An Engineered Plant Metabolic Pathway Results in High Yields of Hydroxytyrosol Due to a Modified Whole-Cell Biocatalysis in Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid and Strain Construction
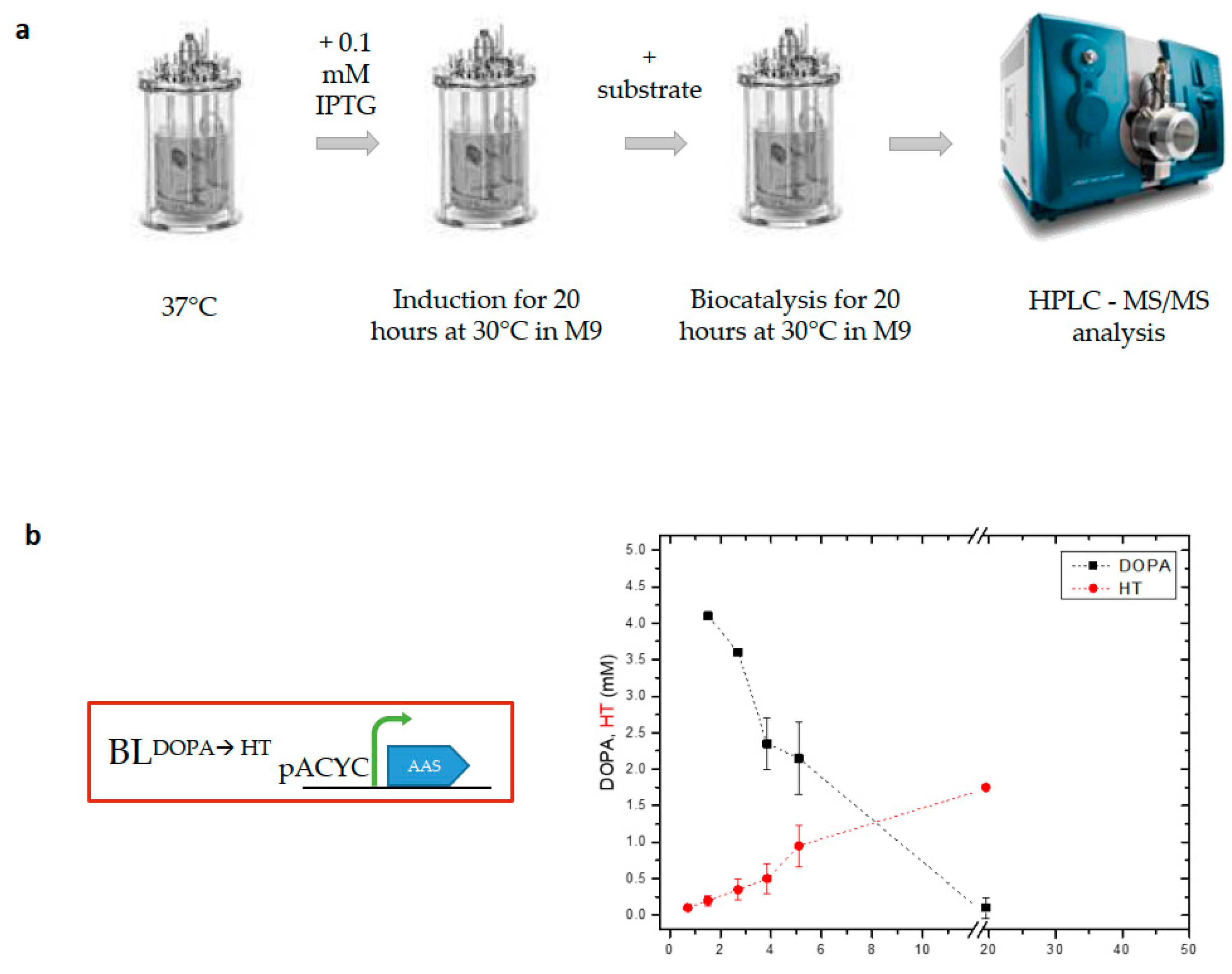
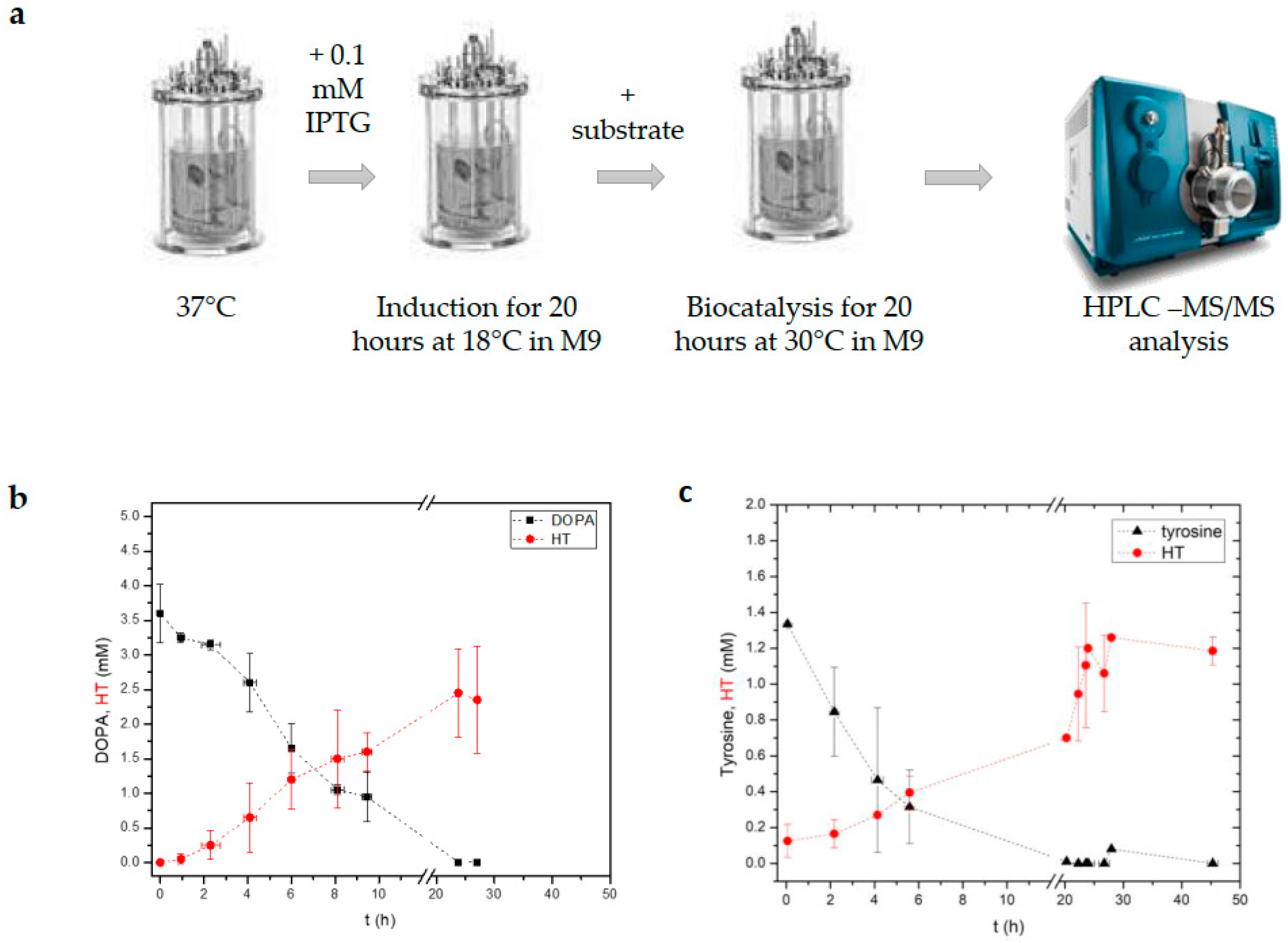
2.2. Hydroxytyrosol Production in 1 L Bioreactor
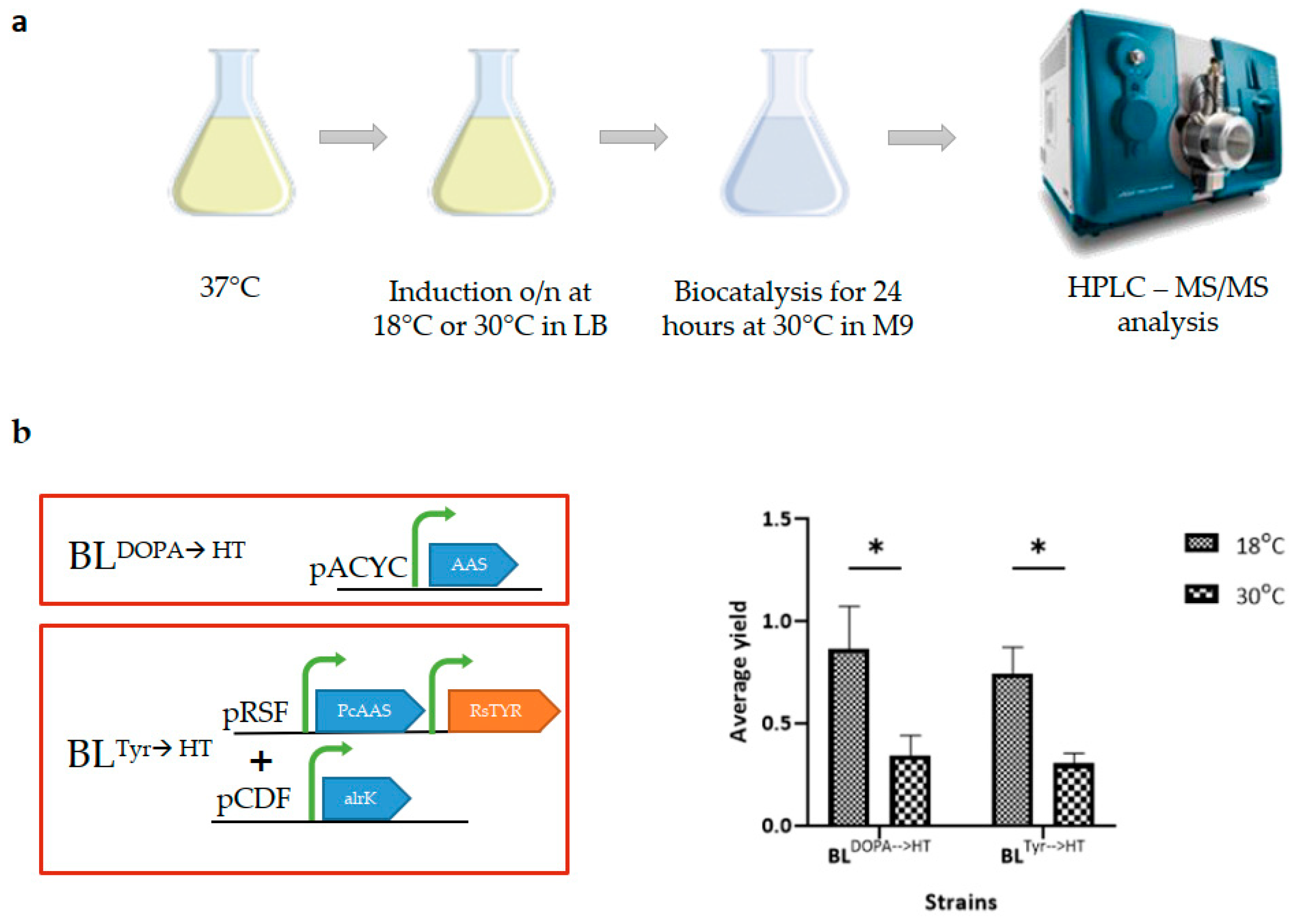
2.3. Culture Conditions and Preparation of Whole-Cell Biocatalysts
2.4. Whole-Cell Bioatalysis Reaction
2.5. Liquid Chromatography and Mass Spectrometry Metabolite Analysis
3. Results
3.1. Scaling up Flask Experiments to Produce Hydroxytyrosol from DOPA in a Bioreactor
3.2. Evaluation of Different Induction Temperatures Utilizing a Whole-Cell Biocatalysis Approach
3.3. Implementation of a Modified Whole-Cell Biocatalysis Method in a 1 L Bioreactor Led to Increased HT Yields
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef] [PubMed]
- Zoidou, E.; Melliou, E.; Gikas, E.; Tsarbopoulos, A.; Magiatis, P.; Skaltsounis, A.-L. Identification of Throuba Thassos, a Traditional Greek Table Olive Variety, as a Nutritional Rich Source of Oleuropein. J. Agric. Food Chem. 2010, 58, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Agalias, A.; Magiatis, P.; Skaltsounis, A.-L.; Mikros, E.; Tsarbopoulos, A.; Gikas, E.; Spanos, I.; Manios, T. A New Process for the Management of Olive Oil Mill Waste Water and Recovery of Natural Antioxidants. J. Agric. Food Chem. 2007, 55, 2671–2676. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From Laboratory Investigations to Future Clinical Trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL-Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Kyriazis, J.D.; Aligiannis, N.; Polychronopoulos, P.; Skaltsounis, A.-L.; Dotsika, E. Leishmanicidal Activity Assessment of Olive Tree Extracts. Phytomedicine 2013, 20, 275–281. [Google Scholar] [CrossRef]
- Crisante, F.; Taresco, V.; Donelli, G.; Vuotto, C.; Martinelli, A.; D’Ilario, L.; Pietrelli, L.; Francolini, I.; Piozzi, A. Antioxidant Hydroxytyrosol-Based Polyacrylate with Antimicrobial and Antiadhesive Activity Versus Staphylococcus Epidermidis. In Advances in Microbiology, Infectious Diseases and Public Health; Donelli, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 901, pp. 25–36. ISBN 978-3-319-27934-3. [Google Scholar]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the In-Vitro Antimicrobial Activity of Oleuropein and Hydroxytyrosol. J. Pharm. Pharmacol. 2010, 51, 971–974. [Google Scholar] [CrossRef]
- Medina-Martínez, M.S.; Truchado, P.; Castro-Ibáñez, I.; Allende, A. Antimicrobial Activity of Hydroxytyrosol: A Current Controversy. Biosci. Biotechnol. Biochem. 2016, 80, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Shen, X.; Wang, J.; Yuan, Q. Recent Advances in Microbial Production of Phenolic Compounds. Chin. J. Chem. Eng. 2021, 30, 54–61. [Google Scholar] [CrossRef]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, Physical and Biotechnological Approaches to the Production of the Potent Antioxidant Hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef]
- Chung, D.; Kim, S.Y.; Ahn, J.-H. Production of Three Phenylethanoids, Tyrosol, Hydroxytyrosol, and Salidroside, Using Plant Genes Expressing in Escherichia coli. Sci. Rep. 2017, 7, 2578. [Google Scholar] [CrossRef]
- Choo, H.J.; Kim, E.J.; Kim, S.Y.; Lee, Y.; Kim, B.-G.; Ahn, J.-H. Microbial Synthesis of Hydroxytyrosol and Hydroxysalidroside. Appl. Biol. Chem. 2018, 61, 295–301. [Google Scholar] [CrossRef]
- Trantas, E.; Navakoudis, E.; Pavlidis, T.; Nikou, T.; Halabalaki, M.; Skaltsounis, L.; Ververidis, F. Dual Pathway for Metabolic Engineering of Escherichia coli to Produce the Highly Valuable Hydroxytyrosol. PLoS ONE 2019, 14, e0212243. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Wu, Y.; Yan, Y.; Sun, X.; Yuan, Q. Establishing an Artificial Pathway for Efficient Biosynthesis of Hydroxytyrosol. ACS Synth. Biol. 2018, 7, 647–654. [Google Scholar] [CrossRef]
- Chen, W.; Yao, J.; Meng, J.; Han, W.; Tao, Y.; Chen, Y.; Guo, Y.; Shi, G.; He, Y.; Jin, J.-M.; et al. Promiscuous Enzymatic Activity-Aided Multiple-Pathway Network Design for Metabolic Flux Rearrangement in Hydroxytyrosol Biosynthesis. Nat. Commun. 2019, 10, 960. [Google Scholar] [CrossRef]
- Liebgott, P.-P.; Amouric, A.; Comte, A.; Tholozan, J.-L.; Lorquin, J. Hydroxytyrosol from Tyrosol Using Hydroxyphenylacetic Acid-Induced Bacterial Cultures and Evidence of the Role of 4-HPA 3-Hydroxylase. Res. Microbiol. 2009, 160, 757–766. [Google Scholar] [CrossRef]
- Allouche, N.; Sayadi, S. Synthesis of Hydroxytyrosol, 2-Hydroxyphenylacetic Acid, and 3-Hydroxyphenylacetic Acid by Differential Conversion of Tyrosol Isomers Using Serratia marcescens Strain. J. Agric. Food Chem. 2005, 53, 6525–6530. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Sayadi, S. Bioconversion of p -Tyrosol into Hydroxytyrosol under Bench-Scale Fermentation. BioMed Res. Int. 2018, 2018, 7390751. [Google Scholar] [CrossRef] [PubMed]
- Brouk, M.; Fishman, A. Improving Process Conditions of Hydroxytyrosol Synthesis by Toluene-4-Monooxygenase. J. Mol. Catal. B Enzym. 2012, 84, 121–127. [Google Scholar] [CrossRef]
- Bernath-Levin, K.; Shainsky, J.; Sigawi, L.; Fishman, A. Directed Evolution of Nitrobenzene Dioxygenase for the Synthesis of the Antioxidant Hydroxytyrosol. Appl. Microbiol. Biotechnol. 2014, 98, 4975–4985. [Google Scholar] [CrossRef] [PubMed]
- Koma, D.; Fujisawa, M.; Ohashi, H.; Yamanaka, H.; Moriyoshi, K.; Nagamori, E.; Ohmoto, T. Production of 3-Hydroxytyrosol from Glucose by Chromosomally Engineered Escherichia coli by Fed-Batch Cultivation in a Jar Fermenter. J. Agric. Food Chem. 2023, 71, 9451–9459. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jia, P.; Bai, Y.; Fan, T.; Zheng, X.; Cai, Y. Efficient Synthesis of Hydroxytyrosol from l -3,4-Dihydroxyphenylalanine Using Engineered Escherichia coli Whole Cells. J. Agric. Food Chem. 2019, 67, 6867–6873. [Google Scholar] [CrossRef]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.Z.; Zhou, T. Challenges Associated With the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 10, 630551. [Google Scholar] [CrossRef]
- Knapp, B.D.; Huang, K.C. The Effects of Temperature on Cellular Physiology. Annu. Rev. Biophys. 2022, 51, 499–526. [Google Scholar] [CrossRef]
- Fahnert, B.; Lilie, H.; Neubauer, P. Inclusion Bodies: Formation and Utilisation. In Physiological Stress Responses in Bioprocesses; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 89, pp. 93–142. ISBN 978-3-540-20311-7. [Google Scholar]
| Plasmid Name | Genotype |
|---|---|
| pRSFDuet-1 | RSF ori kan lacI T7prom T7term |
| pCDFDuet-1 | CDF ori aadA lacI T7prom T7term |
| pACYCDuet-1 | P15A ori CmR lacI T7prom T7term |
| pRSF:TYR | RSF ori kan lacI T7prom:RsTYR:T7term [17] |
| pACYC:AAS | P15A ori CmR lacI T7prom:PcAAS:T7term [17] |
| pCDF:ALRK | CDF ori aadA lacI T7prom:EcALRK:T7term [17] |
| Strain name | Genotype |
| BL21(DE3) | F-ompT gal dcm lon hsdSB(rB - mB -) λ(DE3 [lacI lac-UV5-T7 gene 1 ind1 sam7 nin5]) |
| BLTyr→HT | BL21(DE3) pRSF:TYR:AAS pCDF:ALRK [17] |
| BLDOPA→HT | BL21(DE3) pACYC:AAS [17] |
| Analyte | Q1 Mass (Da) | Q3 Mass (Da) | Dwell Time (msec) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Hydroxytyrosol | 153.00 | 123.00 | 50.0 | −25.00 | −10.00 | −22.00 | −9.00 |
| Tyrosine | 180.00 | 162.80 | 50.0 | −75.00 | −10.00 | −18.00 | −7.00 |
| DOPA | 198.00 | 152.00 | 50.0 | 70.00 | 10.00 | 19.00 | 9.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mermigka, G.; Vavouraki, A.I.; Nikolaou, C.; Cheiladaki, I.; Vourexakis, M.; Goumas, D.; Ververidis, F.; Trantas, E. An Engineered Plant Metabolic Pathway Results in High Yields of Hydroxytyrosol Due to a Modified Whole-Cell Biocatalysis in Bioreactor. Metabolites 2023, 13, 1126. https://doi.org/10.3390/metabo13111126
Mermigka G, Vavouraki AI, Nikolaou C, Cheiladaki I, Vourexakis M, Goumas D, Ververidis F, Trantas E. An Engineered Plant Metabolic Pathway Results in High Yields of Hydroxytyrosol Due to a Modified Whole-Cell Biocatalysis in Bioreactor. Metabolites. 2023; 13(11):1126. https://doi.org/10.3390/metabo13111126
Chicago/Turabian StyleMermigka, Glykeria, Aikaterini I. Vavouraki, Chrysoula Nikolaou, Ioanna Cheiladaki, Michail Vourexakis, Dimitrios Goumas, Filippos Ververidis, and Emmanouil Trantas. 2023. "An Engineered Plant Metabolic Pathway Results in High Yields of Hydroxytyrosol Due to a Modified Whole-Cell Biocatalysis in Bioreactor" Metabolites 13, no. 11: 1126. https://doi.org/10.3390/metabo13111126
APA StyleMermigka, G., Vavouraki, A. I., Nikolaou, C., Cheiladaki, I., Vourexakis, M., Goumas, D., Ververidis, F., & Trantas, E. (2023). An Engineered Plant Metabolic Pathway Results in High Yields of Hydroxytyrosol Due to a Modified Whole-Cell Biocatalysis in Bioreactor. Metabolites, 13(11), 1126. https://doi.org/10.3390/metabo13111126









