Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder?
Abstract
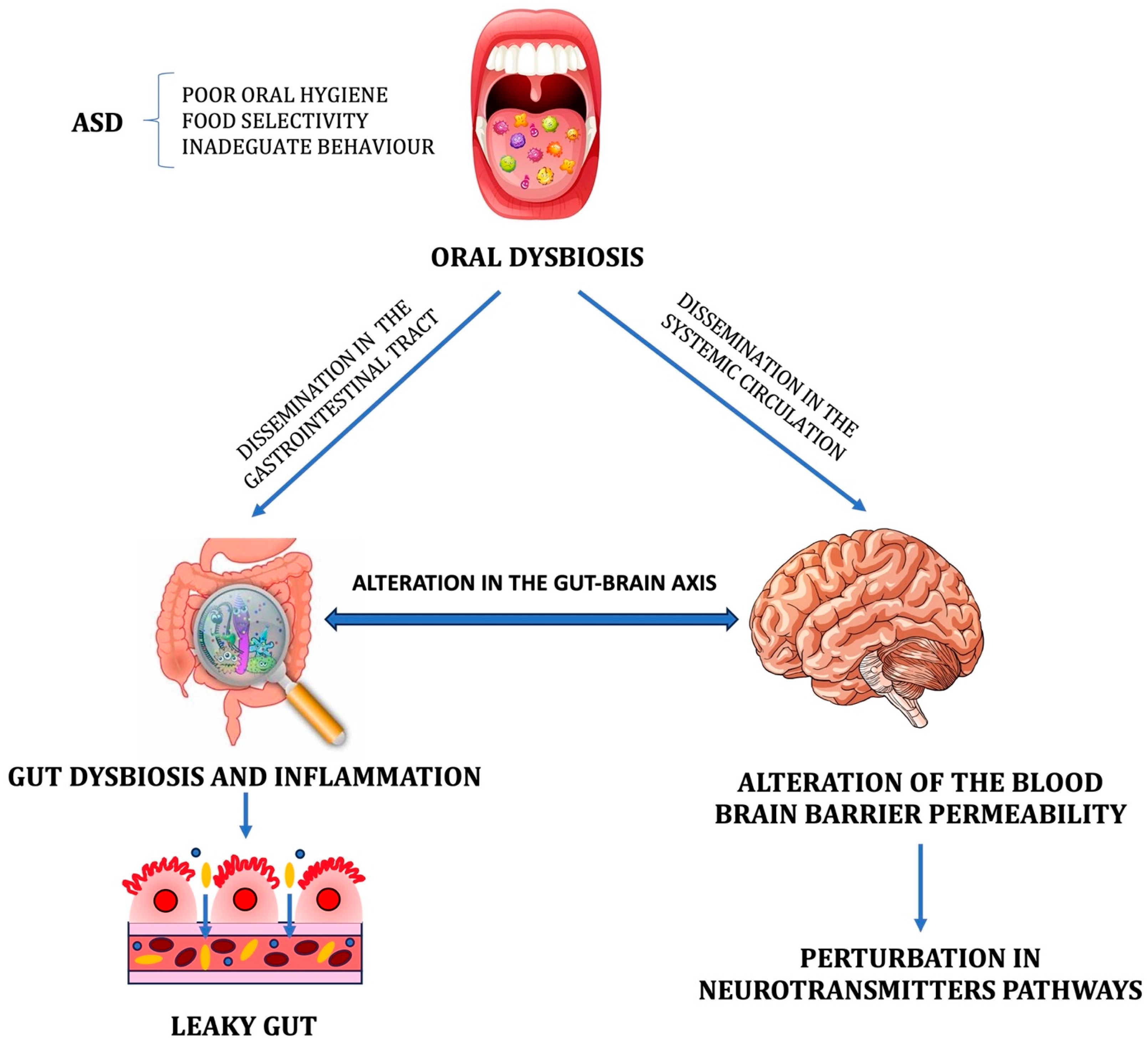
:1. Introduction
1.1. Microbiota and Autism Spectrum Disorder
1.2. Oral Microbiota
2. Methods
3. Results
| Year | Patients | Sample | Method | Oral Microbiome in ASD | Ref. |
|---|---|---|---|---|---|
| 2022 | 15 mice inoculated with saliva from ASD donors (AOMO) 15 mice inoculated with saliva from TD donors (TOMO) 15 mice not receiving any microbes (CON) | Mice saliva and feces | 16S rRNA | Alpha diversity decreased in the AOMO group as compared with the TOMO and CON groups in oral and fecal samples from mice Overgrowth (saliva): Bacteroidetes [G-7], Bacteroidetes [G-3], Tannerella, Porphyromonas sp. HMT, and unclassified Bacteroidales Depletion (feces): Kytococcus and Desulfobulbus | [56] |
| 2018 | 32 ASD aged 7–14 y 27 HC aged 8–11 y | Saliva Gingival plaques from caries-free molars | 16S rRNA | Microbial richness and diversity significantly lower in dental plaques Overgrowth: Proteobacteria, Streptococcus (in saliva), Haemophilus (in dental plaques) Depletion: Actinobacteria (in saliva), Bacteroidetes (in dental plaques), Prevotella, Alloprevotella, Selenomonas, Actinomyces, Fusobacterium, Porphyromonas | [58] |
| 2018 | 180 ASD aged 53 ± 16 y 106 TD aged 43 ± 16 y 60 DD aged 50 ± 13 y | Saliva | Shotgun sequencing by NGS | Overgrowth (ASD vs. TD): Limnohabitans, Planctomycetales spp., Cyanobacteria, Cellulomonas fimi ATCC 484 Overgrowth (ASD vs. DD): Brucella, Enterococcus faecalis Depletion (ASD vs. TD): Bacteroides ovatus, Bacteroides vulgatus, Mucilaginibacter sp. Depletion (ASD vs. DD): Flavobacterium spp., Chamaesiphon minutus PCC 6605, Pseudomonadaceae | [59] |
| 2019 | 20 ASD aged 7–21 y 19 NT aged 7–55 y | Saliva | 16S rRNA | No significant difference in alpha and beta diversity Overgrowth: unspecified Bacilli genus, Gemellaceae, Rickettsiales, Propionibacterium Depletion: Parvimonas, TM7 Saccharibacteria | [62] |
| 2021 | 25 ASD aged 9.2 ± 1.9 y 38 NT aged 10 ± 1.5 y | Tongue scrapping | 16S rRNA | No significant difference in species richness, alpha and beta diversity Overgrowth: Actinomyces odontolyticus, Actinomyces lingnae Depletion: Campylobacter concisus, Streptococcus vestibularis, Bergeyella sp. oral taxon 322 | [64] |
4. Discussion and Conclusive Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Panisi, C.; Guerini, F.R.; Abruzzo, P.M.; Balzola, F.; Biava, P.M.; Bolotta, A.; Brunero, M.; Burgio, E.; Chiara, A.; Clerici, M.; et al. Autism Spectrum Disorder from the Womb to Adulthood: Suggestions for a Paradigm Shift. J. Pers. Med. 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Charman, T.; Havdahl, A.; Carbone, P.; Anagnostou, E.; Boyd, B.; Carr, T.; de Vries, P.J.; Dissanayake, C.; Divan, G.; et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 2021, 399, 271–334. [Google Scholar] [CrossRef] [PubMed]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Psychiatr. Clin. North Am. 2021, 44, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Hsu, J.W.; Huang, K.L.; Bai, Y.M.; Su, T.P.; Chen, T.J.; Chen, M.H. Autism spectrum disorder and periodontitis risk: A cohort study of 38,203 adolescents. J. Am. Dent. Assoc. 2023, 154, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, S.; Hari, N. Salivary biomarker levels and oral health status of children with autistic spectrum disorders: A comparative study. Eur. Arch. Paediatr. Dent. 2017, 18, 91–96. [Google Scholar] [CrossRef]
- Kalemaj, Z.; Marino, M.M.; Santini, A.C.; Tomaselli, G.; Auti, A.; Cagetti, M.G.; Borsello, T.; Costantino, A.; Inchingolo, F.; Boccellino, M.; et al. Salivary microRNA profiling dysregulation in autism spectrum disorder: A pilot study. Front. Neurosci. 2022, 16, 945278. [Google Scholar] [CrossRef]
- Dall’Aglio, L.; Muka, T.; Cecil, C.A.M.; Bramer, W.M.; Verbiest, M.M.P.J.; Nano, J.; Hidalgo, A.C.; Franco, O.H.; Tiemeier, H. The role of epigenetic modifications in neurodevelopmental disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 94, 17–30. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Solmi, M.; Song, M.; Yon, D.K.; Lee, S.W.; Fombonne, E.; Kim, M.S.; Park, S.; Lee, M.H.; Hwang, J.; Keller, R.; et al. Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Mol. Psychiatry 2022, 27, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 29, e5. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- The Centers for Disease Control and Prevention (CDC). CDC estimate on autism prevalence increases by nearly 10 percent, to 1 in 54 children in the U.S. 2020. Available online: https://www.autismspeaks.org/press-release/cdc-estimate-autism-prevalence-increases-nearly-10-percent-1-54-children-us?utm_source=email&utm_medium=text-link&utm_campaign=CDC (accessed on 1 July 2022).
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Brett, D.; Warnell, F.; McConachie, H.; Parr, J.R. Factors Affecting Age at ASD Diagnosis in UK: No Evidence that Diagnosis Age has Decreased Between 2004 and 2014. J. Autism Dev. Disord. 2016, 46, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Emerging signs of autism spectrum disorder in infancy: Putative neural substrate. Dev. Med. Child Neurol. 2022, 64, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Ding, H.T.; Taur, Y.; Walkup, J.T. Gut Microbiota and Autism: Key Concepts and Findings. J. Autism Dev. Disord. 2016, 47, 480–489. [Google Scholar] [CrossRef]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mazmanian, S.K. Microbiota–brain axis: Context and causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition revisited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Brain-Derived Neurotrophic Factor Levels in Autism: A Systematic Review and Meta-Analysis. J. Autism Dev. Disord. 2017, 47, 1018–1029. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Kapila, Y. Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontology 2000 2021, 87, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [PubMed]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the Brain and Oral Microbiota Talk to Each Other? A Review of the Literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef]
- Bowland, G.B.; Weyrich, L.S. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front. Psychiatry 2022, 13, 810008. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Milioli, G.; Elias, S.G.; Teixeira, A.L. Pathophysiology of Bacterial Infection of the Central Nervous System and its Putative Role in the Pathogenesis of Behavioral Changes. Rev. Bras. Psiquiatr. 2013, 35, 81–87. [Google Scholar] [CrossRef]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; John, J.A.S.; Ekberg, J.A.K.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens Penetrating the Central Nervous System: Infection Pathways and the Cellular and Molecular Mechanisms of Invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef]
- Gillig, P.M.; Sanders, R.D. Cranial Nerves IX, X, XI, and XII. Psychiatry 2010, 7, 37–41. [Google Scholar]
- Smoliar, E.; Smoliar, A.; Sorkin, L.; Belkin, V. Microcirculatory bed of the human trigeminal nerve. Anat. Rec. 1998, 250, 245–249. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Salerno, C.; Bravaccio, C.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Autism spectrum disorders and oral health status: Review of the literature. Eur. J. Paediatr. Dent. 2020, 21, 9–12. [Google Scholar] [CrossRef]
- Conti, E.; Retico, A.; Palumbo, L.; Spera, G.; Bosco, P.; Biagi, L.; Fiori, S.; Tosetti, M.; Cipriani, P.; Cioni, G.; et al. Autism Spectrum Disorder and Childhood Apraxia of Speech: Early Language-Related Hallmarks across Structural MRI Study. J. Pers. Med. 2020, 10, 275. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J. Clin. Med. 2022, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Ashwood, P. Anti-Candida albicans IgG Antibodies in Children with Autism Spectrum Disorders. Front. Psychiatry 2018, 9, 627. [Google Scholar] [CrossRef]
- Andreo-Martinez, P.; Garcia-Martinez, N.; Quesada-Medina, J.; Sanchez-Samper, E.P.; Martinez-Gonzalez, A.E. Candida spp. en la microbiota intestinal de las personas con autismo: Revisión sistemática/Candida spp. in the gut microbiota of people with autism: A systematic review. Rev. Neurol. 2019, 68, 1–6. (In Spanish) [Google Scholar]
- Pang, L.; Zhi, Q.; Jian, W.; Liu, Z.; Lin, H. The Oral Microbiome Impacts the Link between Sugar Consumption and Caries: A Preliminary Study. Nutrients 2022, 14, 3693. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hage, S.R.V.; Lopes-Herrera, S.A.; Santos, T.H.F.; Defense-Netvral, D.A.; Martins, A.; Sawasaki, L.Y.; Fernandes, F.D.M. Oral hygiene and habits of children with autism spectrum disorders and their families. J. Clin. Exp. Dent. 2020, 12, e719–e724. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Gong, W.; Li, B.; Xu, R.; Wang, M.; Shen, L.; Shi, H.; Li, Y. Oral Microbiota Changes Contribute to Autism Spectrum Disorder in Mice. J. Dent. Res. 2022, 101, 821–831. [Google Scholar] [CrossRef]
- Olsen, I.; Hicks, S.D. Oral microbiota and autism spectrum disorder (ASD). J. Oral Microbiol. 2019, 12, 1702806. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wu, M.; Feng, Y.; Zhou, Z.; Chen, L.; Chen, F. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef]
- Groc, L.; Choquet, D. Linking glutamate receptor movements and synapse function. Science 2020, 368, eaay4631. [Google Scholar] [CrossRef]
- Magi, S.; Piccirillo, S.; Amoroso, S. The dual face of glutamate: From a neurotoxin to a potential survival factor—Metabolic implications in health and disease. Cell. Mol. Life Sci. 2019, 76, 1473–1488. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaq, A.; Halboub, E.; Homeida, H.E.; Basode, V.K.; Ghzwani, A.H.; Zain, K.A.; Baraniya, D.; Chen, T.; Al-Hebshi, N.N. Tongue microbiome in children with autism spectrum disorder. J. Oral Microbiol. 2021, 13, 1936434. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, J.; He, F.; Cai, C.; Wang, H.; Wang, Y.; Lin, Y.; Rong, H.; Cheng, G.; Xu, R.; et al. Alteration of gut microbiota-associated epitopes in children with autism spectrum disorders. Brain Behav. Immun. 2018, 75, 192–199. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Willyard, C. How gut microbes could drive brain disorders. Nature 2021, 590, 22–25. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Luna, R.A.; Savidge, T.C.; Williams, K.C. The Brain-Gut-Microbiome Axis: What Role Does It Play in Autism Spectrum Disorder? Curr. Dev. Disord. Rep. 2016, 3, 75–81. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Noto, A.; Fanos, V. Metabolomics of autism spectrum disorders: Early insights regarding mammalian-microbial cometabolites. Expert Rev. Mol. Diagn. 2016, 16, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. Int. Rev. J. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicology Teratol. 2013, 36, 82–90. [Google Scholar] [CrossRef]
- Hicks, S.D.; Rajan, A.T.; Wagner, K.E.; Barns, S.; Carpenter, R.L.; Middleton, F.A. Validation of a Salivary RNA Test for Childhood Autism Spectrum Disorder. Front. Genet. 2018, 9, 534. [Google Scholar] [CrossRef]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Guerin, M.; Amador, G.; Denis, F. Pre and Probiotics Involved in the Modulation of Oral Bacterial Species: New Therapeutic Leads in Mental Disorders? Microorganisms 2021, 9, 1450. [Google Scholar] [CrossRef]
- Jiménez-Hernández, N.; Serrano-Villar, S.; Domingo, A.; Pons, X.; Artacho, A.; Estrada, V.; Moya, A.; Gosalbes, M.J. Modulation of Saliva Microbiota through Prebiotic Intervention in HIV-Infected Individuals. Nutrients 2019, 11, 1346. [Google Scholar] [CrossRef]
- Jiang, Q.; Stamatova, I.; Kainulainen, V.; Korpela, R.; Meurman, J.H. Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol. 2016, 16, 149. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Ligezka, A.N.; Sonmez, A.I.; Corral-Frias, M.P.; Golebiowski, R.; Lynch, B.; Croarkin, P.E.; Romanowicz, M. A systematic review of microbiome changes and impact of probiotic supplementation in children and adolescents with neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110187. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, S.; Zhang, Y.; Li, E. A review of probiotics in the treatment of autism spectrum disorders: Perspectives from the gut–brain axis. Front. Microbiol. 2023, 14, 1123462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussap, M.; Beretta, P.; Esposito, E.; Fanos, V. Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder? Metabolites 2023, 13, 1183. https://doi.org/10.3390/metabo13121183
Mussap M, Beretta P, Esposito E, Fanos V. Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder? Metabolites. 2023; 13(12):1183. https://doi.org/10.3390/metabo13121183
Chicago/Turabian StyleMussap, Michele, Paola Beretta, Elena Esposito, and Vassilios Fanos. 2023. "Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder?" Metabolites 13, no. 12: 1183. https://doi.org/10.3390/metabo13121183
APA StyleMussap, M., Beretta, P., Esposito, E., & Fanos, V. (2023). Once upon a Time Oral Microbiota: A Cinderella or a Protagonist in Autism Spectrum Disorder? Metabolites, 13(12), 1183. https://doi.org/10.3390/metabo13121183









