Brain Regulation of Cardiac Function during Hypoglycemia
Abstract
:1. Introduction
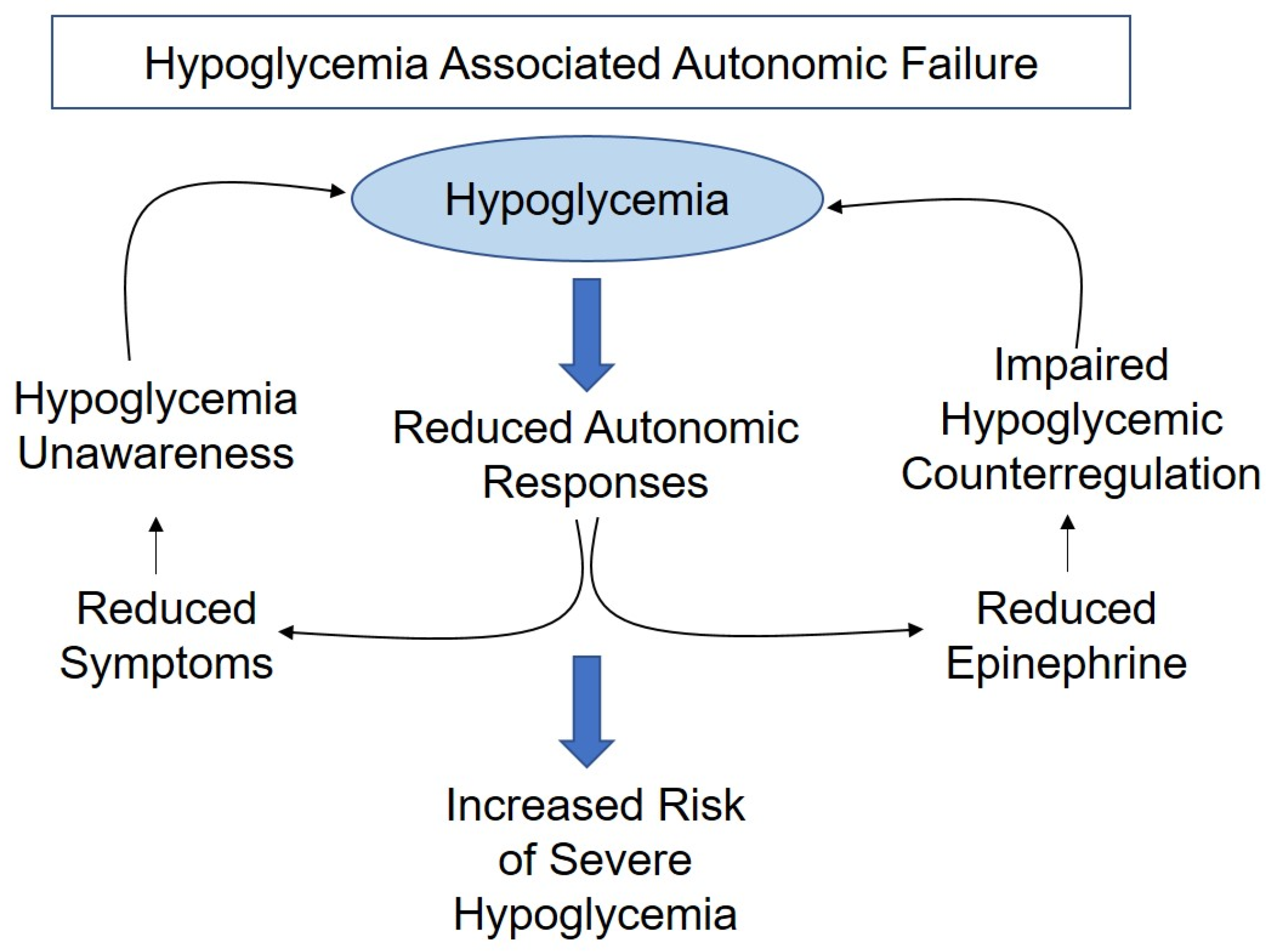
1.1. Impaired Awareness of Hypoglycemia
1.2. Severe Hypoglycemia
2. Methods
3. Hypoglycemia and the Counterregulatory Response
4. Brain Connection to the Heart
5. Sympathetic vs. Parasympathetic
6. Hypoglycemia-Induced Cardiac Arrhythmias
6.1. Sympathetic Nervous System and Cardiac Arrhythmias
6.2. Parasympathetic Nervous System and Cardiac Arrhythmias
6.3. Other Factors Involved in Cardiac Arrhythmias
6.3.1. Potassium
6.3.2. Sodium
6.3.3. Calcium
7. Hypoglycemia’s Alteration of Cardiac Function
7.1. Hemodynamic
7.2. Prothrombotic
7.3. Pro-Inflammatory
7.4. Oxidative Stress
8. Clamp Studies vs. Spontaneous Hypoglycemia Studies
8.1. Clamp Studies and ECG
8.2. Spontaneous Hypoglycemia Studies and ECG
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Cryer, P.E. The Barrier of Hypoglycemia in Diabetes. Diabetes 2008, 57, 3169–3176. [Google Scholar] [CrossRef]
- Frier, B.M. Hypoglycaemia in diabetes mellitus: Epidemiology and clinical implications. Nat. Rev. Endocrinol. 2014, 10, 711–722. [Google Scholar] [CrossRef]
- Group, U.K.H.S. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007, 50, 1140–1147. [Google Scholar] [CrossRef]
- Khunti, K.; Alsifri, S.; Aronson, R.; Berkovic, C.; Enters-Weijnen, C.; Forsen, T.; Galstyan, G.; Geelhoed-Duijvestijn, P.; Goldfracht, M.; Gydesen, H.; et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: The global HAT study. Diabetes Obes. Metab. 2016, 18, 907–915. [Google Scholar] [CrossRef]
- Agiostratidou, G.; Anhalt, H.; Ball, D.; Blonde, L.; Gourgari, E.; Harriman, K.N.; Kowalski, A.J.; Madden, P.; McAuliffe-Fogarty, A.H.; McElwee-Malloy, M.; et al. Standardizing Clinically Meaningful Outcome Measures Beyond HbA(1c) for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017, 40, 1622–1630. [Google Scholar] [CrossRef]
- Cryer, P.E. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes 1992, 41, 255–260. [Google Scholar] [CrossRef]
- Terpstra, M.; Moheet, A.; Kumar, A.; Eberly, L.E.; Seaquist, E.; Oz, G. Changes in human brain glutamate concentration during hypoglycemia: Insights into cerebral adaptations in hypoglycemia-associated autonomic failure in type 1 diabetes. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 876–882. [Google Scholar] [CrossRef]
- Routh, V.H. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes/Metab. Res. Rev. 2003, 19, 348–356. [Google Scholar] [CrossRef]
- American Diabetes, A. Glycemic Targets. Diabetes Care 2015, 38, S33–S40. [Google Scholar] [CrossRef]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef]
- Terry, T.; Raravikar, K.; Chokrungvaranon, N.; Reaven, P.D. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr. Cardiol. Rep. 2012, 14, 79–88. [Google Scholar] [CrossRef]
- Turner, R.C.; Holman, R.R.; Stratton, I.M.; Cull, C.A.; Matthews, D.R.; Manley, S.E.; Frighi, V.; Wright, D.; Neil, A.; Kohner, E.; et al. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Feltbower, R.G.; Bodansky, H.J.; Patterson, C.C.; Parslow, R.C.; Stephenson, C.R.; Reynolds, C.; McKinney, P.A. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: Results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 2008, 31, 922–926. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group; Jacobson, A.M.; Musen, G.; Ryan, C.M.; Silvers, N.; Cleary, P.; Waberski, B.; Burwood, A.; Weinger, K.; Bayless, M.; et al. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007, 356, 1842–1852. [Google Scholar] [CrossRef]
- Skrivarhaug, T.; Bangstad, H.J.; Stene, L.C.; Sandvik, L.; Hanssen, K.F.; Joner, G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006, 49, 298–305. [Google Scholar] [CrossRef]
- Secrest, A.M.; Becker, D.J.; Kelsey, S.F.; Laporte, R.E.; Orchard, T.J. Characterizing sudden death and dead-in-bed syndrome in Type 1 diabetes: Analysis from two childhood-onset Type 1 diabetes registries. Diabet. Med. A J. Br. Diabet. Assoc. 2011, 28, 293–300. [Google Scholar] [CrossRef]
- Tattersall, R.B.; Gill, G.V. Unexplained deaths of type 1 diabetic patients. Diabet. Med. 1991, 8, 49–58. [Google Scholar] [CrossRef]
- Tu, E.; Twigg, S.M.; Duflou, J.; Semsarian, C. Causes of death in young Australians with type 1 diabetes: A review of coronial postmortem examinations. Med. J. Aust. 2008, 188, 699–702. [Google Scholar] [CrossRef]
- O’Reilly, M.; O’Sullivan, E.P.; Davenport, C.; Smith, D. “Dead in bed”: A tragic complication of type 1 diabetes mellitus. Ir. J. Med. Sci. 2010, 179, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Tanenberg, R.J.; Newton, C.A.; Drake, A.J. Confirmation of Hypoglycemia in the “Dead-in-Bed” Syndrome, as Captured by a Retrospective Continuous Glucose Monitoring System. Endocr. Pract. 2010, 16, 244–248. [Google Scholar] [CrossRef]
- Patel, F. Diabetic death bed: Post-mortem determination of hypoglycaemia. Med. Sci. Law 1994, 34, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D. Cardiovascular autonomic neuropathy: Clinical manifestations and measurement. Diabetes Rev. 1999, 7, 342–357. [Google Scholar]
- International Hypoglycaemia Study, G. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019, 7, 385–396. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R.; et al. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. J. Clin. Endocrinol. Metab. 2013, 98, 1845–1859. [Google Scholar] [CrossRef]
- Bisgaard Bengtsen, M.; Moller, N. Mini-review: Glucagon responses in type 1 diabetes—A matter of complexity. Physiol. Rep. 2021, 9, e15009. [Google Scholar] [CrossRef]
- Lopez-Gambero, A.J.; Martinez, F.; Salazar, K.; Cifuentes, M.; Nualart, F. Brain Glucose-Sensing Mechanism and Energy Homeostasis. Mol. Neurobiol. 2019, 56, 769–796. [Google Scholar] [CrossRef]
- Coll, A.P.; Yeo, G.S. The hypothalamus and metabolism: Integrating signals to control energy and glucose homeostasis. Curr. Opin. Pharmacol. 2013, 13, 970–976. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Figlewicz, D.P.; Baskin, D.G.; Woods, S.C.; Porte, D., Jr. Insulin in the brain: A hormonal regulator of energy balance. Endocr. Rev. 1992, 13, 387–414. [Google Scholar] [CrossRef]
- Robinson, R.T.; Harris, N.D.; Ireland, R.H.; Lee, S.; Newman, C.; Heller, S.R. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003, 52, 1469–1474. [Google Scholar] [CrossRef]
- Chow, E.; Bernjak, A.; Walkinshaw, E.; Lubina-Solomon, A.; Freeman, J.; Macdonald, I.A.; Sheridan, P.J.; Heller, S.R. Cardiac Autonomic Regulation and Repolarization During Acute Experimental Hypoglycemia in Type 2 Diabetes. Diabetes 2017, 66, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.M.; Watts, A.G. Peripheral and Central Glucose Sensing In Hypoglycemic Detection. Physiology 2014, 29, 314–324. [Google Scholar] [CrossRef]
- Watts, A.G.; Donovan, C.M. Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Front. Neuroendocr. 2010, 31, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Meynell, A.A.; Routh, V.H.; Kang, L.; Gaspers, L.; Levin, B.E. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 2002, 51, 2056–2065. [Google Scholar] [CrossRef]
- Guan, H.Z.; Dong, J.; Jiang, Z.Y.; Chen, X. alpha-MSH Influences the Excitability of Feeding-Related Neurons in the Hypothalamus and Dorsal Vagal Complex of Rats. Biomed. Res. Int. 2017, 2017, 2034691. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Hentges, S.T.; Dunn-Meynell, A.A.; Levin, B.E.; Wang, W.; Routh, V.H. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 2004, 53, 1959–1965. [Google Scholar] [CrossRef]
- Katafuchi, T.; Oomura, Y.; Yoshimatsu, H. Single Neuron Activity in the Rat Lateral Hypothalamus during 2-Deoxy-D-Glucose Induced and Natural Feeding-Behavior. Brain Res. 1985, 359, 1–9. [Google Scholar] [CrossRef]
- Zhu, J.N.; Guo, C.L.; Li, H.Z.; Wang, J.J. Dorsomedial hypothalamic nucleus neurons integrate important peripheral feeding-related signals in rats. J. Neurosci. Res. 2007, 85, 3193–3204. [Google Scholar] [CrossRef]
- Funahashi, M.; Adachi, A. Glucose-Responsive Neurons Exist within the Area Postrema of the Rat—In-Vitro Study on the Isolated Slice Preparation. Brain Res. Bull. 1993, 32, 531–535. [Google Scholar] [CrossRef]
- Mimee, A.; Ferguson, A.V. Glycemic state regulates melanocortin, but not nesfatin-1, responsiveness of glucose-sensing neurons in the nucleus of the solitary tract. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R690–R699. [Google Scholar] [CrossRef]
- Flak, J.N.; Goforth, P.B.; Dell’Orco, J.; Sabatini, P.V.; Li, C.E.; Bozadjieva, N.; Sorensen, M.; Valenta, A.; Rupp, A.; Affinati, A.H.; et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J. Clin. Investig. 2020, 130, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Meek, T.H.; Nelson, J.T.; Matsen, M.E.; Dorfman, M.D.; Guyenet, S.J.; Damian, V.; Allison, M.B.; Scarlett, J.M.; Nguyen, H.T.; Thaler, J.P.; et al. Functional identification of a neurocircuit regulating blood glucose. Proc. Natl. Acad. Sci. USA 2016, 113, E2073–E2082. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.L.; Matsen, M.E.; Velasco, K.R.; Damian, V.; Phan, B.A.; Adam, D.; Therattil, A.; Schwartz, M.W.; Morton, G.J. Distinct Neuronal Projections From the Hypothalamic Ventromedial Nucleus Mediate Glycemic and Behavioral Effects. Diabetes 2018, 67, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Routh, V.H.; Kuzhikandathil, E.V.; Gaspers, L.D.; Levin, B.E. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004, 53, 549–559. [Google Scholar] [CrossRef]
- Routh, V.H. Glucose Sensing Neurons in the Ventromedial Hypothalamus. Sensors 2010, 10, 9002–9025. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Shaw, M.; Fan, X.N.; Cheng, H.Y.; Ding, Y.Y.; Vella, M.C.; Zhou, L.G.; Mcnay, E.C.; Sherwin, R.S. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008, 57, 444–450. [Google Scholar] [CrossRef]
- Fioramonti, X.; Marsollier, N.; Song, Z.T.; Fakira, K.A.; Patel, R.M.; Brown, S.; Duparc, T.; Pica-Mendez, A.; Sanders, N.M.; Knauf, C.; et al. Ventromedial Hypothalamic Nitric Oxide Production Is Necessary for Hypoglycemia Detection and Counterregulation. Diabetes 2010, 59, 519–528. [Google Scholar] [CrossRef]
- Amiel, S.A.; Simonson, D.C.; Tamborlane, W.V.; Defronzo, R.A.; Sherwin, R.S. Rate of Glucose Fall Does Not Affect Counterregulatory Hormone Responses to Hypoglycemia in Normal and Diabetic Humans. Diabetes 1987, 36, 518–522. [Google Scholar] [CrossRef]
- Frizzell, R.T.; Jones, E.M.; Davis, S.N.; Biggers, D.W.; Myers, S.R.; Connolly, C.C.; Neal, D.W.; Jaspan, J.B.; Cherrington, A.D. Counterregulation during Hypoglycemia Is Directed by Widespread Brain-Regions. Diabetes 1993, 42, 1253–1261. [Google Scholar] [CrossRef]
- Musen, G.; Simonson, D.C.; Bolo, N.R.; Driscoll, A.; Weinger, K.; Raji, A.; Theberge, J.; Renshaw, P.F.; Jacobson, A.M. Regional brain activation during hypoglycemia in type 1 diabetes. J. Clin. Endocr. Metab. 2008, 93, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, N.; Dai, L.; Ferguson, A.V. Glucose-responsive neurons in the subfornical organ of the rat--a novel site for direct CNS monitoring of circulating glucose. Neuroscience 2012, 201, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dallaporta, M.; Himmi, T.; Perrin, J.; Orsini, J.C. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport 1999, 10, 2657–2660. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Miselis, R.R. The Central Neural Connections of the Area Postrema of the Rat. J. Comp. Neurol. 1985, 234, 344–364. [Google Scholar] [CrossRef]
- Martinez, F.; Cifuentes, M.; Tapia, J.C.; Nualart, F. The median eminence as the hypothalamic area involved in rapid transfer of glucose to the brain: Functional and cellular mechanisms. J. Mol. Med. 2019, 97, 1085–1097. [Google Scholar] [CrossRef]
- Kohnke, S.; Buller, S.; Nuzzaci, D.; Ridley, K.; Lam, B.; Pivonkova, H.; Bentsen, M.A.; Alonge, K.M.; Zhao, C.; Tadross, J.; et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep. 2021, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.M.; Tompkins, L.S.; Brooks, H.L.; Dunn-Meynell, A.A.; Levin, B.E. Localization of glucokinase gene expression in the rat brain. Diabetes 2000, 49, 693–700. [Google Scholar] [CrossRef]
- Boychuk, C.R.; Gyarmati, P.; Xu, H.; Smith, B.N. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J. Neurophysiol. 2015, 114, 999–1007. [Google Scholar] [CrossRef]
- Lamy, C.M.; Sanno, H.; Labouebe, G.; Picard, A.; Magnan, C.; Chatton, J.Y.; Thorens, B. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014, 19, 527–538. [Google Scholar] [CrossRef]
- Loewy, A.; Spyer, K. Vagal preganglionic neurons. In Central Regulation of Autonomic Functions; Loewy, A., Spyer, K.M., Eds.; Oxford University Press: Oxford, UK; New York, NY, USA, 1990; pp. 69–87. [Google Scholar]
- Frangos, E.; Komisaruk, B.R. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: fMRI Evidence in Healthy Humans. Brain Stimul. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Buller, K.; Xu, Y.; Dayas, C.; Day, T. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1beta-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinology 2001, 73, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.; Benarroch, E.E. Neural control of the heart: Recent concepts and clinical correlations. Neurology 2014, 83, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.L.; Sollers, J.J.; Lane, R.D.; Labiner, D.M.; Herring, A.M.; Weinand, M.E.; Hutzler, R.; Thayer, J.F. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia 2001, 42, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Teves, D.; Videen, T.O.; Cryer, P.E.; Powers, W.J. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc. Natl. Acad. Sci. USA 2004, 101, 6217–6221. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Parikh, L.; Lacadie, C.; Seo, D.; Lam, W.; Hamza, M.; Schmidt, C.; Dai, F.; Sejling, A.S.; Belfort-DeAguiar, R.; et al. Hypoglycemia unawareness in type 1 diabetes suppresses brain responses to hypoglycemia. J. Clin. Investig. 2018, 128, 1485–1495. [Google Scholar] [CrossRef]
- Saha, S. Role of the central nucleus of the amygdala in the control of blood pressure: Descending pathways to medullary cardiovascular nuclei. Clin. Exp. Pharmacol. Physiol. 2005, 32, 450–456. [Google Scholar] [CrossRef]
- Evans, S.B.; Wilkinson, C.W.; Gronbeck, P.; Bennett, J.L.; Taborsky, G.J.; Figlewicz, D.P. Inactivation of the PVN during hypoglycemia partially simulates hypoglycemia-associated autonomic failure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R57–R65. [Google Scholar] [CrossRef]
- Borg, W.P.; During, M.J.; Sherwin, R.S.; Borg, M.A.; Brines, M.L.; Shulman, G.I. Ventromedial Hypothalamic-Lesions in Rats Suppress Counterregulatory Responses to Hypoglycemia. J. Clin. Investig. 1994, 93, 1677–1682. [Google Scholar] [CrossRef]
- Puskas, N.; Papp, R.S.; Gallatz, K.; Palkovits, M. Interactions between orexin-immunoreactive fibers and adrenaline or noradrenaline-expressing neurons of the lower brainstem in rats and mice. Peptides 2010, 31, 1589–1597. [Google Scholar] [CrossRef]
- Melville, K.I.; Blum, B.; Shister, H.E.; Silver, M.D. Cardiac Ischemic Changes and Arrhythmias Induced by Hypothalamic Stimulation. Am. J. Cardiol. 1963, 12, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, S.A.; Briski, K.P. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience 2005, 130, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Diggs-Andrews, K.A.; Zhang, X.; Song, Z.; Daphna-Iken, D.; Routh, V.H.; Fisher, S.J. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes 2010, 59, 2271–2280. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Seguela, P.; Wadiche, J.; Dineley-Miller, K.; Dani, J.A.; Patrick, J.W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Wada, E.; Wada, K.; Boulter, J.; Deneris, E.; Heinemann, S.; Patrick, J.; Swanson, L.W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. J. Comp. Neurol. 1989, 284, 314–335. [Google Scholar] [CrossRef]
- Carlson, A.B.; Kraus, G.P. Physiology, Cholinergic Receptors. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2023. [Google Scholar]
- Colomer, C.; Olivos-Ore, L.A.; Vincent, A.; McIntosh, J.M.; Artalejo, A.R.; Guerineau, N.C. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: Implication in stress-induced functional plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 6732–6742. [Google Scholar] [CrossRef]
- Bachmann, S.; Auderset, A.; Burckhardt, M.A.; Szinnai, G.; Hess, M.; Zumsteg, U.; Denhaerynck, K.; Donner, B. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in children with type 1 diabetes. Pediatr. Diabetes 2021, 22, 1023–1030. [Google Scholar] [CrossRef]
- Chow, E.; Bernjak, A.; Williams, S.; Fawdry, R.A.; Hibbert, S.; Freeman, J.; Sheridan, P.J.; Heller, S.R. Risk of cardiac arrhythmnias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014, 63, 1738–1747. [Google Scholar] [CrossRef]
- Novodvorsky, P.; Bernjak, A.; Chow, E.; Iqbal, A.; Sellors, L.; Williams, S.; Fawdry, R.A.; Parekh, B.; Jacques, R.M.; Marques, J.L.B.; et al. Diurnal Differences in Risk of Cardiac Arrhythmias During Spontaneous Hypoglycemia in Young People With Type 1 Diabetes. Diabetes Care 2017, 40, 655–662. [Google Scholar] [CrossRef]
- Ahmet, A.; Dagenais, S.; Barrowman, N.J.; Collins, C.J.; Lawson, M.L. Prevalence of nocturnal hypoglycemia in pediatric type 1 diabetes: A pilot study using continuous glucose monitoring. J. Pediatr. 2011, 159, 297–302 e291. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, E.J.; Newton, K.; McTavish, L. Unrecognised hypoglycaemia in children and adolescents with type 1 diabetes using the continuous glucose monitoring system: Prevalence and contributors. J. Paediatr. Child Health 2006, 42, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.G.; Kittleson, M.D. Drugs used in the management of heart disease and cardiac arrhythmias. In Small Animal Clinical Pharmacology, 2nd ed.; Maddison, J.E., Page, S.W., Church, D.B., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2008; pp. 380–457. [Google Scholar] [CrossRef]
- Reno, C.M.; Bayles, J.; Huang, Y.; Oxspring, M.; Hirahara, A.M.; Dosdall, D.J.; Fisher, S.J. Severe Hypoglycemia-Induced Fatal Cardiac Arrhythmias Are Mediated by the Parasympathetic Nervous System in Rats. Diabetes 2019, 68, 2107–2119. [Google Scholar] [CrossRef]
- Reno, C.M.; Daphna-Iken, D.; Chen, Y.S.; Vanderweele, J.; Jethi, K.; Fisher, S.J. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013, 62, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; VanderWeele, J.; Bayles, J.; Litvin, M.; Skinner, A.; Jordan, A.; Daphna-Iken, D.; Fisher, S.J. Severe Hypoglycemia-Induced Fatal Cardiac Arrhythmias are Augmented by Diabetes and Attenuated by Recurrent Hypoglycemia. Diabetes 2017, 66, 3091–3097. [Google Scholar] [CrossRef]
- Gill, G.; Woodward, A.; Casson, I.; Weston, P. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes-the ‘dead in bed’ syndrome revisited. Diabetologia 2009, 52, 42–45. [Google Scholar] [CrossRef]
- Marques, J.L.; George, E.; Peacey, S.R.; Harris, N.D.; Macdonald, I.A.; Cochrane, T.; Heller, S.R. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet. Med. 1997, 14, 648–654. [Google Scholar] [CrossRef]
- Landstedt-Hallin, L.; Englund, A.; Adamson, U.; Lins, P.E. Increased QT dispersion during hypoglycaemia in patients with type 2 diabetes mellitus. J. Intern. Med. 1999, 246, 299–307. [Google Scholar] [CrossRef]
- Kubiak, T.; Wittig, A.; Koll, C.; Mraz, B.; Gustav, J.; Herrmann, U.; Weber, H.; Kerner, W. Continuous glucose monitoring reveals associations of glucose levels with QT interval length. Diabetes Technol. Ther. 2010, 12, 283–286. [Google Scholar] [CrossRef]
- Christensen, T.F.; Cichosz, S.L.; Tarnow, L.; Randlov, J.; Kristensen, L.E.; Struijk, J.J.; Eldrup, E.; Hejlesen, O.K. Hypoglycaemia and QT interval prolongation in type 1 diabetes—Bridging the gap between clamp studies and spontaneous episodes. J. Diabetes Complicat. 2014, 28, 723–728. [Google Scholar] [CrossRef]
- Mylona, M.; Liatis, S.; Anastasiadis, G.; Kapelios, C.; Kokkinos, A. Severe iatrogenic hypoglycaemia requiring medical assistance is associated with concurrent prolongation of the QTc interval. Diabetes Res. Clin. Pract. 2020, 161, 108038. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Azeem, A.; Yeboah, J.; Soliman, E.Z.; Aggarwal, S.R.; Bertoni, A.G.; Carr, J.J.; Freedman, B.I.; Herrington, D.M.; Bowden, D.W. Heart Rate- Corrected QT Interval Is an Independent Predictor of AllCause and Cardiovascular Mortality in Individuals WithType 2 Diabetes: The Diabetes Heart Study. Diabetes Care 2014, 37, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Breum, L.; Major-Pedersen, A.; Sato, A.; Winding, H.; Pietersen, A.; Kastrup, J.; Parving, H.H. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet. Med. 2001, 18, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Frier, B.M.; Schernthaner, G.; Heller, S.R. Hypoglycemia and cardiovascular risks. Diabetes Care 2011, 34 (Suppl. 2), S132–S137. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, T.; Lyyra-Laitinen, T.; Huopio, H.; Vauhkonen, I.; Halonen, T.; Hartikainen, J.; Niskanen, L.; Laakso, M. Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann. Noninvasive Electrocardiol. Off. J. Int. Soc. Holter Noninvasive Electrocardiol. Inc. 2008, 13, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Soydan, N.; Bretzel, R.G.; Fischer, B.; Wagenlehner, F.; Pilatz, A.; Linn, T. Reduced capacity of heart rate regulation in response to mild hypoglycemia induced by glibenclamide and physical exercise in type 2 diabetes. Metab. Clin. Exp. 2013, 62, 717–724. [Google Scholar] [CrossRef]
- Andersen, A.; Bagger, J.I.; Baldassarre, M.P.A.; Christensen, M.B.; Abelin, K.U.; Faber, J.; Pedersen-Bjergaard, U.; Holst, J.J.; Lindhardt, T.B.; Gislason, G.; et al. Acute hypoglycemia and risk of cardiac arrhythmias in insulin-treated type 2 diabetes and controls. Eur. J. Endocrinol. 2021, 185, 343–353. [Google Scholar] [CrossRef]
- Andersen, A.; Bagger, J.I.; Sorensen, S.K.; Baldassarre, M.P.A.; Pedersen-Bjergaard, U.; Forman, J.L.; Gislason, G.; Lindhardt, T.B.; Knop, F.K.; Vilsboll, T. Associations of hypoglycemia, glycemic variability and risk of cardiac arrhythmias in insulin-treated patients with type 2 diabetes: A prospective, observational study. Cardiovasc. Diabetol. 2021, 20, 241. [Google Scholar] [CrossRef]
- Pollock, G.; Brady, W.J., Jr.; Hargarten, S.; DeSilvey, D.; Carner, C.T. Hypoglycemia manifested by sinus bradycardia: A report of three cases. Acad. Emerg. Med. 1996, 3, 700–707. [Google Scholar] [CrossRef]
- Bolognesi, R.; Tsialtas, D.; Bolognesi, M.G.; Giumelli, C. Marked sinus bradycardia and QT prolongation in a diabetic patient with severe hypoglycemia. J. Diabetes Complicat. 2011, 25, 349–351. [Google Scholar] [CrossRef]
- Nordin, C. The proarrhythmic effect of hypoglycemia: Evidence for increased risk from ischemia and bradycardia. Acta Diabetol. 2014, 51, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Skinner, A.; Bayles, J.; Chen, Y.S.; Daphna-Iken, D.; Fisher, S.J. Severe hypoglycemia-induced sudden death is mediated by both cardiac arrhythmias and seizures. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E240–E249. [Google Scholar] [CrossRef] [PubMed]
- Greenhoot, J.H.; Reichenbach, D.D. Cardiac injury and subarachnoid hemorrhage. A clinical, pathological, and physiological correlation. J. Neurosurg. 1969, 30, 521–531. [Google Scholar] [CrossRef]
- Reno, C.M.; Bayles, J.; Skinner, A.; Fisher, S.J. Glibenclamide Prevents Hypoglycemia-Induced Fatal Cardiac Arrhythmias in Rats. Endocrinology 2018, 159, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Graffunder, F.P.; Ternes, C.M.P.; Fernandes, A.; Rocha, A.V.; Fernandes, G.; Bhatt, D.L. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100933. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Fawzy, A.M.; Rivera-Caravaca, J.M.; Underhill, P.; Fauchier, L.; Lip, G.Y.H. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: Insights from a global federated electronic medical record database. Diabetes Obes. Metab. 2023, 25, 602–610. [Google Scholar] [CrossRef]
- Yang, F.; Meng, R.; Zhu, D.L. Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chronic Dis. Transl. Med. 2020, 6, 239–245. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Dobrev, D.; Wehrens, X.H. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 2014, 114, 1311–1319, discussion 1319. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Terrenoire, C.; Reiken, S.; Wehrens, X.H.; Song, L.S.; Tillman, E.J.; Mancarella, S.; Coromilas, J.; Lederer, W.J.; Kass, R.S.; et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc. Natl. Acad. Sci. USA 2006, 103, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Betzenhauser, M.J.; Kushnir, A.; Reiken, S.; Meli, A.C.; Wronska, A.; Dura, M.; Chen, B.X.; Marks, A.R. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J. Clin. Investig. 2010, 120, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.T.; Belevych, A.E.; Liu, B.; Bonilla, I.M.; Radwanski, P.B.; Kubasov, I.V.; Valdivia, H.H.; Schober, K.; Carnes, C.A.; Gyorke, S. Muscarinic Stimulation Facilitates Sarcoplasmic Reticulum Ca Release by Modulating Ryanodine Receptor 2 Phosphorylation Through Protein Kinase G and Ca/Calmodulin-Dependent Protein Kinase II. Hypertension 2016, 68, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Baine, S.; Thomas, J.; Bonilla, I.; Ivanova, M.; Belevych, A.; Li, J.; Veeraraghavan, R.; Radwanski, P.B.; Carnes, C.; Gyorke, S. Muscarinic-dependent phosphorylation of the cardiac ryanodine receptor by protein kinase G is mediated by PI3K-AKT-nNOS signaling. J. Biol. Chem. 2020, 295, 11720–11728. [Google Scholar] [CrossRef]
- Bround, M.J.; Asghari, P.; Wambolt, R.B.; Bohunek, L.; Smits, C.; Philit, M.; Kieffer, T.J.; Lakatta, E.G.; Boheler, K.R.; Moore, E.D.W.; et al. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc. Res. 2012, 96, 372–380. [Google Scholar] [CrossRef]
- Fisher, B.M.; Gillen, G.; Hepburn, D.A.; Dargie, H.J.; Frier, B.M. Cardiac responses to acute insulin-induced hypoglycemia in humans. Am. J. Physiol. 1990, 258, H1775–H1779. [Google Scholar] [CrossRef]
- Hilsted, J.; Bonde-Petersen, F.; Norgaard, M.B.; Greniman, M.; Christensen, N.J.; Parving, H.H.; Suzuki, M. Haemodynamic changes in insulin-induced hypoglycaemia in normal man. Diabetologia 1984, 26, 328–332. [Google Scholar] [CrossRef]
- Yakubovich, N.; Gerstein, H.C. Serious cardiovascular outcomes in diabetes: The role of hypoglycemia. Circulation 2011, 123, 342–348. [Google Scholar] [CrossRef]
- Hsu, P.F.; Sung, S.H.; Cheng, H.M.; Yeh, J.S.; Liu, W.L.; Chan, W.L.; Chen, C.H.; Chou, P.; Chuang, S.Y. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: A nationwide population-based study. Diabetes Care 2013, 36, 894–900. [Google Scholar] [CrossRef]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, F.W.; Loesment, W.A.; Kaehler, H. Beta-receptor blockade, physical activity, and metabolism. J. Cardiovasc. Pharmacol. 1990, 16 (Suppl. 5), S45–S52. [Google Scholar] [CrossRef]
- Wright, R.J.; Frier, B.M. Vascular disease and diabetes: Is hypoglycaemia an aggravating factor? Diabetes-Metab. Res. 2008, 24, 353–363. [Google Scholar] [CrossRef]
- Gogitidze Joy, N.; Hedrington, M.S.; Briscoe, V.J.; Tate, D.B.; Ertl, A.C.; Davis, S.N. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010, 33, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Prince, L.R.; Novodvorsky, P.; Bernjak, A.; Thomas, M.R.; Birch, L.; Lambert, D.; Kay, L.J.; Wright, F.J.; Macdonald, I.A.; et al. Effect of Hypoglycemia on Inflammatory Responses and the Response to Low-Dose Endotoxemia in Humans. J. Clin. Endocrinol. Metab. 2019, 104, 1187–1199. [Google Scholar] [CrossRef]
- Fisher, B.M.; Quin, J.D.; Rumley, A.; Lennie, S.E.; Small, M.; MacCuish, A.C.; Lowe, G.D. Effects of acute insulin-induced hypoglycaemia on haemostasis, fibrinolysis and haemorheology in insulin-dependent diabetic patients and control subjects. Clin. Sci. 1991, 80, 525–531. [Google Scholar] [CrossRef]
- Chow, E.; Iqbal, A.; Walkinshaw, E.; Phoenix, F.; Macdonald, I.A.; Storey, R.F.; Ajjan, R.; Heller, S.R. Prolonged Prothrombotic Effects of Antecedent Hypoglycemia in Individuals With Type 2 Diabetes. Diabetes Care 2018, 41, 2625–2633. [Google Scholar] [CrossRef]
- Verhulst, C.E.M.; van Heck, J.I.P.; Fabricius, T.W.; Stienstra, R.; Teerenstra, S.; McCrimmon, R.J.; Tack, C.J.; Pedersen-Bjergaard, U.; de Galan, B.E. Sustained Proinflammatory Effects of Hypoglycemia in People With Type 2 Diabetes and in People Without Diabetes. Diabetes 2022, 71, 2716–2727. [Google Scholar] [CrossRef]
- Ceriello, A.; Novials, A.; Ortega, E.; La Sala, L.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Esposito, K.; Giugliano, D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012, 61, 2993–2997. [Google Scholar] [CrossRef]
- Wright, R.J.; Newby, D.E.; Stirling, D.; Ludlam, C.A.; Macdonald, I.A.; Frier, B.M. Effects of acute insulin-induced hypoglycemia on indices of inflammation: Putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010, 33, 1591–1597. [Google Scholar] [CrossRef]
- Dagher, Z.; Ruderman, N.; Tornheim, K.; Ido, Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001, 88, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Razavi Nematollahi, L.; Kitabchi, A.E.; Stentz, F.B.; Wan, J.Y.; Larijani, B.A.; Tehrani, M.M.; Gozashti, M.H.; Omidfar, K.; Taheri, E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metab. Clin. Exp. 2009, 58, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Santos, R.X.; Correia, S.C.; Carvalho, C.; Santos, M.S.; Baldeiras, I.; Oliveira, C.R.; Moreira, P.I. Insulin-induced recurrent hypoglycemia exacerbates diabetic brain mitochondrial dysfunction and oxidative imbalance. Neurobiol. Dis. 2013, 49, 1–12. [Google Scholar] [CrossRef]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiology. Heart Circ. Physiol. 2006, 291, H1489–H1506. [Google Scholar] [CrossRef]
- Reno-Bernstein, C.M.; Oxspring, M.; Bayles, J.; Huang, E.Y.; Holiday, I.; Fisher, S.J. Vitamin E treatment in insulin-deficient diabetic rats reduces cardiac arrhythmias and mortality during severe hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E428–E434. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.R.; Andersen, A.; Hagelqvist, P.G.; Maytham, K.; Lauritsen, J.V.; Engberg, S.; Faber, J.; Pedersen-Bjergaard, U.; Knop, F.K.; Vilsboll, T. Sustained heart rate-corrected QT prolongation during recovery from hypoglycaemia in people with type 1 diabetes, independently of recovery to hyperglycaemia or euglycaemia. Diabetes Obes. Metab. 2023, 25, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Stahn, A.; Pistrosch, F.; Ganz, X.; Teige, M.; Koehler, C.; Bornstein, S.; Hanefeld, M. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: Silent hypoglycemias and silent arrhythmias. Diabetes Care 2014, 37, 516–520. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambers, M.E.; Nuibe, E.H.; Reno-Bernstein, C.M. Brain Regulation of Cardiac Function during Hypoglycemia. Metabolites 2023, 13, 1089. https://doi.org/10.3390/metabo13101089
Chambers ME, Nuibe EH, Reno-Bernstein CM. Brain Regulation of Cardiac Function during Hypoglycemia. Metabolites. 2023; 13(10):1089. https://doi.org/10.3390/metabo13101089
Chicago/Turabian StyleChambers, Matthew E., Emily H. Nuibe, and Candace M. Reno-Bernstein. 2023. "Brain Regulation of Cardiac Function during Hypoglycemia" Metabolites 13, no. 10: 1089. https://doi.org/10.3390/metabo13101089




