Barouh, N.; Durand, E.; Baréa, B.; Robert, M.; Micard, V.; Lullien-Pellerin, V.; Villeneuve, P.; Cameron, L.C.; Ryan, E.P. Metabolomics of pigmented rice coproducts applying conventional or deep eutectic extraction solvents reveal a potential antioxidant source for human nutrition. Metabolites 2021, 11, 110. [Google Scholar] [CrossRef]
- Jiang, B.; Song, J.; Jin, Y. A flavonoid monomer tricin in Gramineous plants: Metabolism, bio/chemosynthesis, biological properties, and toxicology. Food Chem. 2020, 320, 126617. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Yin, H.; Du, B.; Niu, K.; Yang, Y.; Wang, S. Anti-inflammatory effect of flavonoids from chestnut flowers in lipopolysaccharide-stimulated RAW 264.7 macrophages and acute lung injury in mice. J. Ethnopharmacol. 2022, 290, 115086. [Google Scholar] [CrossRef] [PubMed]
- Şahin, T.D.; Gocmez, S.S.; Duruksu, G.; Yazir, Y.; Utkan, T. Resveratrol and quercetin attenuate depressive-like behavior and restore impaired contractility of vas deferens in chronic stress-exposed rats: Involvement of oxidative stress and inflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. IJPB 2017, 3, 1–9. [Google Scholar]
- Hsu, R.-J.; Hsu, Y.-C.; Chen, S.-P.; Fu, C.-L.; Yu, J.-C.; Chang, F.-W.; Chen, Y.-H.; Liu, J.-M.; Ho, J.-Y.; Yu, C.-P. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Damle, A.A.; Pawar, Y.P.; Narkar, A.A. Anticancer Activity of Betulinic Acid on MCF-7 Tumors in Nude Mice; NISCAIR-CSIR: New Delhi, India, 2013. [Google Scholar]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Luk, C.-Y.; Ludidi, N.; Chung, G.; Chen, S.-P.T.; Lam, H.-M. Chapter Ten—Soybean secondary metabolites and flavors: The art of compromise among climate, natural enemies, and human culture. In Advances in Botanical Research; Lam, H.-M., Li, M.-W., Eds.; Academic Press: New York, NY, USA, 2022; Volume 102, pp. 295–347. [Google Scholar]
- Vásquez, S.M.; Abascal, G.G.W.; Leal, C.E.; Cardineau, G.A.; Lara, S.G. Application of metabolic engineering to enhance the content of alkaloids in medicinal plants. Metab. Eng. Commun. 2022, 14, e00194. [Google Scholar] [CrossRef] [PubMed]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An update of the sanguinarine and benzophenanthridine alkaloids’ biosynthesis and their applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef]
- Khalili, M.; Alavi, M.; Esmaeil-Jamaat, E.; Baluchnejadmojarad, T.; Roghani, M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int. Immunopharmacol. 2018, 61, 355–362. [Google Scholar] [CrossRef]
- Marie, F.; Benjamin, H.; Marie, V.; Marie, P.; Robert, C.; Violeta, R.; Hélène, V.; Audrey, L.; Gilles, J.G.; Loic, Y.; et al. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS ONE 2016, 11, e0158051. [Google Scholar] [CrossRef]
- Gümüş, M.; Yakan, M.; Koca, İ. Recent advances of thiazole hybrids in biological applications. Future Med. Chem. 2019, 11, 1979–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-R.; Yang, J.-W.; Ji, C.-S.; Zeng, X.-H.; Shi, G.-X.; Fisher, M.; Liu, C.-Z. Inhibition of NADPH oxidase–dependent oxidative stress in the rostral ventrolateral medulla mediates the antihypertensive effects of acupuncture in spontaneously hypertensive rats. Hypertension 2018, 71, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-B.; Li, Y.-Z.; Huang, S.; Chen, L.; Luo, Y.-Y.; Gao, F.; Zhou, X.-L. Diterpenoid alkaloids from the whole herb of Delphinium grandiflorum L. Phytochemistry 2021, 190, 112866. [Google Scholar] [CrossRef]
- Shuveksh, P.S.; Ahmed, K.; Padhye, S.; Schobert, R.; Biersack, B. Chemical and biological aspects of the natural 1,4-benzoquinone embelin and its (semi-) synthetic derivatives. Curr. Med. Chem. 2017, 24, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11—Terpenoids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.T.; Liu, L.; Rose, T.J.; Waters, D.L.; Benkendorff, K. Arbuscular mycorrhizal fungi: Effects on plant terpenoid accumulation. Plant Biol. 2016, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.J.H.; Freschi, L.; Ferrari, R.C.; Peroni-Okita, F.H.G.; Cordenunsi-Lysenko, B.R. Exploring the significance of photosynthetic activity and carbohydrate metabolism in peel tissues during banana fruit ripening. Sci. Hortic. 2022, 295, 110811. [Google Scholar] [CrossRef]
- Jafari, S.M. A Review of Potential Anti-Cancer Effect of Sesquiterpene Lactones in Breast Cancer. Jorjani Biomed. J. 2022, 10, 48–59. [Google Scholar]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 105817. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Olabiyi, A.A.; de Castro Brás, L.E. Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions. Biomedicines 2023, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Jetten, A.M. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010, 287, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Y.; Chen, Y.; Xiao, L.; Zhang, X.; Yang, C.; Li, Z.; Zhu, M.; Liu, Z.; Wang, Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS–SPME/GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef] [PubMed]
- Durak, A.; Gawlik-Dziki, U.; Pecio, Ł. Coffee with cinnamon–Impact of phytochemicals interactions on antioxidant and anti-inflammatory in vitro activity. Food Chem. 2014, 162, 81–88. [Google Scholar] [CrossRef]
- Drevin, G.; Palayer, M.; Compagnon, P.; Zabet, D.; Jousset, N.; Briet, M.; Abbara, C. A fatal case report of acute yohimbine intoxication. Forensic Toxicol. 2020, 38, 287–291. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.; Leiria, L.O.; Alexandre, E.C.; Davel, A.P.C.; Mónica, F.Z.; De Nucci, G.; Antunes, E. Prolonged therapy with the soluble guanylyl cyclase activator BAY 60-2770 restores the erectile function in obese mice. J. Sex. Med. 2014, 11, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Porcel, M.; Chade, A.; Miller, J. Studies on atherosclerosis. In Oxidative Stress in Applied Basic Research and Clinical Practice; Springer: Berlin, Germany, 2017; Volume 10, pp. 971–978. [Google Scholar]
- Judkins, C.P.; Diep, H.; Broughton, B.R.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef] [PubMed]
- Ieda, N.; Hotta, Y.; Yamauchi, A.; Nishikawa, A.; Sasamori, T.; Saitoh, D.; Kawaguchi, M.; Kimura, K.; Nakagawa, H. Development of a red-light-controllable nitric oxide releaser to control smooth muscle relaxation in vivo. ACS Chem. Biol. 2020, 15, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Roychoudhury, S. Pathological roles of reactive oxygen species in male reproduction. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility-Volume One; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–62. [Google Scholar]
- Zhou, B.; Chen, Y.; Yuan, H.; Wang, T.; Feng, J.; Li, M.; Liu, J. NOX1/4 inhibitor GKT-137831 improves erectile function in diabetic rats by ROS reduction and endothelial nitric oxide synthase reconstitution. J. Sex. Med. 2021, 18, 1970–1983. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Kurnik-Łucka, M.; Panula, P.; Bugajski, A.; Gil, K. Salsolinol: An unintelligible and double-faced molecule—Lessons learned from in vivo and in vitro experiments. Neurotox. Res. 2018, 33, 485–514. [Google Scholar] [CrossRef]
- Melis, M.; Carboni, E.; Caboni, P.; Acquas, E. Key role of salsolinol in ethanol actions on dopamine neuronal activity of the posterior ventral tegmental area. Addict. Biol. 2015, 20, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Chen, W. Chemical constituents from Illicium brevistylum and their anti-inflammatory activities. Chin. Tradit. Pat. Med. 2017, 2081–2085. [Google Scholar]
- Lee, B.; Weon, J.B.; Eom, M.R.; Jung, Y.S.; Ma, C.J. Neuroprotective compounds of Tilia amurensis. Pharmacogn. Mag. 2015, 11, S303. [Google Scholar]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green tea and epigallocatechin gallate (EGCG) for the management of nonalcoholic fatty liver diseases (NAFLD): Insights into the role of oxidative stress and antioxidant mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, J.; Li, W.; Wu, W. The color difference of rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT 2021, 149, 111825. [Google Scholar] [CrossRef]
- Reshef, N.; Hayari, Y.; Goren, C.; Boaz, M.; Madar, Z.; Knobler, H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005, 18, 1360–1363. [Google Scholar] [CrossRef]
- Onakpoya, I.; O’Sullivan, J.; Heneghan, C.; Thompson, M. The effect of grapefruits (Citrus paradisi) on body weight and cardiovascular risk factors: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 602–612. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L. Variations in ellagic acid, quercetin and myricetin in berry cultivars after preharvest methyl jasmonate treatments. J. Food Compos. Anal. 2015, 39, 55–61. [Google Scholar] [CrossRef]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Matsuda, M.; Kauss, T.; Al-Kharrat, A.; Rambert, J.; Fawaz, F.; Thiolat, D.; Moynet, D.; Coves, S.; Malvy, D.; Mossalayi, M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 2006, 72, 1304–1310. [Google Scholar] [CrossRef] [PubMed]




| Tentative Identification | Molecular Formula | Adduct | Precursor m/z | MS2 | NP Superclass |
|---|---|---|---|---|---|
| (R)-1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquinoline-6,7-diol | C16H17NO4 | M+H | 288.12 | 123.04, 143.05, 149.00, 161.06, 164.0, 225.09 | Alkaloids |
| 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (Salsolinol) | C18H24N2O3 | M+H | 180.102 | 117.07, 137.06, 145.0, 151.07 | Alkaloids |
| Cinchonidine | C19H22N2O | M-H | 293.166 | 59.01, 96.96 | Alkaloids |
| Coniferyl aldehyde | C10H10O3 | M+H | 179.07 | 55.02, 91.06, 105.07, 119.05, 123.04, 147.0 | Cinnamic acids and derivatives |
| 3-Hydroxy-4-methoxycinnamic acid | C10H10O4 | M+H-H2O | 177.054 | 89.05, 117.03, 145.0, 149.06 | Cinnamic acids and derivatives |
| 6-Methylcoumarin | C10H8O2 | M+H | 161.06 | 91.06, 105.07, 115.06, 119.0 | Coumarins |
| (2R,3R,4S,5S,6R)-2-[[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | C27H30O13 | M+H | 453.139 | 85.03, 123.04, 139.0, 163.04, 205.05, 273.08 | Flavonoids |
| Kaempferol * | C15H10O6 | M+H | 287.055 | 153.02, 165.02, 213.06, 287.0 | Flavonoids |
| Quercetin | C15H10O7 | M+H | 303.049 | 137.02, 151.0, 165.02, 229.05, 257.04 | Flavonoids |
| M-H | 301.035 | 121.02, 151.0, 179.00 | |||
| Maesopsin | C15H12O6 | M-H | 287.056 | 57.03, 83.01, 125.0, 151.00, 215.07, 259.06 | Flavonoids |
| (-)-Epicatechin | C15H14O6 | M+H | 291.086 | 115.0, 123.04, 139.04 | Flavonoids |
| (+)-Catechin | 123.04, 139.0, 147.04, 161.06, 165.05, 179.07, 207.07 | Flavonoids | |||
| Epigallocatechin | C15H14O7 | M+H | 307.081 | 84.08, 111.04, 123.04, 139.0, 151.04 | Flavonoids |
| Gallocatechin | M-H | 305.066 | 109.3, 125.0, 137.02, 161.02, 165.02, 219.07 | Flavonoids | |
| Apigetrin [Cosmosiine] | C21H20O10 | M+H | 433.113 | 256.73, 271.0 | Flavonoids |
| Astragalin [Kaempferol-3-O-glucoside] | C21H20O11 | M+H * | 449.108 | 85.03, 153.02, 287.0 | Flavonoids |
| M-H | 447.091 | 227.03, 255.03, 284.0 | Flavonoids | ||
| Kaempferol-4-glucoside | M+H | 449.108 | 85.03, 97.03, 127.04, 287.0 | Flavonoids | |
| Isoquercetin * | C21H20O12 | M-H | 463.088 | 151.00, 255.03, 271.02, 300.0 | Flavonoids |
| 1-(4-hydroxy-3-methoxyphenyl)-6-methoxy-2,3-dimethyl-3,4-dihydro-1H-naphthalene-2,7-diol [6-Methoxyluteolin] * | C21H22O6 | M+H-H2O | 327.158 | 137.06, 151.0, 171.08, 203.11 | Flavonoids |
| Phlorizin [3-(beta-D-glucopyranosyloxy)-2-methyl-4H-Pyran-4-one] | C21H24O10 | M-H | 435.13 | 125.02, 167.0, 179.03, 273.08 | Flavonoids |
| M+H * | 289.092 | 85.03, 127.0 | Flavonoids | ||
| (-)-Catechin gallate | C22H18O10 | M+H | 443.096 | 123.04, 139.0, 153.02, 273.07 | Flavonoids |
| Epicatechin gallate | M-H | 441.08 | 109.03, 125.02, 169.0, 193.01, 245.08, 289.07 | Flavonoids | |
| M+H * | 443.097 | 139.0, 153.02, 165.05, 273.07, 291.09 | Flavonoids | ||
| [6-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-3-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl 3,4,5-trihydroxybenzoate | C28H24O15 | M-H | 599.105 | 125.02, 151.00, 169.0, 284.03, 285.04, 313.06 | Flavonoids |
| Procyanidin B1 | C30H26O12 | M-H | 577.135 | 109.0, 125.0, 161.03, 203.07, 245.08, 289.07, 407.08 | Flavonoids |
| Procyanidin B2 | M+H | 579.15 | 127.0, 139.04, 191.03, 233.04, 247.06, 271.06, 287.06, 409.09 | Flavonoids | |
| Isatin | C8H5NO2 | M+H | 148.04 | 92.05, 120.0 | Indoles and derivatives |
| [3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyl)oxyoxan-2-yl]methyl3,4,5-trihydroxybenzoate | C20H20O14 | M-H | 483.078 | 125.02, 151.01, 169.0, 211.02, 271.04, 313.07 | Phenolic acids |
| 3,4-Dihydroxy-5-[(6-O-{[4-(2-hydroxy-2-propanyl)-1-cyclohexen-1-yl]carbonyl}-beta-D-glucopyranosyl)oxy]benzoic acid | C23H30O12 | M-H | 497.166 | 125.02, 169.0, 313.06, 331.07 | Phenolic acids |
| Gallic acid | C7H6O5 | M+H-H2O | 153.018 | 79.02, 97.03, 107.0, 125.02 | Phenolic acids |
| M-H * | 169.014 | 81.03, 97.03, 125.0, 169.01 | |||
| 4-Allyl-2,6-dimethoxyphenol (Eugenol) * | C10H12O3 | M+H | 195.102 | 107.0, 135.08, 154.06, 163.07, 195.10 | Phenolic compounds |
| Genipin * | C11H14O5 | M+H-H2O | 209.081 | 121.0, 149.06, 177.06, 181.05, 209.08 | Phenolic compounds |
| Coumaroyl quinic acid * | C16H18O9 | M-H | 337.092 | 93.03, 163.04, 173.04, 191.0 | Phenolic compounds |
| Protocatechuic acid * | C7H6O4 | M-H | 137.024 | 93.03, 108.02, 109.03, 137.0 | Phenolic compounds |
| p-Coumaric acid * | C9H8O3 | M+H-H2O | 147.044 | 91.05, 119.0, 123.96, 147.04 | Phenolic compounds |
| Methyl gallate * | C8H8O5 | M+H | 185.045 | 113.01, 126.03, 153.02, 185.0 | Phenolic compounds (gallotannins) |
| Cuminyl alcohol * | C10H12O2 | M+H-H2O | 133.101 | 91.05, 105.07, 118.08, 133.1 | Phenolic compounds (phenylpropanoids) |
| Alpha-Pinene * | C10H16 | M+H | 137.13 | 67.05, 79.05, 81.07, 95.09, 109.10, 137.10 | Terpenoids |
| 1S,2S,6R,7R,9R)-6-methyl-10,12-dioxatricyclo [7.2.1.0<2,7>]dodec-4-en-8-one [Ascaridole] * | C10H16O2 | M+H | 195.102 | 95.05, 123.08, 149.06, 167.07 | Terpenoids |
| (1R)-(-)-Nopol | C11H18O | M+H-H2O | 149.132 | 65.04, 81.07, 93.07, 107.09, 121.0 | Terpenoids |
| 3-(beta-D-glucopyranosyloxy)-2-methyl-4H-Pyran-4-one | C13H20O8 | M+H | 289.092 | 97.03, 127.0 | Terpenoids |
| 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)- 2-Butanone | C15H22O | M+H | 177.164 | 79.06, 81.07, 93.07, 95.09, 107.09, 1121.1, 149.06 | Terpenoids |
| Farnesol * | C15H26O | M+H | 223.205 | 69.07, 95.09, 83.09, 109.10, 121.10, 135.12, 205.20, 223.21 | Terpenoids |
| Betulinic acid | C30H48O3 | M+H-H2O | 439.357 | 57.07, 81.07, 95.09, 109.10, 123.12, 137.13, 189.16, 241.19, 255.21 | Terpenoids |
| Betulonic acid | C30H48O4 | M+H-H2O | 437.341 | 69.07, 95.09, 107.09, 121.10, 135.12, 189.16, 203.18, 215.18, 241.19, 255.21, 391.33 | Terpenoids |
| Sumaresinolic acid | C30H48O4 | M+H-H2O | 455.352 | 57.07, 81.07, 95.09, 109.10, 137.13, 189.16, 203.18, 391.33, 409.35 | Terpenoids |
| Betulin | C30H50O2 | M+H-H2O | 425.378 | 67.06, 81.07, 95.09, 109.10, 123.12, 137.13, 189.16, 201.16,215.18, 227.18, 255.21, 269.23 | Terpenoids |
| Tentative Identification | Molecular Formula | Adduct | Precursor m/z | MS2 | NP Superclass |
|---|---|---|---|---|---|
| Epicatechin gallate | C22H18O10 | M+H | 443.0972 | 139.039; 153.0184; 165.0548 | Flavonoids (catechin gallates) |
| Quercetin | C15H10O7 | M+H | 303.0499 | 137.0240, 229.0488 | Flavonoids (flavonols) |
| Methylophiopogonanone A * | C19H18O6 | M+Na | 365.1052 | 185.0422; 203.0528; 365.1057 | Flavonoids (homoisoflavanones) |
| Emodin * | C15H10O5 | M-H | 269.0452 | 201.0566; 241.0503; 269.0546 | Phenolic compounds (hydroxyanthraquinones) |
| Catechol | C6H6O2 | M-H | 109.0293 | 81.0345, 91.0189, 108.0217, 109.0294 | Phenolic compounds |
| 3-Coumaric acid * | C9H8O3 | M-H | 163.0402 | 119.0499, 163.0034 | Phenolic compounds (hydroxycinnamic acids) |
| p-Coumaric acid | C9H8O3 | M+H | 165.0546 | 91.0540, 95.0858, 119.0493, 147.0442 | Phenolic compounds (hydroxycinnamic acids) |
| Caffeic acid * | C9H8O4 | M-H-CO2 | 135.0451 | 107.0499, 135.0450 | Phenolic compounds (hydroxycinnamic acids) |
| 2-[(2S,4aR,8aS)-2-hydroxy-4a-methyl-8-methylidene-3,4,5,6,7,8a-hexahydro-1H-naphthalen-2-yl]prop-2-enoic acid * | C15H22O3 | M-H | 249.1495 | 205.1594, 249.1494 | Terpenoids (eudesmane, isoeudesmane, or cyclic terpenoids) |
| Saikikogenin D * | C30H48O4 | M+H | 473.3625 | 473.3460 (only MS1 available) | Terpenoids (triterpenoids) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, E.; Lewis, A.; Narine, S.S.; Emery, R.J.N. Unlocking Potentially Therapeutic Phytochemicals in Capadulla (Doliocarpus dentatus) from Guyana Using Untargeted Mass Spectrometry-Based Metabolomics. Metabolites 2023, 13, 1050. https://doi.org/10.3390/metabo13101050
Smith E, Lewis A, Narine SS, Emery RJN. Unlocking Potentially Therapeutic Phytochemicals in Capadulla (Doliocarpus dentatus) from Guyana Using Untargeted Mass Spectrometry-Based Metabolomics. Metabolites. 2023; 13(10):1050. https://doi.org/10.3390/metabo13101050
Chicago/Turabian StyleSmith, Ewart, Ainsely Lewis, Suresh S. Narine, and R. J. Neil Emery. 2023. "Unlocking Potentially Therapeutic Phytochemicals in Capadulla (Doliocarpus dentatus) from Guyana Using Untargeted Mass Spectrometry-Based Metabolomics" Metabolites 13, no. 10: 1050. https://doi.org/10.3390/metabo13101050









