Impact of Different Treatment Regimens and Timeframes in the Plasmatic Metabolic Profiling of Patients with Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Therapeutic Regimens
2.3. Sample Collection
2.4. NMR Spectroscopy
2.5. Statistical Analysis
3. Results
3.1. General Metabolomic Profile—Univariate Analysis
3.2. General Metabolomic Profile—Multivariate Analysis
3.3. Long Responders’ Metabolomic Profile—Univariate Analysis
3.4. Long Responders’ Metabolomic Profile—Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; on behalf of the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. Available online: https://gco.iarc.fr (accessed on 3 June 2023).
- Madama, D.; Martins, R.; Pires, A.S.; Botelho, M.F.; Alves, M.G.; Abrantes, A.M.; Cordeiro, C.R. Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabollites 2021, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.; Morra, F.; Guggino, G.; Celetti, A. The between Now and Then of Lung Cancer Chemotherapy and Immunotherapy. Int. J. Mol. Sci. 2017, 18, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Wallace, A.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.E.; DeCamp, M.; et al. NCCN Clinical Practical Guidelines in Oncology. Non-Small Cell Lung Cancer, Version 5.2023. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Escuín, J.S.D.C. New Immunotherapy and Lung Cancer. Arch. Bronconeumol. 2017, 53, 682–687. [Google Scholar] [CrossRef]
- Cordero, R.R.; Devine, W.P. Targeted Therapy and checkpoint Immunotherapy in Lung Cancer. Surg. Path 2020, 13, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The concept of immune surveillance against tumors: The first theories. Oncotarget 2017, 8, 7175–7180. [Google Scholar] [CrossRef]
- Prantesh, J.; Chhavi, J.; Vamsidhar, V. Role of Immune-checkpoint inhibitors in lung cancer. Ther. Adv. Respir. Dis. 2018, 12, 1–13. [Google Scholar]
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef]
- Rihawi, K.; Gelsomino, F.; Sperandi, F.; Melotti, B.; Fiorentino, M.; Casolari, L.; Ardizzoni, A. Pembrolizumab in the treatment of metastatic non-small cell lung cancer: A review of current evidence. Ther. Adv. Respir. Dis. 2017, 11, 353–373. [Google Scholar] [CrossRef]
- Brahmer, J.; Rodriguez-Abreu, D.; Robinson, A.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥ 50%. Ann. Oncol. 2020, 30, 1181–1182. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in metastatic non-small-cell-lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Laera, L.; Fantechi, B.; Monte, F.; Benelli, M.; McCartney, A.; Leonardo, T.; Luchinat, C.; Pozzessere, D. Metabolomics to Assess Response to Immune Checkpoint Inhibitors in Patients with Non-Small-Cell Lung Cancer. Cancers 2020, 12, 3574. [Google Scholar] [CrossRef]
- De Vries, R.; Muller, M.; van der Noort, V.; Theelen, W.S.M.E.; Schouten, R.D.; Hummelink, K.; Muller, S.H.; Wolf-Lansdorf, M.; Dagelet, J.W.F.; Monkhorst, K.; et al. Prediction of response to anti-PD-1 therapy in patients with non-small-cell lung cancer by electronic nose analysis of exhaled breath. Ann. Oncol. 2019, 30, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Duruisseaux, M.; Martínez-Cardús, A.; Calleja-Cervantes, M.E.; Moran, S.; de Moura, C.M.; Davalos, V.; Piñeyro, D.; Sanchez-Cespedes, M.; Girard, N.; Brevet, M.; et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: A multicentre, retrospective analysis. Lancet Respir. Med. 2018, 6, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Mazurek, S. Tumour Cell Energetic Metabolome. In Molecular System Bioenergetic: Energy for Life; Saks, V., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 521–540. [Google Scholar]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2017, 2, 881–898. [Google Scholar] [CrossRef]
- Rocha, C.M. Metabolic Signature of Lung Cancer: A Metabolomic Study of Human Tissues and Biofluids. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2015. [Google Scholar]
- Shen, J.; Ye, Y.; Chang, D.W.; Huang, M.; Heymach, J.V.; Roth, J.A.; Wu, X.; Zhao, H. Circulating metabolite profiles to predict overall survival in advanced non-small cell lung cancer patients receiving first-line chemotherapy. Lung Cancer 2017, 114, 70–78. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Liu, X.; Duan, J.; Feng, G.; Yin, Y.; Gu, J.; Chen, Z.; Gao, S.; Bai, H.; et al. Prediction of chemotherapeutic efficacy in non-small cell lung cancer by plasma metabolomic profiling. Clin. Cancer Res. 2018, 24, 2100–2109. [Google Scholar] [CrossRef]
- Hao, D.; Sengupta, A.; Ding, K.; Ubeydullah, E.R.; Krishnaiah, S.; Leighl, N.B.; Shepherd, F.A.; Seymour, L.; Weljie, A. Metabolites as Prognostic Markers for Metastatic Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Platinum-Doublet Chemotherapy. Cancers 2020, 12, 1926. [Google Scholar] [CrossRef]
- Hao, D.; Sarfaraz, M.O.; Farshidfar, F.; Bebb, D.B.; Lee, C.Y.; Card, C.M.; David, M.; Weljie, A.M. Temporal characterization of plasma metabolite signatures in lung cancer patients undergoing treatment. Metabolomics 2016, 12, 58. [Google Scholar] [CrossRef]
- Hatae, R.; Chamoto, K.; Kim, Y.H.; Sonomura, K.; Taneishi, K.; Kawaguchi, S.; Yoshida, H.; Ozasa, H.; Sakamori, Y.; Akrami, M.; et al. Combination of host immune metabolic biomarkers for the PD-1 blockade cancer immunotherapy. JCI Insight 2020, 5, e133501. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Xia, L.; Gao, F.; Liu, L.; Yang, Y.; Chen, Y.; Duan, H.; Yao, Y.; Chen, Z.; Lu, S.; et al. Plasma metabolite biomarkers predictive of response to PD-1 blockade therapy in non-small cell lung cancer. Front. Mol. Biosci. 2021, 8, 678753. [Google Scholar] [CrossRef]
- Brusselaers, N.; Lagergren, J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar]
- Eisenhauera, E.A.; Therasseb, P.; Bogaertsc, J.; Schwartzd, L.H.; Sargente, D.; Fordf, R.; Danceyg, J.; Arbuckh, S.; Gwytheri, S.; Mooneyg, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 247. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iReCIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Grapov, D.; Fahrmann, J.F.; DeFelice, B.; Rom, W.; Pass, H.; Kim, K.; Nguyen, U.T.; Taylor, S.L.; Kelly, K.; et al. Metabolomic Markers of Altered Nucleotide Metabolism in Early-Stage Adenocarcinoma. Cancer Prev. Res. 2015, 8, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Jimenez-Jim, C.; Garrido-Rodrıguez, M.; Calderón-Santiago, M.; Molina, S.; Lara-Chica, M.; Priego-Capote, F.; Salvatierra, A.; Munoz, E.; Calzado, M.A. Metabolomic profiling of human lung tumor tissues—Nucleotide metabolism as a candidate for therapeutic interventions and biomarkers. Mol. Oncol. 2018, 12, 1778–1796. [Google Scholar] [CrossRef]
- Bordag, N.; Klie, S.; Jurchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Lane, A.N.; Higashi, R.M.; Farag, M.A.; Gao, H.; Bousamra, M.; Miller, D.M. Altered regulation of metabolic pathways in human lung cancer discerned by 13C stable isotope-resolved metabolomics (SIRM). Mol. Cancer 2009, 8, 41. [Google Scholar] [CrossRef]
- Kami, K.; Fujimori, T.; Sato, H.; Sato, M.; Yamamoto, H.; Ohashi, Y.; Sugiyama, N.; Ishihama, Y.; Onozuka, H.; Ochiai, A.; et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 2013, 9, 444–453. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Gupta, R.; Kumar, V.; Kumar, V.; Rai, E. Intervention on lactate in cancer: A promising approach for the development of cancer therapeutics. Adv. Cancer Biol. Metastasis 2022, 5, 100058. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2021, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Y.; Atefi, M.; Liu, Y.; Elshimali, Y.; Vadgama, J.V. Lactate, a neglected factor for diabetes and cancer interaction. Mediat. Inflamm. 2016, 2016, 6456018. [Google Scholar] [CrossRef] [PubMed]
- Klupczynska, A.; Derezinski, P.; Dyskiewicz, W.; Paylak, K.; Kasprzyk, M.; Kokot, Z.J. Evaluation of plasma amino acid profiles’ utility in non-small cell lung cancer detection in Polish population. Lung Cancer 2016, 100, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Higashiyama, M.; Imaizumi, A.; Nakayama, T.; Yamamoto, H.; Daimon, T.; Yamakado, M.; Imamura, F.; Kodama, K. Possibility of multivariate function composed of plasma amino acid profiles as a novel screening index for non-small cell lung cancer: A case control study. BMC Cancer 2010, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Ramaty, E.; Maor, E.; Peltz-Sinvani, N.; Brom, A.; Grinfeld, A.; Kivity, S.; Segev, S.; Sidi, Y.; Kessler, T.; Sela, B.A.; et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur. J. Intern. Med. 2014, 25, 919–921. [Google Scholar] [CrossRef]
- Ramati, E.; Israel, A.; Kessler, T.; Petz-Sinuani, N.; Sela, B.A.; Goren, I.; Grinfeld, A.; Lavi, B.; Segal, G. Low ALT activity amongst patients hospitalized in internal medicine wards is a widespread phenomenon associated with low vitamin B6 levels in their blood. Harefuah 2015, 154, 89–93, 137. [Google Scholar]
- Dagan, A.; Sella, T.; Urban, D.; Onn, A.; Bar, J.; Segal, G. Low alanine transaminase is not associate with increased rate of mortality in patients with advanced lung cancer. JCSM Clin. Rep. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Stocke, A.B.; van Berkel, V.; Miller, D.M.; Frieboes, H.B. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics 2018, 14, 81. [Google Scholar] [CrossRef]









| CTX Group N = 9 | IT Group N = 9 | p-Value | |
|---|---|---|---|
| Age (years) | 61.56 ± 9.50 | 60.67 ± 8.44 | 0.840 |
| Smoking habits (pack-years) | 41.11 ± 41.67 | 14.67 ± 23.15 | 0.116 |
| Gender—Female (%) | 17.20 | 44.40 | 0.317 |
| CCI | 2 (0–5) | 3 (0–3) | 0.446 |
| Treatment duration (months) | 9.78 ± 4.24 | 13 ± 7.19 | 0.247 |
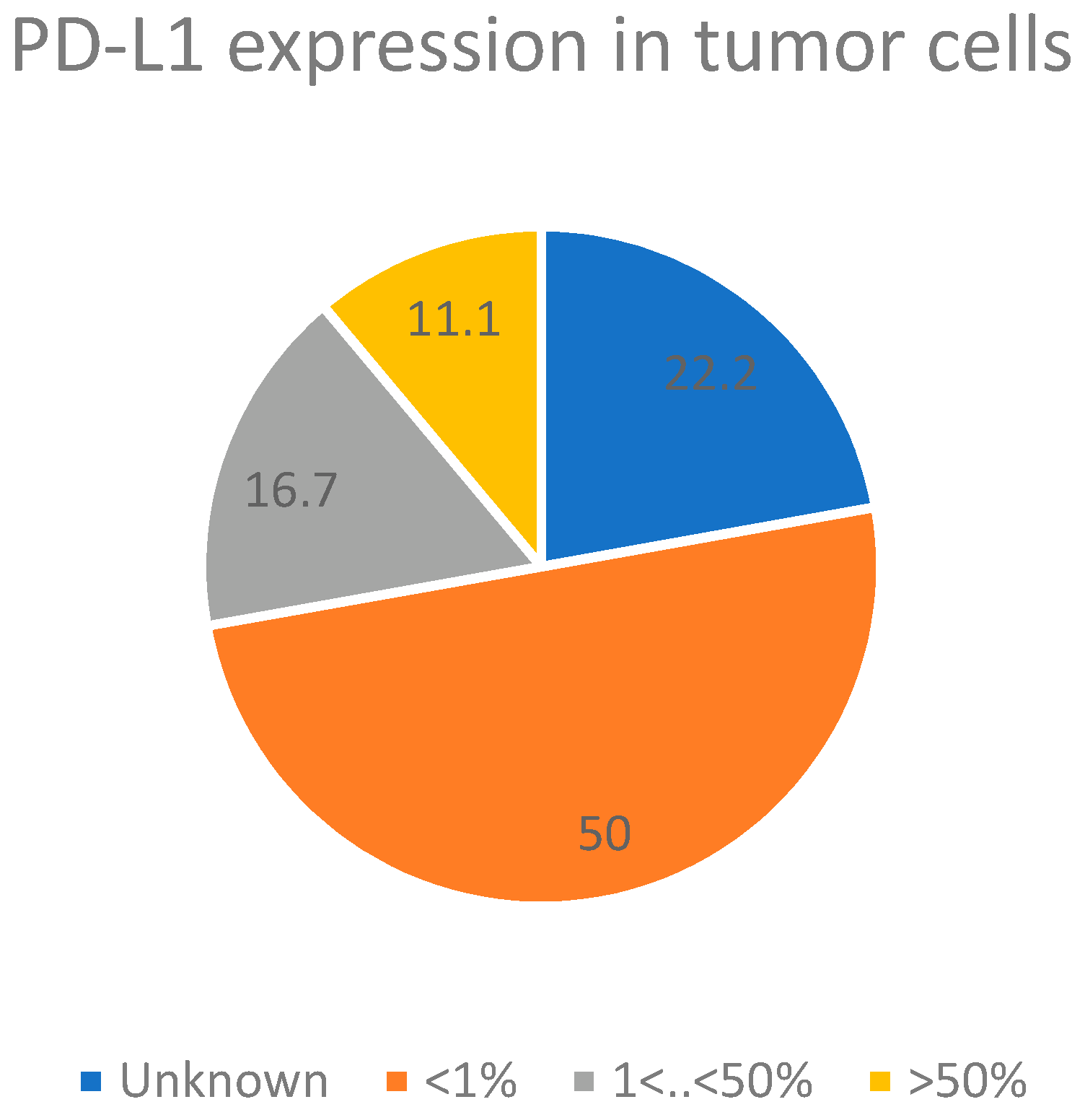
| Patient Number | Diagnosis | Staging | PD-L1 Level | Age | Sex | Smoking Habits | Profession | Housing Type | Co-Morbidities (CCI) | Family History of Cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AdC | IIIA T4N0M0 | Neg | 69 | M | S—60 SPY | Retired (legal assistant) | Rural house | HT, COPD, Dysl (3) | Stomach |
| 2 | AdC | IVA T4N1M1a | 10% | 63 | M | FS—40 SPY | Retired (budgetist) | Rural house | HT, Dysl, OSA, AMI (3) | Prostate |
| 3 | AdC | IVA T4N0M1a | ? | 69 | F | NS | Farmer | Rural house | 0 (2) | Lung |
| 4 | AdC | IVA T4N0M1a | Neg | 57 | F | NS | Stay-at-home mom | Rural house | 0 (1) | Breast |
| 5 | AdC | IVA T4N3M1a | Neg | 75 | M | FS—70 SPY | Retired (policeman) | Rural house | COPD, DM, HT, Dysl (5) | Larynx |
| 6 | AdC | IIIC T4N3M0 | Neg | 53 | F | NS | Stay-at-home mom | Rural house | Sarcoidosis (1) | Prostate |
| 7 | AdC | IIIC T3N3M0 | 90% | 62 | M | S—100 SPY | Construction | Rural house | Dysl (2) | 0 |
| 8 | AdC | IIIA T2bN2M0 | Neg | 65 | M | FS—32 SPY | Electrician | City apartment | HT, Dysl (2) | 0 |
| 9 | AdC | IVA T3N3M1b | 70% | 47 | M | NS | ? | City apartment | 0 (0) | 0 |
| 10 | AdC | IIIA T2bN2M0 | ? | 44 | M | NS | Scrap worker | City apartment | 0 (0) | 0 |
| 11 | AdC | IVB T3N0M1c | Neg | 65 | M | NS | Construction | Rural house | AF, HT, Dysl (2) | 0 |
| 12 | AdC | IVA T4N0M1a | ? | 69 | F | NS | Stay-at-home mom | Rural house | 0 (5) | Lung |
| 13 | AdC | IVA T3N2M1b | 20% | 52 | F | NS | Stay-at-home mom | Rural house | Dysl (2) | 0 |
| 14 | AdC | IVA T4N0M1a | Neg | 56 | M | S—90 SPY | Cleaning open spaces | Rural house | COPD, alcoholism (2) | Colon |
| 15 | AdC | IIIB T3N2M0 | ? | 61 | M | FS—70 SPY | Retired (construction) | City apartment | Dysl, HT, myocardiopathy (3) | 0 |
| 16 | AdC | IIIC T4N3M0 | Neg | 54 | F | NS | Stay-at-home mom | Rural house | Sarcoidosis, Amaurosis (1) | Prostate |
| 17 | AdC | IVB T3N3M1c | 10% | 69 | M | FS—50 SPY | Retired (bank officer) | City apartment | Colon tumor (2) | Larynx |
| 18 | AdC | IVA T1N1M1b | Neg | 70 | M | NS | Retired (truck driver) | Rural house | HT (19 | Colon |
| Patient No | Weight (Kg) | BMI (Kg/m2) | Hemoglobin (g/dL) | Glyc Hb (%) | Leucocytes (×109/L) | Platelets (×109/L) | Sodium (mmol/L) | Potassium (mmol/L) | Urea Nitrogen (mg/dL) | Creatinine (mg/dL) | Glucose (mg/dL) | Osmolarity (mOSM/Kg) | Triglycerides (mg/dL) | Cholesterol (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71/71/71 | 26.7/26.7/26.7 | 14.1/14.9/14.8 | 5.8 | 5.9/6.4/7.4 | 213/227/226 | 132/135/134 | 4.8/4.4/4.4 | 11/12/13 | 0.98/1.02/1.19 | 99/99/97 | 264/271/268 | 181/170/281 | 203/214/200 |
| 2 | 63/67/71 | 21.7/23.2/24.7 | 12.4/13.3/14 | 6 | 8.5/7.1/9.3 | 266/296/278 | 140/139/138 | 3.9/4.3/4.3 | 16/13/15 | 0.74/0.73/0.72 | 111/104/115 | 280/278/277 | 118 | 118 |
| 3 | 73/72/73 | 27.9/27.5/27.6 | 13.8/13.2/11.3 | 6.5 | 12.3/8/7.6 | 223/260/195 | 138/138/139 | 4/3.6/3.2 | 19/24/23 | 0.66/0.66/0.69 | 276/203/244 | 286/286/289 | 247 | 224 |
| 4 | 52/52/51 | 22/22/21.5 | 13.2/13.4/13 | 6.6 | 12.7/10.8/12.7 | 299/279/371 | 140/139/136 | 4.7/3.8/3.7 | 15/16/13 | 0.72/0.7/0.62 | 127/116/122 | 281/270/273 | 111 | 309 |
| 5 | 63/65/65 | 21.8/22.5/22.5 | 14.1/10.7/10.5 | 6.6 | 6.6/3.7/3.7 | 205/364/307 | 137/136/135 | 3.9/4.8/5.1 | 14/26/20 | 0.79/0.93/0.95 | 277/289/284 | 284/287/283 | 73 | 133 |
| 6 | 99/101/97 | 31.9/32.6/31.3 | 11.3/11.1/11 | 5.4 | 8.9/6.5/8.9 | 249/417/373 | 132/130/132 | 4.6/5.1/5.4 | 11/8/12 | 0.73/0.67/0.72 | 128/112/114 | 266/260/265 | 304 | 93 |
| 7 | 60/62/64 | 20.3/20.1/21.6 | 13.8/13.5/12.1 | 6.2 | 15/6.2/6.6 | 200/346/404 | 139/140/139 | 4.1/3.9/3.9 | 15/14/14 | 0.6/0.74/0.69 | 93/149/154 | 278/283/276 | 62 | 115 |
| 8 | 84/84/77 | 26.8/26.8/24.3 | 12.2/11.2/10.1 | 6.6 | 6.1/7.7/7.8 | 206/288/347 | 139/141/137 | 5.1/4.6/4 | 13/23/17 | 0.86/0.87/0.75 | 87/72/116 | 272/284/276 | 193 | 32 |
| 9 | 74/70/71 | 22.4/21.1/21.4 | 9.7/13.3/13.8 | 5.7 | 13.7/5.3/5.7 | 754/343/344 | 137/138/140 | 4.2/4.5/3.9 | 9/16/23 | 0.74/0.77/0.84 | 131/87/89 | 274/274/283 | 176 | 182 |
| 10 | 75/68/65 | 24.6/22.2/21.2 | 14/13.4/12.2 | 5.8 | 11.5/3.5/3.6 | 250/289/222 | 137/138/138 | 4/4.2/4.4 | 16/10/10 | 0.87/0.77/0.93 | 113/113/115 | 275/276/278 | 51 | 185 |
| 11 | 68/67/69 | 27.3/26.9/27.6 | 16/15.2/15 | 5.8 | 7.0/5.1/6.9 | 279/252/214 | 141/142/141 | 3.9/3.8/3.9 | 10/19/17 | 0.66/0.82/1 | 115/94/95 | 282/285/283 | 115 | 180 |
| 12 | 75/71/69 | 28.6/27.1/26.1 | 14/13.5/13.1 | 7.5 | 6.9/6.9/6.2 | 170/196/105 | 143/138/141 | 3.8/4.1/3.9 | 17/17/19 | 0.65/0.61/0.89 | 128/139/92 | 286/285/283 | 247 | 224 |
| 13 | 42/44/44 | 20/21/21 | 9.1/10.9/11 | 5.3 | 8.8/3.6/4.9 | 583/381/351 | 136/136/139 | 4.2/4,1/4.4 | 10/13/18 | 0.75/0.74/0.69 | 198/107/131 | 277/278/281 | 80 | 183 |
| 14 | 50/47/49 | 16.9/16.3/16.6 | 12.8/11.7/8.5 | 5.5 | 7.3/4.1/5.5 | 186/316/395 | 138/142/139 | 4.8/5.1/5.1 | 8/6/9 | 0.81/0.73/0.83 | 141/154/305 | 276/284/288 | 69 | 259 |
| 15 | 79/73/73 | 24.9/22.9/22.9 | 16/12.8/12.7 | 6.8 | 9.4/5.9/7.5 | 338/419/415 | 140/140/140 | 5.1/5.3/4.7 | 33/22/25 | 1.17/1.32/1.36 | 196/165/233 | 272/286/293 | 95 | 163 |
| 16 | 99/95/95 | 37.7/36.2/36.2 | 11.2/10.9/11.2 | 5.4 | 5.2/4.2/5.4 | 213/241/233 | 134/132/131 | 3.9/4.7/4.5 | 8/8/10 | 0.91/0.68/0.83 | 103/85/86 | 267/262/261 | 304 | 310 |
| 17 | 107/104/105 | 32.3/31.4/32 | 14.3/11/11.1 | 5.9 | 10.7/5.4/8.9 | 277/333/397 | 134/135/136 | 4.5/5/4.9 | 15/11/10 | 0.78/0.78/0.74 | 169/141/158 | 273/272/274 | 59 | 189 |
| 18 | 113/101/99 | 35.2/33.4/31 | 8.8/10/10.3 | 5.4 | 6.4/5.6/6.9 | 223/213/243 | 142/140/140 | 4/3.6/3.4 | 11/12/11 | 0.75/0.74/0.64 | 102/144/144 | 283/282/281 | 49 | 219 |
| Patient No | T3 | RECIST 1.1 iRECIST | T6 | RECIST 1.1 iRECIST |
|---|---|---|---|---|
| 1 | Reduction of main tumor nodule (26 × 23→19 × 16 mm) | PR | Reduction of main tumor nodule, stable size of lymph nodes | PR |
| 2 | Heterogeneous response—reduction and increase of different nodules | SD | Reduction of 30% of the volume of the main lesion, reduction of lymph nodes and liver metastasis | PR |
| 3 | Stability | SD | Stability | SD |
| 4 | Suspected pseudoprogression | PP | Emergence of pleural effusion | PD |
| 5 | Small reduction of main tumor (47 × 28→40 × 25 mm) | SD | Increase of the main tumor, carcinomatous lymphangitis | PD |
| 6 | Small reduction of main tumor nodule | SD | Stability | SD |
| 7 | Small reduction of main tumor | SD | Reduction of main tumor (38 × 26→26 × 19 mm) | PR |
| 8 | Small reduction of main tumor | SD | Increase of the main tumor (36 × 44→52 × 59 mm) | PD |
| 9 | Small reduction of main tumor | SD | Reduction of main tumor (54 × 74 × 54→30 mm) | PR |
| 10 | Stability | SD | Increase in number and size of lymph nodes, carcinomatous lymphangitis | PD |
| 11 | Stability | SD | Stability | SD |
| 12 | Stability | SD | Small reduction of main tumor | SD |
| 13 | Suspected metastatic involvement of D11 | PD | Stability | SD |
| 14 | Stability | SD | Stability | SD |
| 15 | Stability | SD | Stability of main tumor, reduction in number and size of lymph nodes | SD |
| 16 | Stability | SD | Increase of main tumor and lymph nodes | PD |
| 17 | Stability of main tumor, reduction of lymph nodes | SD | Reduction of main tumor (48 × 26→29 × 16 mm) | PR |
| 18 | Stability | SD | Reduction of main tumor and number and size of lymph nodes | PR |
| Drug | Dose |
|---|---|
| Chemotherapy | |
| Cisplatin | 75 mg/m2, day 1, every 21 days |
| Carboplatin | AUC 5 or 6, day 1, every 21 days |
| Pemetrexed | 500 mg/m2, day 1, every 21 days |
| Immunotherapy | |
| Nivolumab | 240 mg, every 2 weeks |
| Pembrolizumab | 200 mg, every 3 weeks |
| Atezolizumab | 1200 mg, every 3 weeks |
| Patient | Therapeutic Scheme | T0 Sample Code | T3 Sample Code | T6 Sample Code |
|---|---|---|---|---|
| 1 | Nivolumab | 1P | 7P | 14P |
| 2 | Pembrolizumab | 11P | 33P | 49P |
| 3 | Carboplatin + Pemetrexed | 19P | 35P | 48P |
| 4 | Nivolumab | 28P | 40P | 51P |
| 5 | Cisplatin + Pemetrexed | 29P | 71P | 92P |
| 6 | Cisplatin + Pemetrexed | 47P | 64P | 90P |
| 7 | Cisplatin + Pemetrexed | 52P | 81P | 100P |
| 8 | Nivolumab | 54P | 95P | 108P |
| 9 | Pembrolizumab | 53P | 76P | 95P |
| 10 | Carboplatin + Pemetrexed | 62P | 82P | 105P |
| 11 | Carboplatin + Pemetrexed | 65P | 141P | 153P |
| 12 | Nivolumab | 70P | 99P | 124P |
| 13 | Pembrolizumab | 96P | 126P | 159P |
| 14 | Carboplatin + Pemetrexed | 106P | 134P | 148P |
| 15 | Carboplatin + Pemetrexed | 123P | 140P | 161P |
| 16 | Atezolizumab | 146P | 165P | 174P |
| 17 | Carboplatin + Pemetrexed | 155P | 175P | 185P |
| 18 | Nivolumab | 127P | 144P | 176P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madama, D.; Carrageta, D.F.; Guerra-Carvalho, B.; Botelho, M.F.; Oliveira, P.F.; Cordeiro, C.R.; Alves, M.G.; Abrantes, A.M. Impact of Different Treatment Regimens and Timeframes in the Plasmatic Metabolic Profiling of Patients with Lung Adenocarcinoma. Metabolites 2023, 13, 1180. https://doi.org/10.3390/metabo13121180
Madama D, Carrageta DF, Guerra-Carvalho B, Botelho MF, Oliveira PF, Cordeiro CR, Alves MG, Abrantes AM. Impact of Different Treatment Regimens and Timeframes in the Plasmatic Metabolic Profiling of Patients with Lung Adenocarcinoma. Metabolites. 2023; 13(12):1180. https://doi.org/10.3390/metabo13121180
Chicago/Turabian StyleMadama, Daniela, David F. Carrageta, Bárbara Guerra-Carvalho, Maria F. Botelho, Pedro F. Oliveira, Carlos R. Cordeiro, Marco G. Alves, and Ana M. Abrantes. 2023. "Impact of Different Treatment Regimens and Timeframes in the Plasmatic Metabolic Profiling of Patients with Lung Adenocarcinoma" Metabolites 13, no. 12: 1180. https://doi.org/10.3390/metabo13121180










