Metabolomics to Understand Alterations Induced by Physical Activity during Pregnancy
Abstract
:1. Introduction
2. Effects of PA in Non-Pregnant Populations Using Metabolomics
2.1. Changes in Amino Acids
2.2. Changes in Lipids
| Study Population and Sample Size | Biosample | Study Design | Metabolomic Profiling Platform | PA Intervention or Measurement | Main Results |
|---|---|---|---|---|---|
| 214 people, including 20 women (18–65 years of age) | Plasma | Intervention | LC-MS | The test used a cycle ergometer for a three-minute warm-up at baseline, followed by four-minute steady-state workloads at 15, 35, 55, and 75% of VO2 max | PE(34:3), PC(34:2), PC(34:3), PC(36:3), and Cer(10:0) were some of the lipids higher after the exercise intervention [15]. |
| 14 obese sedentary individuals (5 women, 40 ± 2 years of age) and 14 persons with type 2 diabetes (5 women, 43 ± 2 years of age) | Skeletal muscle | Intervention | LC-MS | Cycle ergometer for 1.5 h at 50% of VO2 max | The total PE amount was higher in people with insulin resistance after a 90-min bout of PA [57]. |
| 12–15 women with obesity (30–50 years of age) | Plasma | Intervention | LC-MS | 30 min of aerobic exercise for 4 days/wk at an intensity of 60–70% of maximal HR | Palmitoyl and oleoyl carnitines were slightly reduced after exercise [58]. |
| 5197 people, including 2120 healthy women (25–42 years of age) | Plasma | Cross-sectional | LC-MS | Self-reported questionnaires every 2–4 years | PA was positive associated with LPC(18:1), PC(36:0), PC(34:3p), PE(38:3p), CE(18:1), CE(16:0), and SM(22:1) and negative associated with DG(34:2) and TG(50:2) [1]. |
| 12–15 women with obesity (30–50 years of age) | Plasma | Intervention | LC-MS | Data from calorie-restricted diet + 14 wks of 4 day/wk aerobic exercise at 60–75% of maximal HR combined with data from an acute bout of exercise | Epoxides (19(20)-EpDPE, 13-HOTE, 11-HETE), prostaglandins (PGE1, PGE2, PGD2), and endocannabinoids (2-AG, AEA) were significantly reduced by physical training [34]. |
| 14 women with obesity (no exercise, 24 ± 4 years of age) and 19 obese women (exercise, 23 ± 3 years of age) | Skeletal muscle | Intervention | GC-MS AND LC-MS | 12 weeks of combined aerobic (75–80% of peak HR) and resistance (60–70% of maximum HR) exercise training | Cardiolipins, PLs, and acylcarnitines increased, while LPC content decreased after PA [56]. |
| 20 women with obesity (35 ± 6 years of age) | Plasma | Intervention | LC-MS | 8 weeks of aerobic and strength exercises, alternately, for 55 min at 75–90% of maximum HR, 3 times a week | Downregulated lipid species were PC(36:0), PC(40:6), PC(36:5p), PC(42:1p), PE(40:4p), PE(36:5), PE(38:3p), TG(54:8), TG(56:9), TG(60:11), SM(d18:1/20:0), and stearic acid, while upregulated lipid species were LPC(16:0p), LPC(18:0p), LPC(20:2), PC(20:2), TG(48:0), TG(50:0), and SM(d18:1/26:1) [3]. |
| Adults who are sedentary; 14 lean and 10 obese | Plasma Stool | Intervention | LC-MS | 6 wks of supervised, aerobic exercise 3×/wk (60–75% of heart rate reserve 30–60 min | Palmitic acid was significantly increased post-intervention [35]. |
2.3. Other Metabolites
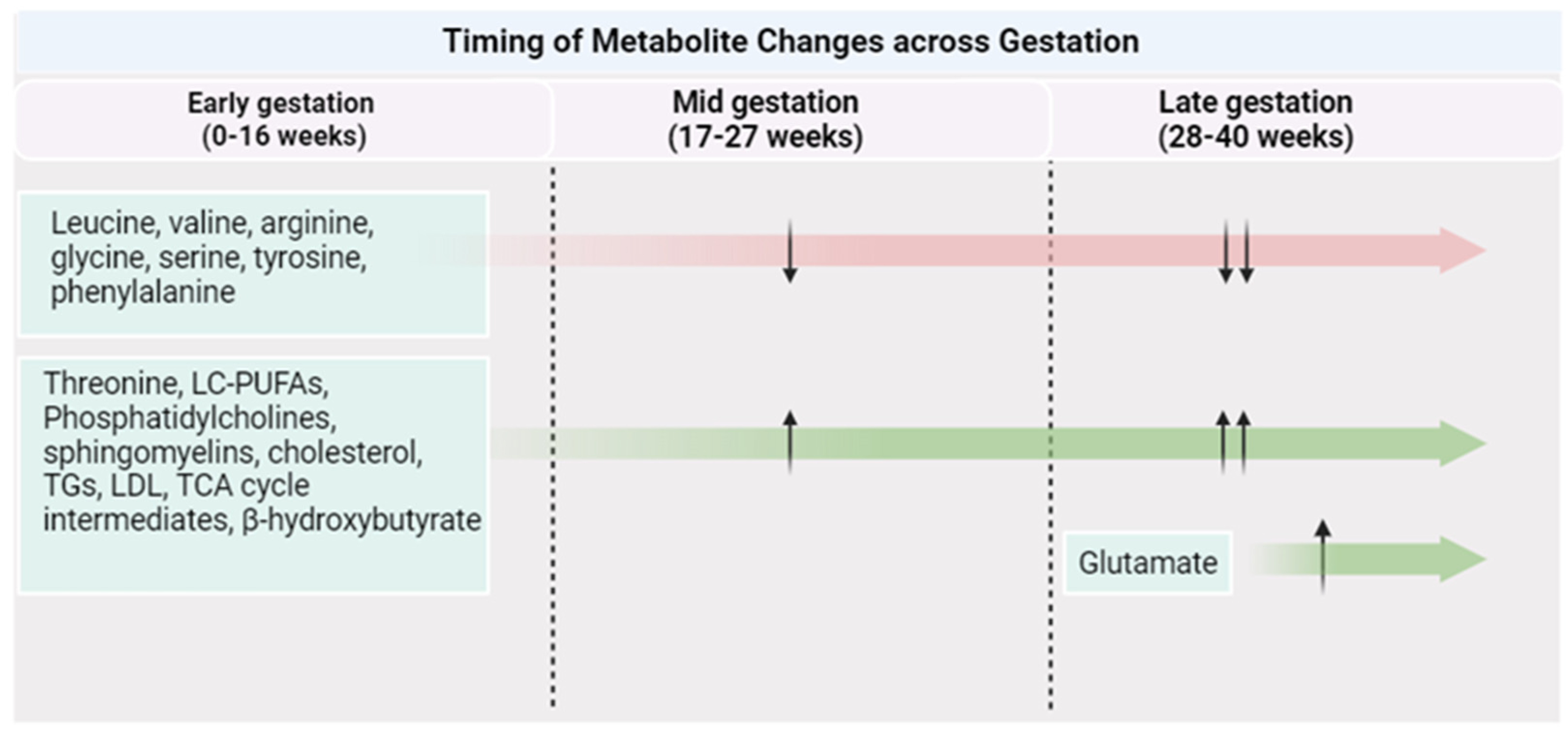
3. Effects of PA in Pregnant Populations Using Metabolomics
4. Future Research Focus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ding, M.; Zeleznik, O.A.; Guasch-Ferre, M.; Hu, J.; Lasky-Su, J.; Lee, I.M.; Jackson, R.D.; Shadyab, A.H.; Lamonte, M.J.; Clish, C.; et al. Metabolome-Wide Association Study of the Relationship Between Habitual Physical Activity and Plasma Metabolite Levels. Am. J. Epidemiol. 2019, 188, 1932–1943. [Google Scholar] [CrossRef]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, Physical Activity, Exercise and Health: A Review of the Current Evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef]
- San Martin, R.; Brandao, C.F.C.; Junqueira-Franco, M.V.M.; Junqueira, G.P.; de Freitas, E.C.; de Carvalho, F.G.; Rodrigues, C.H.P.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; et al. Untargeted Lipidomic Analysis of Plasma from Obese Women Submitted to Combined Physical Exercise. Sci. Rep. 2022, 12, 11541. [Google Scholar] [CrossRef]
- Vanweert, F.; Boone, S.C.; Brouwers, B.; Mook-Kanamori, D.O.; de Mutsert, R.; Rosendaal, F.R.; Lamb, H.J.; Schrauwen-Hinderling, V.B.; Schrauwen, P.; Hesselink, M.K.C.; et al. The Effect of Physical Activity Level and Exercise Training on the Association between Plasma Branched-Chain Amino Acids and Intrahepatic Lipid Content in Participants with Obesity. Int. J. Obes. 2021, 45, 1510–1520. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Mottola, M.F.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian Guideline for Physical Activity Throughout Pregnancy: Methodology. J. Obstet. Gynaecol. Can. 2018, 40, 1468–1483. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of Prenatal Exercise on Neonatal and Childhood Outcomes: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal Exercise for the Prevention of Gestational Diabetes Mellitus and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Mottola, M.F.; Artal, R. Fetal and Maternal Metabolic Responses to Exercise during Pregnancy. Early Hum. Dev. 2016, 94, 33–41. [Google Scholar] [CrossRef]
- Fazzi, C.; Saunders, D.H.; Linton, K.; Norman, J.E.; Reynolds, R.M. Sedentary Behaviours during Pregnancy: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Everest, C.; da Silva, D.F.; Puranda, J.; Souza, S.C.S.; Goudreau, A.D.; Nagpal, T.S.; Edwards, C.M.; Gupta, R.; Adamo, K.B. Physical Activity and Weight Gain Throughout Pregnancy Are Associated With Umbilical Cord Markers. J. Obstet. Gynaecol. Can. 2022, 44, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Saavedra, D.; Markunas, C.; Takahashi, H.; Baer, L.A.; Harris, J.E.; Hirshman, M.F.; Ilkayeva, O.; Newgard, C.B.; Stanford, K.I.; Goodyear, L.J. Maternal Exercise and Paternal Exercise Induce Distinct Metabolite Signatures in Offspring Tissues. Diabetes 2022, 71, 2094–2105. [Google Scholar] [CrossRef]
- Maitre, L.; Villanueva, C.M.; Lewis, M.R.; Ibarluzea, J.; Santa-Marina, L.; Vrijheid, M.; Sunyer, J.; Coen, M.; Toledano, M.B. Maternal Urinary Metabolic Signatures of Fetal Growth and Associated Clinical and Environmental Factors in the INMA Study. BMC Med. 2016, 14, 177. [Google Scholar] [CrossRef]
- Mills, H.L.; Patel, N.; White, S.L.; Pasupathy, D.; Briley, A.L.; Santos Ferreira, D.L.; Seed, P.T.; Nelson, S.M.; Sattar, N.; Tilling, K.; et al. The Effect of a Lifestyle Intervention in Obese Pregnant Women on Gestational Metabolic Profiles: Findings from the UK Pregnancies Better Eating and Activity Trial (UPBEAT) Randomised Controlled Trial. BMC Med. 2019, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Moore, S.C.; Keadle, S.K.; Xiang, Y.B.; Zheng, W.; Peters, T.M.; Leitzmann, M.F.; Ji, B.T.; Sampson, J.N.; Shu, X.O.; et al. Objectively Measured Physical Activity and Plasma Metabolomics in the Shanghai Physical Activity Study. Int. J. Epidemiol. 2016, 45, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; O’Grada, C.M.; Ryan, M.F.; Gibney, M.J.; Roche, H.M.; Gibney, E.R.; Brennan, L. Modulation of the Lipidomic Profile Due to a Lipid Challenge and Fitness Level: A Postprandial Study. Lipids Health Dis. 2015, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Mariotti, F. Editorial: Beyond Traditional Roles for Amino Acids. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel Metabolic and Physiological Functions of Branched Chain Amino Acids: A Review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Han, Q.; Phillips, R.S.; Li, J. Editorial: Aromatic Amino Acid Metabolism. Front. Mol. Biosci. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. Biomed. Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. Sulfur Containing Amino Acids and Human Disease. Biomed. Pharmacother. 2004, 58, 47–55. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136 (Suppl. S6), 1636s–1640s. [Google Scholar] [CrossRef] [PubMed]
- Osowska, S.; Moinard, C.; Neveux, N.; Loï, C.; Cynober, L. Citrulline Increases Arginine Pools and Restores Nitrogen Balance after Massive Intestinal Resection. Gut 2004, 53, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased Dietary Protein Modifies Glucose and Insulin Homeostasis in Adult Women during Weight Loss. Hum. Nutr. Metab. 2003, 133, 405–410. [Google Scholar] [CrossRef]
- Roberts, E.L. The Support of Energy Metabolism in the Central Nervous System with Substrates Other than Glucose. In Handbook of Neurochemistry and Molecular Neurobiology Brain Energetics. Integration of Molecular and Cellular Processes; Springer: Berlin/Heidelberg, Germany, 2007; pp. 137–179. [Google Scholar]
- Watford, M. Amino Acids | Glutamine. Encycl. Biol. Chem. Third Ed. 2021, 1, 56–70. [Google Scholar] [CrossRef]
- Newsholme, P. Glutamine Metabolism: Nutritional and Clinical Significance Why Is L-Glutamine Metabolism Important to Cells of the Immune System in Health, Postinjury, Surgery or Infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef]
- Chen, S.; Akter, S.; Kuwahara, K.; Matsushita, Y.; Nakagawa, T.; Konishi, M.; Honda, T.; Yamamoto, S.; Hayashi, T.; Noda, M.; et al. Serum Amino Acid Profiles and Risk of Type 2 Diabetes among Japanese Adults in the Hitachi Health Study. Sci. Rep. 2019, 9, 7010. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Yang, S.; Shen, N.; Wang, Y.; Zhu, Y.; Guo, Z.; Yang, S.Y.; Xing, D.; Li, H.; et al. Glycine-Serine-Threonine Metabolic Axis Delays Intervertebral Disc Degeneration through Antioxidant Effects: An Imaging and Metabonomics Study. Oxidative Med. Cell. Longev. 2021, 2021, 5579736. [Google Scholar] [CrossRef]
- de Koning, T.J.; Fuchs, S.A.; Klomp, L.W.J. Serine, Glycine, and Threonine. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: Berlin, Germany, 2007; pp. 23–45. [Google Scholar]
- Chang, W.; Hatch, G.M.; Wang, Y.; Yu, F.; Wang, M. The Relationship between Phospholipids and Insulin Resistance: From Clinical to Experimental Studies. J. Cell. Mol. Med. 2019, 23, 702–710. [Google Scholar] [CrossRef]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Buring, J.E.; Lee, I.M.; Manson, J.A.E.; Tobias, D.K. Association of Modifiable Lifestyle Factors with Plasma Branched-Chain Amino Acid Metabolites in Women. J. Nutr. 2022, 152, 1515–1524. [Google Scholar] [CrossRef]
- Den Hoed, M.; Hesselink, M.K.C.; Van Kranenburg, G.P.J.; Westerterp, K.R. Habitual Physical Activity in Daily Life Correlates Positively with Markers for Mitochondrial Capacity. J. Appl. Physiol. 2008, 105, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Grapov, D.; Fiehn, O.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Keim, N.L.; Newman, J.W.; Hunter, G.R.; et al. Exercise Plasma Metabolomics and Xenometabolomics in Obese, Sedentary, Insulin-Resistant Women: Impact of a Fitness and Weight Loss Intervention. Am. J. Physiol. Endocrinol. Metab. 2019, 317, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Kasperek, M.C.; Mailing, L.; Piccolo, B.D.; Moody, B.; Lan, R.; Gao, X.; Hernandez-Saavedra, D.; Woods, J.A.; Adams, S.H.; Allen, J.M. Exercise Training Modifies Xenometabolites in Gut and Circulation of Lean and Obese Adults. Physiol. Rep. 2023, 11, e15638. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Bala, C.G.; Rusu, A.; Ciobanu, D.; Bucsa, C.; Roman, G. Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research. Antioxidants 2021, 10, 610. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the Tyrosine Isomers Meta-Tyrosine and Ortho-Tyrosine in Oxidative Stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef]
- De Chiara, B.; Sedda, V.; Parolini, M.; Campolo, J.; De Maria, R.; Caruso, R.; Pizzi, G.; Disoteo, O.; Dellanoce, C.; Corno, A.R.; et al. Plasma Total Cysteine and Cardiovascular Risk Burden: Action and Interaction. Sci. World J. 2012, 2012, 303654. [Google Scholar] [CrossRef]
- Adams, S.H. Emerging Perspectives on Essential Amino Acid Metabolism in Obesity and the Insulin-Resistant State. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Kuehnbaum, N.L.; Gillen, J.B.; Gibala, M.J.; Britz-McKibbin, P. Personalized Metabolomics for Predicting Glucose Tolerance Changes in Sedentary Women after High-Intensity Interval Training. Sci. Rep. 2014, 4, 6166. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Männistö, V.; Tauriainen, M.M.; Pentikäinen, H.; Savonen, K.; Klåvus, A.; Koistinen, V.; Hanhineva, K.; Schwab, U. Effects of Exercise on NAFLD Using Non-Targeted Metabolomics in Adipose Tissue, Plasma, Urine, and Stool. Sci. Rep. 2022, 12, 6485. [Google Scholar] [CrossRef]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of Oral L-Arginine Supplementation on Blood Pressure: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Someya, A.; Nagaoka, I. Citrulline Cooperatively Exerts an Anti-Inflammatory Effect on Synovial Cells with Glucosamine and n-Acetylglucosamine. Biomed. Rep. 2020, 13, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs, M.S.A.; Kopciuk, K.A.; Shaykhutdinov, R.A.; Robson, P.J.; Mignault, D.; Rabasa-Lhoret, R.; Vogel, H.J.; Csizmadi, I. Serum Metabolomics of Activity Energy Expenditure and Its Relation to Metabolic Syndrome and Obesity. Sci. Rep. 2018, 8, 3308. [Google Scholar] [CrossRef]
- Kojouri, M.; Pinto, R.; Mustafa, R.; Huang, J.; Gao, H.; Elliott, P.; Tzoulaki, I.; Dehghan, A. Metabolome-Wide Association Study on Physical Activity. Sci. Rep. 2023, 13, 2374. [Google Scholar] [CrossRef]
- Fahs, C.A.; Heffernan, K.S.; Fernhall, B. Hemodynamic and Vascular Response to Resistance Exercise with L-Arginine. Med. Sci. Sports Exerc. 2009, 41, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Pang, Y.; Kartsonaki, C.; Du, H.; Millwood, I.Y.; Guo, Y.; Chen, Y.; Bian, Z.; Yang, L.; Walters, R.; Bragg, F.; et al. Physical Activity, Sedentary Leisure Time, Circulating Metabolic Markers, and Risk of Major Vascular Diseases. Circ. Genom. Precis. Med. 2019, 12, 386–396. [Google Scholar] [CrossRef]
- Jian-Chun, Y.U.; Jiang, Z.-M.; De-Min, L.I. Glutamine: A Precursor of Glutathione and Its Effect on Liver. World J. Gastroenterol. 1999, 5, 143–146. [Google Scholar]
- Gao, X.; Randell, E.; Zhou, H.; Sun, G. Higher Serum Choline and Betaine Levels Are Associated with Better Body Composition in Male but Not Female Population. PLoS ONE 2018, 13, e0193114. [Google Scholar] [CrossRef]
- Seibert, R.; Abbasi, F.; Hantash, F.M.; Caulfield, M.P.; Reaven, G.; Kim, S.H. Relationship between Insulin Resistance and Amino Acids in Women and Men. Physiol. Rep. 2015, 3, e12392. [Google Scholar] [CrossRef]
- Halama, A.; Aye, M.M.; Dargham, S.R.; Kulinski, M.; Suhre, K.; Atkin, S.L. Metabolomics of Dynamic Changes in Insulin Resistance before and after Exercise in PCOS. Front. Endocrinol. 2019, 10, 116. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for Studying Metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Meikle, P.J.; Summers, S.A. Sphingolipids and Phospholipids in Insulin Resistance and Related Metabolic Disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-De Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E.; et al. Exercise Training Improves Mitochondrial Respiration and Is Associated with an Altered Intramuscular Phospholipid Signature in Women with Obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Newsom, S.A.; Brozinick, J.T.; Kiseljak-Vassiliades, K.; Strauss, A.N.; Bacon, S.D.; Kerege, A.A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; et al. Skeletal Muscle Phosphatidylcholine and Phosphatidylethanolamine Are Related to Insulin Sensitivity and Respond to Acute Exercise in Humans. J. Appl. Physiol. 2016, 120, 1355–1363. [Google Scholar] [CrossRef]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as Markers of Exercise-Associated Fuel Partitioning, Xenometabolism, and Potential Signals to Muscle Afferent Neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Horowitz, J.F. Exercise-Induced Alterations in Muscle Lipid Metabolism Improve Insulin Sensitivity. Exerc. Sport. Sci. Rev. 2007, 35, 192–196. [Google Scholar] [CrossRef] [PubMed]
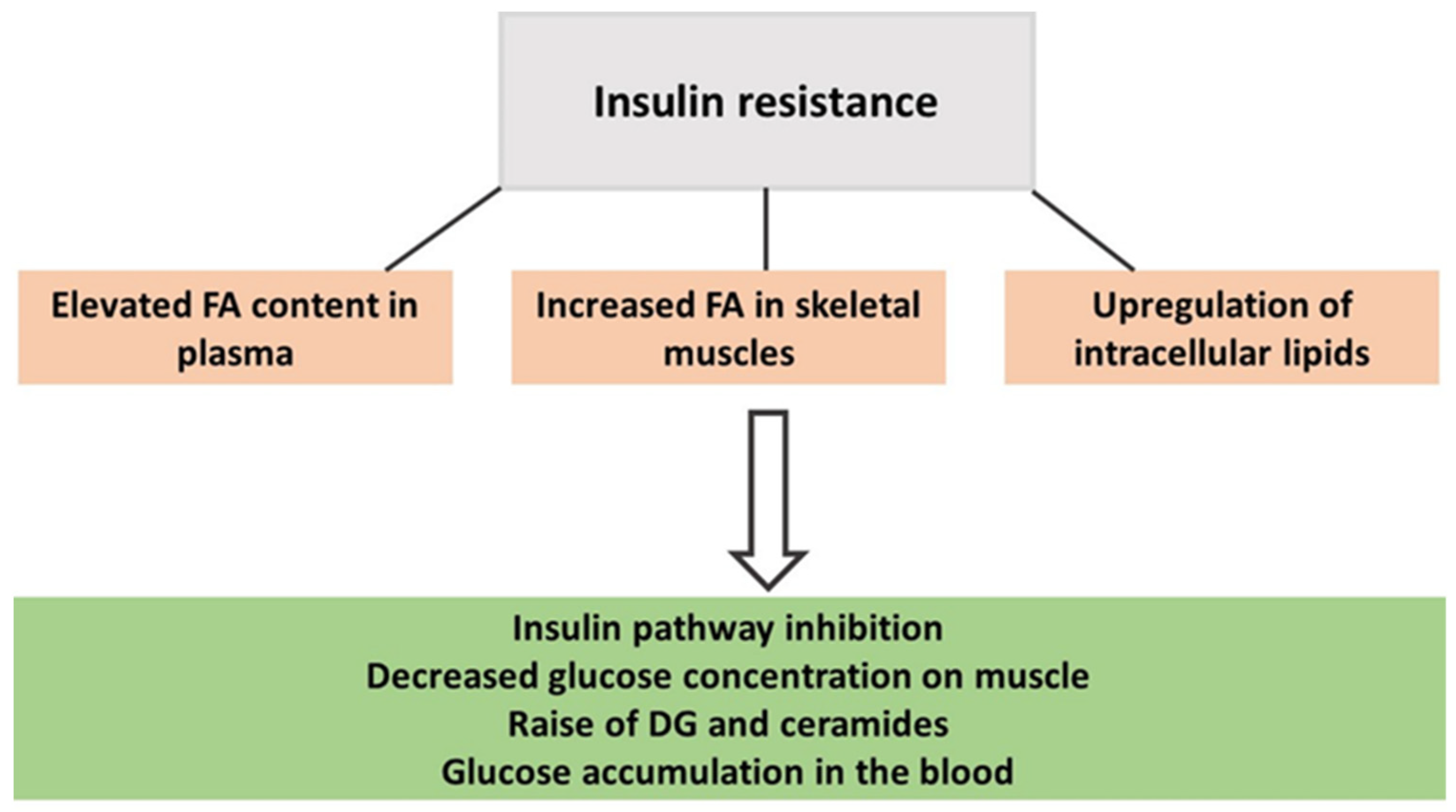
- Imierska, M.; Kurianiuk, A.; Błachnio-Zabielska, A. The Influence of Physical Activity on the Bioactive Lipids Metabolism in Obesity-Induced Muscle Insulin Resistance. Biomolecules 2020, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal Muscle Energy Metabolism in Obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid Metabolite Biomarkers in Cardiovascular Disease: Discovery and Biomechanism Translation from Human Studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’elia, F.; Patti, A.; Bonavolontà, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef]
- Almanza-Aguilera, E.; Brunius, C.; Bernal-Lopez, M.R.; Garcia-Aloy, M.; Madrid-Gambin, F.; Tinahones, F.J.; Gómez-Huelgas, R.; Landberg, R.; Andres-Lacueva, C. Impact in Plasma Metabolome as Effect of Lifestyle Intervention for Weight-Loss Reveals Metabolic Benefits in Metabolically Healthy Obese Women. J. Proteome Res. 2018, 17, 2600–2610. [Google Scholar] [CrossRef]
- Opialla, T.; Gollasch, B.; Kuich, P.H.J.L.; Klug, L.; Rahn, G.; Busjahn, A.; Spuler, S.; Boschmann, M.; Kirwan, J.A.; Luft, F.C.; et al. Exercise Blood-Drop Metabolic Profiling Links Metabolism with Perceived Exertion. Front. Mol. Biosci. 2022, 9, 1042231. [Google Scholar] [CrossRef]
- Nix, C.; Hemmati, M.; Cobraiville, G.; Servais, A.C.; Fillet, M. Blood Microsampling to Monitor Metabolic Profiles During Physical Exercise. Front. Mol. Biosci. 2021, 8, 681400. [Google Scholar] [CrossRef]
- Gol, M.; Özkaya, B.; Yildirim, C.; Bal, R. Regular Exercise, Overweight/Obesity and Sedentary Lifestyle Cause Adaptive Changes in Thiol–Disulfide Homeostasis. An. Acad. Bras. Cienc. 2019, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.; Packer, L. Thiol Homeostasis and Supplements in Physical Exercise. Am. J. Clin. Nutr. 2000, 72, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Kuehnbaum, N.L.; Gillen, J.B.; Kormendi, A.; Lam, K.P.; Dibattista, A.; Gibala, M.J.; Britz-Mckibbin, P. Multiplexed Separations for Biomarker Discovery in Metabolomics: Elucidating Adaptive Responses to Exercise Training. Electrophoresis 2015, 36, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Hadden, D.R.; McLaughlin, C. Normal and Abnormal Maternal Metabolism during Pregnancy. Semin. Fetal Neonatal Med. 2009, 14, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, H.; Faerber, A.; Young, M.; Ballentine, S.J.; Thompson, M.D. Maternal Exercise Protects Male Offspring From Maternal Diet-Programmed Nonalcoholic Fatty Liver Disease Progression. Endocrinology 2023, 164, bqad010. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hellmuth, C.; Uhl, O.; Godfrey, K.; Briley, A.; Welsh, P.; Pasupathy, D.; Seed, P.T.; Koletzko, B.; Poston, L. Cord Metabolic Profiles in Obese Pregnant Women: Insights into Offspring Growth and Body Composition. J. Clin. Endocrinol. Metab. 2018, 103, 346–355. [Google Scholar] [CrossRef]
- Haslam, D.E.; Li, J.; Liang, L.; Martinez, M.; Palacios, C.; Trak-Fellermeier, M.A.; Franks, P.W.; Joshipura, K.; Bhupathiraju, S.N. Changes in Metabolites during an Oral Glucose Tolerance Test in Early and Mid-Pregnancy: Findings from the PEARLS Randomized, Controlled Lifestyle Trial. Metabolites 2020, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, T.; Azab, S.M.; Anand, S.S.; Thabane, L.; Shanmuganathan, M.; Morrison, K.M.; Atkinson, S.A.; Stearns, J.C.; Teo, K.K.; Britz-Mckibbin, P.; et al. Sources of Variation in Food-Related Metabolites during Pregnancy. Nutrients 2022, 14, 2503. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Placental Regulation of Fatty Acid Delivery and Its Effect on Fetal Growth—A Review. Placenta 2002, 23, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue Levels Acids during of Polyunsaturated Fatty Early Human Development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Crocker, I.; Lawson, N.; Daniels, I.; Baker, P.; Fletcher, J. Significance of Fatty Acids in Pregnancy-Induced Immunosuppression. Clin. Diagn. Lab. Immunol. 1999, 6, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tsai, M.Y.; Sun, Q.; Hinkle, S.N.; Rawal, S.; Mendola, P.; Ferrara, A.; Albert, P.S.; Zhang, C. A Prospective and Longitudinal Study of Plasma Phospholipid Saturated Fatty Acid Profile in Relation to Cardiometabolic Biomarkers and the Risk of Gestational Diabetes. Am. J. Clin. Nutr. 2018, 107, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, M.; Rahman, M.L.; Hinkle, S.N.; Wu, J.; Weir, N.L.; Lin, Y.; Yang, H.; Tsai, M.Y.; Ferrara, A.; et al. Plasma Phospholipid N-3 and n-6 Polyunsaturated Fatty Acids in Relation to Cardiometabolic Markers and Gestational Diabetes: A Longitudinal Study within the Prospective NICHD Fetal Growth Studies. PLoS Med. 2019, 16, e1002910. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Maternal PUFAs, Placental Epigenetics, and Their Relevance to Fetal Growth and Brain Development. Reprod. Sci. 2023, 30, 408–427. [Google Scholar] [CrossRef]
- Wild, R.; Feingold, K. Effect of Pregnancy on Lipid Metabolism and Lipoprotein Levels; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Copyright © 2000–2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK498654/ (accessed on 1 October 2023).
- Srinivas, V.; Molangiri, A.; Mallepogu, A.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Maternal N-3 PUFA Deficiency Alters Uterine Artery Remodeling and Placental Epigenome in the Mice. J. Nutr. Biochem. 2021, 96, 108784. [Google Scholar] [CrossRef]
- Grootendorst-van Mil, N.H.; Tiemeier, H.; Steenweg-de Graaff, J.; Koletzko, B.; Demmelmair, H.; Jaddoe, V.W.V.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Maternal Plasma N-3 and n-6 Polyunsaturated Fatty Acids during Pregnancy and Features of Fetal Health: Fetal Growth Velocity, Birth Weight and Duration of Pregnancy. Clin. Nutr. 2018, 37, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Blanco Sequeiros, E.; Tuomaala, A.-K.; Tabassum, R.; Bergman, P.H.; Koivusalo, S.B.; Huvinen, E. Early Ascending Growth Is Associated with Maternal Lipoprotein Profile during Mid and Late Pregnancy and in Cord Blood. Int. J. Obes. 2023, 47, 1081–1087. [Google Scholar] [CrossRef]
- Lam, T.K.T.; Carpentier, A.; Lewis, G.F.; Rald Van De Werve, G.; Fantus, I.G.; Giacca, A. Mechanisms of the Free Fatty Acid-Induced Increase in Hepatic Glucose Production. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E863–E873. [Google Scholar] [CrossRef]
- Ishii, M.; Maeda, A.; Tani, S.; Akagawa, M. Palmitate Induces Insulin Resistance in Human HepG2 Hepatocytes by Enhancing Ubiquitination and Proteasomal Degradation of Key Insulin Signaling Molecules. Arch. Biochem. Biophys. 2015, 566, 26–35. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, X.; Lu, Z.; Perry, D.M.; Li, Y.; Brice Russo, S.; Ashley Cowart, L.; Hannun, Y.A.; Huang, Y. Acid Sphingomyelinase Plays a Key Role in Palmitic Acid-Amplified Inflammatory Signaling Triggered by Lipopolysaccharide at Low Concentrations in Macrophages. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 853–867. [Google Scholar] [CrossRef]
- Yakoob, M.Y.; Shi, P.; Hu, F.B.; Campos, H.; Rexrode, K.M.; Orav, E.J.; Willett, W.C.; Mozaffarian, D. Circulating Biomarkers of Dairy Fat and Risk of Incident Stroke in U.S. Men and Women in 2 Large Prospective Cohorts. Am. J. Clin. Nutr. 2014, 100, 1437–1447. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.J.; et al. Differences in the Prospective Association between Individual Plasma Phospholipid Saturated Fatty Acids and Incident Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Xia, T.; Chen, L.; Fei, Z.; Liu, X.; Dai, J.; Hinkle, S.N.; Zhu, Y.; Wu, J.; Weir, N.L.; Tsai, M.Y.; et al. A Longitudinal Study on Associations of Moderate-to-Vigorous Physical Activity with Plasma Monounsaturated Fatty Acids in Pregnancy. Front. Nutr. 2022, 9, 983418. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, C.; Chen, W.; Meng, Q. Analysis of Specific Lipid Metabolites in Cord Blood of Patients with Gestational Diabetes Mellitus. Biocell 2022, 46, 1565–1573. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine Metabolism and Nutrition in Growth, Health and Disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.M.; Mellott, T.J.; Kovacheva, V.P.; Blusztajn, J.K. Gestational Choline Supply Regulates Methylation of Histone H3, Expression of Histone Methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA Methylation of Their Genes in Rat Fetal Liver and Brain. J. Biol. Chem. 2009, 284, 1982–1989. [Google Scholar] [CrossRef]
- Cleal, J.K.; Lewis, R.M. The Mechanisms and Regulation of Placental Amino Acid Transport to the Human Foetus. J. Neuroendocrinol. 2008, 20, 419–426. [Google Scholar] [CrossRef]
- Jones, H.N.; Powell, T.L.; Jansson, T. Regulation of Placental Nutrient Transport—A Review. Placenta 2007, 28, 763–774. [Google Scholar] [CrossRef]
- Cetin, I.; Ronzoni, S.; Marconi, A.M.; Perugino, G.; Corbetta, C.; Battaglia, F.C.; Pardi, G. Maternal Concentrations and Fetal-Maternal Concentration Differences of Plasma Amino Acids in Normal and Intrauterine Growth-Restricted Pregnancies. Am. J. Obstet. Gyneco 1995, 174, 1575–1583. [Google Scholar] [CrossRef]
- Cetin, I.; Nobile De Santis, M.S.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and Fetal Amino Acid Concentrations in Normal Pregnancies and in Pregnancies with Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ekstrand, Y.; Bjö, C.; Wennergren, M.; Powell, T.L. Alterations in the Activity of Placental Amino Acid Transporters in Pregnancies Complicated by Diabetes. Diabetes 2002, 51, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-Chain Amino Acids in Metabolic Signalling and Insulin Resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- El-Batch, M.; Ibrahim, W.; Said, S. Effect of Histidine on Autotaxin Activity in Experimentally Induced Liver Fibrosis. J. Biochem. Mol. Toxicol. 2011, 25, 143–150. [Google Scholar] [CrossRef]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into Intrauterine Growth Restriction Based on Maternal and Umbilical Cord Blood Metabolomics. Sci. Rep. 2021, 11, 7824. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.; Simões, R.V.; Miranda, J.; García-Martín, M.L.; Paules, C.; Crovetto, F.; Amigó, N.; Cañellas, N.; Gratacos, E.; Crispi, F. Paired Maternal and Fetal Metabolomics Reveal a Differential Fingerprint in Preeclampsia versus Fetal Growth Restriction. Sci. Rep. 2021, 11, 14422. [Google Scholar] [CrossRef] [PubMed]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of Maternal BMI and Gestational Diabetes Mellitus on Maternal and Cord Blood Metabolome: Results from the PREOBE Cohort Study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Favretto, D.; Cosmi, E.; Ragazzi, E.; Visentin, S.; Tucci, M.; Fais, P.; Cecchetto, G.; Zanardo, V.; Viel, G.; Ferrara, S.D. Cord Blood Metabolomic Profiling in Intrauterine Growth Restriction. Anal. Bioanal. Chem. 2012, 402, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | Physiological Functions | Association with PA | |
|---|---|---|---|
| BCAAs | Leucine | Negative | |
| Isoleucine | |||
| Valine | |||
| AAAs | Phenylalanine | Negative | |
| Tyrosine | |||
| Tryptophan | |||
| Sulfur-containing AAs | Methionine | Negative | |
| Cysteine | |||
| Cystine | |||
| The urea cycle AAs | Arginine | Positive | |
| Ornithine | |||
| Citrulline | |||
| Glucogenic AAs | Alanine | Negative | |
| Glutamine | Positive | ||
| Serine, Glycine, and Threonine | Positive | ||
| Study Population and Sample Size | Biosample | Study Design | Metabolomic Profiling Platform | PA Intervention or Measurement | Main Results |
|---|---|---|---|---|---|
| 11 women with overweight/obesity (18–45 years of age) | Plasma | Intervention | Shotgun TOF-MS | HIIT for 6 weeks | Ornithine levels were significantly upregulated after HIIT [41]. |
| 339 healthy adults (147 women, 40–64 years of age) | Plasma | Cohort | LC-MS and GC-MS | PAEE (MET hour per day) measured by accelerometer | Distinct amino acids (e.g., valine, isoleucine, glutamate, alanine, leucine) were associated with PA, even if the intensity was low [14]. |
| 102 adults, including 47 women (30–60 years of age) | Serum | Cross-sectional | NMR | AEE derived from doubly labeled water and Sedentary Time and Activity Reporting Questionnaire | Serine, arginine, and betaine were associated with higher levels of PA [45]. |
| 5197 people, including 2120 healthy women (25–42 years of age) | Plasma | Cross-sectional | LC-MS | Self-reported questionnaires every 2–4 years | Citrulline, asparagine, glutamate, and glycine were the amino acids associated with PA [1]. |
| 12 women with PCOS (aged 25.26 ± 2.0) and 10 aged and BMI-matched healthy controls | Plasma | Intervention | LC-MS | Supervised exercise program for a 1 h session at 60% of baseline VO2 max, three times per week for 8 weeks | Patients with PCOS had higher concentrations of BCAAs and AAAs compared to the controls. Following an 8-week exercise intervention, the difference in amino acid concentrations no longer existed between groups for isoleucine (BCAA), phenylalanine (AAA), and tyrosine (AAA) [53]. |
| 3195 incident CVD cases and 1465 controls (30–79 years of age) | Plasma | Nested case-control | NMR | Self-reported PA and sedentary leisure time | PA had a significant inverse association with alanine and a significant positive association with glutamine. Sedentary leisure time was inversely associated with glutamine and histidine and positively associated with alanine [49]. |
| Women with obesity, sedentary lifestyle, and insulin resistance (30–50 years of age) | Plasma | Intervention | GC–MS | Data from calorie-restricted diet + 14 wks of 4-day/wk aerobic exercise at 60–75% of maximal HR combined with data from an acute bout of exercise | Acute exercise lowered plasma amino acids as a biochemical class. Individual amino acids, including methionine, cysteine, cystine, serine, tryptophan, phenylalanine, serine, and aspartic acid, were significantly reduced during exercise [34]. |
| 1983 individuals categorized as obese and overweight (45–65 years of age) | Plasma | Cross-sectional | NMR | Self-reported questionnaire | Isoleucine and leucine levels were significantly lower in less-active individuals compared to more-active individuals. Compared to men, women had significantly lower levels of BCAAs [4]. |
| 20 adults with MASLD (13 women, 59 years of age) | Plasma, stool, and adipose tissue (AT) | Intervention | LC-MS | HIIT for 12 weeks Control: sedentary lifestyle | The samples studied had distinct metabolic profiles. AT amino acids such as arginine, leucine, phenylalanine, and proline were higher after the PA, while methyl proline was reduced in plasma. Stool samples had different leucine-contained peptides reduced in the intervention group [42]. |
| 18,897 women (aged 45 or older) free of T2D, cancer, and cardiovascular disease at baseline | Plasma | Cross-sectional | NMR | Self-reported questionnaire | Compared to the lowest quartile of LTPA, the highest quartile had significantly lower total BCAAs (1%). For individual BCAAs, the lowest vs. highest LTPA had significantly lower leucine concentrations [32]. |
| 2217 individuals (aged 41.2 ± 8.3) | Plasma | Cohort | LC/GC–MS | Self-reported questionnaire | Vigorous (but not moderate) PA was inversely associated with several metabolites independent of BMI, including glutamate [46]. |
| sedentary adults; 14 lean and 10 obese | Plasma Stool | Intervention | LC-MS/MS | 6 wks of supervised, aerobic exercise 3×/wk (60–75% heart rate reserve) for 30–60min | Serum methionine levels increased significantly by exercise irrespective of obesity status AAA and AA metabolic pathways were upregulated in obese individuals [35]. |
| Study Population and Sample Size | Sample | Study Design | Metabolomic Profiling Platform | PA Intervention/Measurement | Main Results |
|---|---|---|---|---|---|
| 806 pregnant individuals (first trimester); 886 pregnant women (third trimester) | Gestational parent urine | Cohort | NMR spectroscopy | Questionnaires | PA was significantly associated with lower BCAA levels in the third trimester [12]. |
| 343 mothers with obesity and their offspring (intervention = 169, control = 174) | Cord blood serum | Randomized controlled trial and cohort | MS | An initial session with a health trainer, followed by eight weekly sessions + plus dietary advice recommending foods with a low dietary glycemic index, avoidance of sugar-sweetened beverages, and reduced saturated fats | No association between cord blood metabolites and lifestyle intervention. Phosphatidylcholines and LPCs were associated with birth weight [73]. |
| 1158 individuals with obesity (intervention = 577; control = 581) | Gestational parent serum | Randomized controlled trial | NMR spectroscopy | An initial session with a health trainer, followed by eight weekly sessions + plus dietary advice recommending foods with a low dietary glycemic index, avoidance of sugar-sweetened beverages, and reduced saturated fats | The intervention was able to decrease the magnitude of change in several metabolites, including extremely large, very large, large, and medium VLDL particles, specifically those containing triglycerides [13]. |
| Healthy pregnant individuals in mid- and early-pregnancy (intervention = 13; control = 16) | Gestational parent plasma | Randomized controlled trial | LC-MS | A lifestyle modification intervention focused on improving PA levels, diet quality, and calorie intake in a standard care control group before 16 gestational weeks | Lifestyle intervention was able to decrease levels of acylcarnitines, amino acids, 3-hydroxybutyrate, and bile acids in mid-pregnancy [74]. |
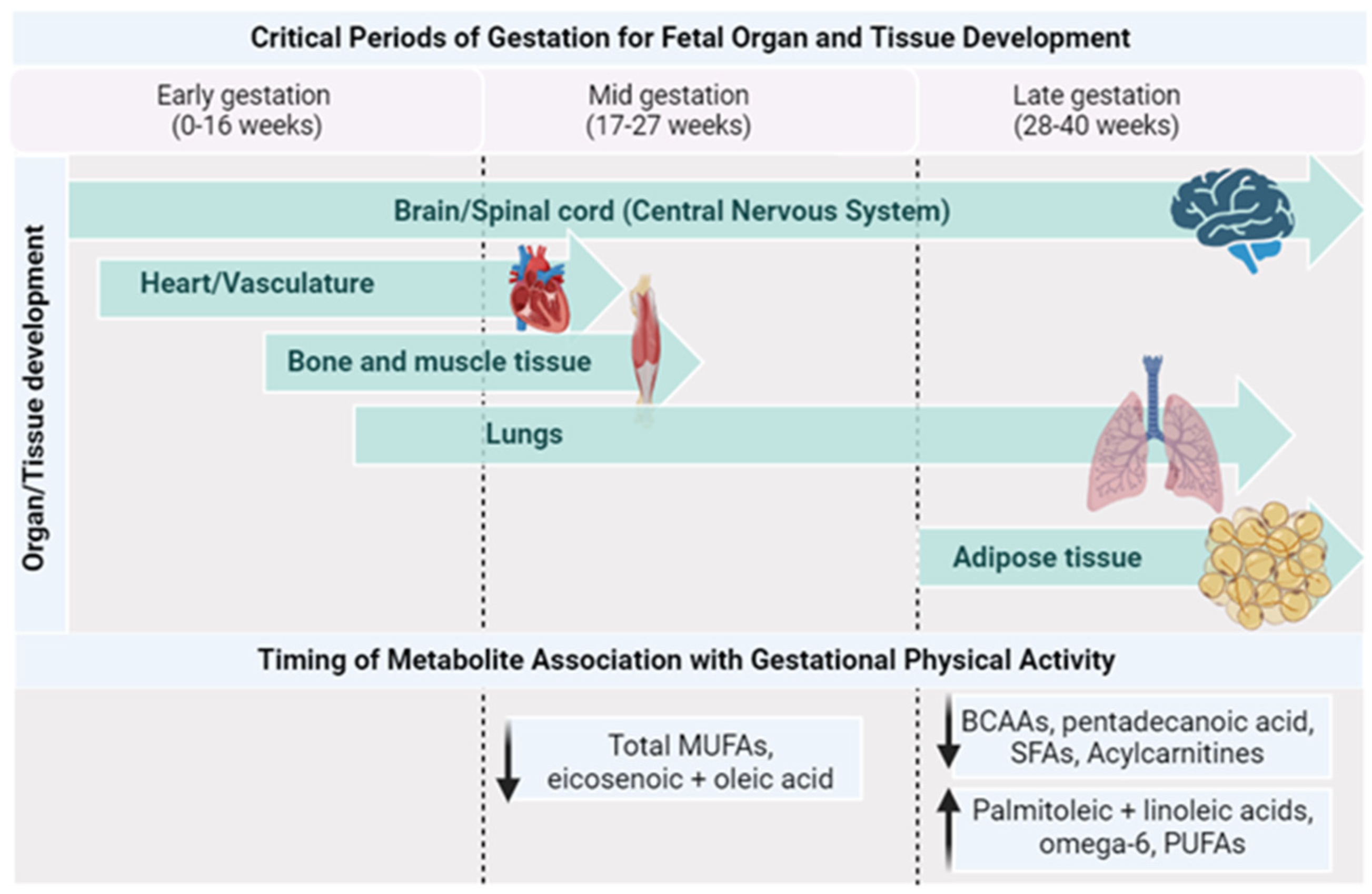
| 600 healthy pregnant individuals (24–36 weeks) | Gestational parent serum | Cohort | Electrophoresis MS | Questionnaires | Higher PA was associated with lower 17:0 SFAs [75]. |
| 318 healthy pregnant individuals | Gestational parent plasma | Cohort | Gas chromatography system with flame ionization detection (GC-FID) | Self-administrated Pregnancy PA Questionnaire | Higher MVPA at 15–26 weeks was positively associated with total MUFAs; higher MVPA at 15–26 weeks was positively associated with oleic acid and eicosenoic acid; higher MVPA at 23–31 weeks was positively associated with palmitoleic acid [14]. |
| Exercise-trained or sedentary male and female C57BL/6 mice | Offspring serum, liver, and triceps muscle | Animal study | LC-MS and GC-MS | Mice housed with running wheels for 2 weeks before conception and during gestation | In the liver, maternal exercise increased tyrosine, histidine, and phenylalanine and decreased pyruvate and short and long fatty acids; in the muscle, maternal exercise increased serine, ATP, and NADP and decreased short AC; in serum, maternal exercise decreased valine and long ACs, and increased short ACs [11]. |
| High-fat, fructose, and cholesterol-fed female C57Bl/6J mice and normal diet control | Liver tissue | Animal study | LC-MS | Mice housed for voluntary exercise of at least 2 km per day for 6 weeks before conception and during gestation | Maternal exercise modified hepatic levels of taurocholate and cholate sulfate, eicosoenoylcarnitin (C20:1), behenoylcarnitine (C22:0), histidine, choline, and sarcosine [72]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.C.R.; Yadegari, A.; Tzaneva, V.; Vasanthan, T.; Laketic, K.; Shearer, J.; Bainbridge, S.A.; Harris, C.; Adamo, K.B. Metabolomics to Understand Alterations Induced by Physical Activity during Pregnancy. Metabolites 2023, 13, 1178. https://doi.org/10.3390/metabo13121178
da Silva ACR, Yadegari A, Tzaneva V, Vasanthan T, Laketic K, Shearer J, Bainbridge SA, Harris C, Adamo KB. Metabolomics to Understand Alterations Induced by Physical Activity during Pregnancy. Metabolites. 2023; 13(12):1178. https://doi.org/10.3390/metabo13121178
Chicago/Turabian Styleda Silva, Ana Carolina Rosa, Anahita Yadegari, Velislava Tzaneva, Tarushika Vasanthan, Katarina Laketic, Jane Shearer, Shannon A. Bainbridge, Cory Harris, and Kristi B. Adamo. 2023. "Metabolomics to Understand Alterations Induced by Physical Activity during Pregnancy" Metabolites 13, no. 12: 1178. https://doi.org/10.3390/metabo13121178








