Miltiradiene Production by Cytoplasmic Metabolic Engineering in Nicotiana benthamiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Construction of Plant Transformation Vectors
2.3. Subcellular Localization
2.4. Miltiradiene Production in N. benthamiana
2.5. Metabolite Extraction
2.6. GC–MS Analyses of Products
2.7. Statistical Analysis
3. Results
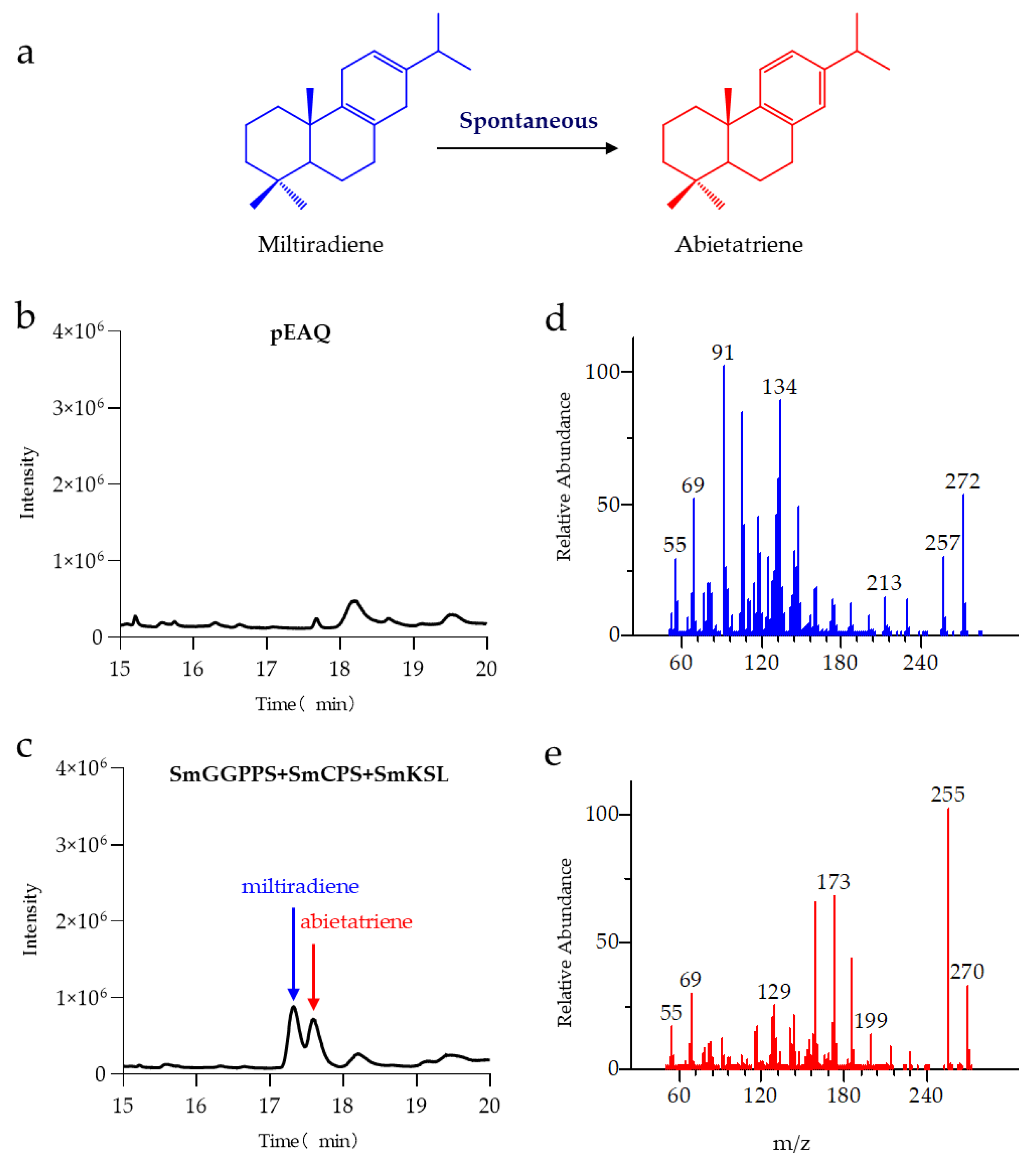
3.1. Production of Miltiradiene in N. benthamiana
3.2. Organelle Localization of Heterologous Proteins
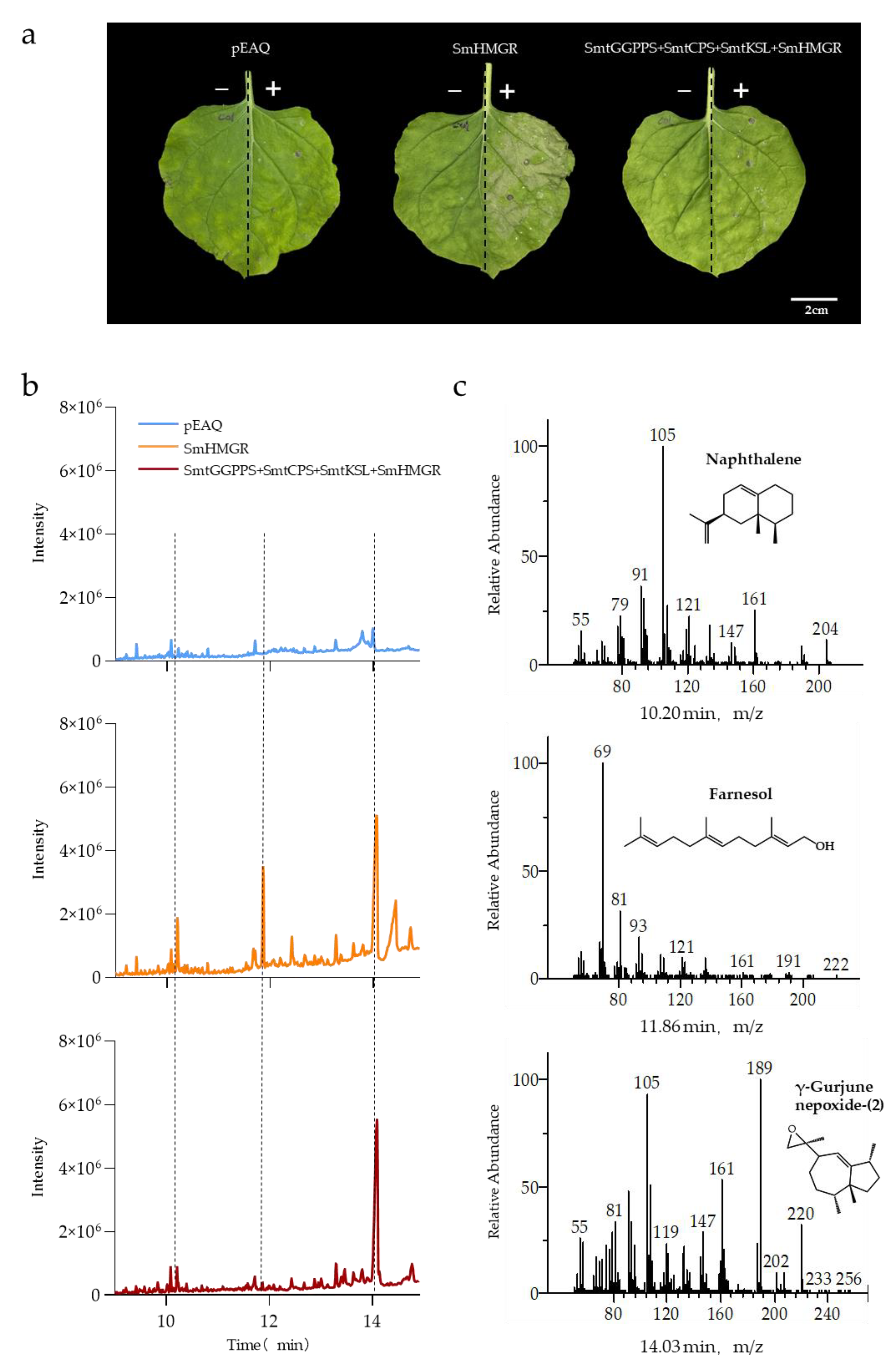
3.3. Comparison of Cytoplasmic Pathway and Plastid Pathway Products
3.4. Overexpression of SmHMGR Accelerates Apoptosis in Tobacco Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Peters, R.J. Tanshinones: Leading the way into Lamiaceae labdane-related diterpenoid biosynthesis. Curr. Opin. Plant Biol. 2022, 66, 102189. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Song, J.; Du, L.; Zhang, L.; Qiang, G.; Wang, S.; Yang, X.; Fang, L. Chemical and pharmacological research on the polyphenol acids isolated from Danshen: A review of salvianolic acids. Adv. Pharmacol. 2020, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Wang, Y.P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Wang, Y. The mechanisms of tanshinone in the treatment of tumors. Front. Pharmacol. 2023, 14, 1282203. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 58, 152871. [Google Scholar] [CrossRef]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The Anticancer Properties of Tanshinones and the Pharmacological Effects of Their Active Ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef]
- Alam, S.S.M.; Samanta, A.; Uddin, F.; Ali, S.; Hoque, M. Tanshinone IIA targeting cell signaling pathways: A plausible paradigm for cancer therapy. Pharmacol. Rep. 2023, 75, 907–922. [Google Scholar] [CrossRef]
- Liu, W.; Wei, Y.Y.; Liu, D.H.; Zhou, J.; Wang, X.; Li, F.S.; Zhu, F.Y. Biological and Phenological Properties of Salvia miltiorrhiza f. alba for Anti-continuous Cropping. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2015, 38, 5–7. [Google Scholar]
- Wang, T.L.; Guan, W.; Sun, K.; Wang, S.; Chi, X.L.; Guo, L.P. Progress in researches on pathogens, epidemiology and integrated control of diseases on Salvia miltiorrhiza in China. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2018, 43, 2402–2406. [Google Scholar] [CrossRef]
- Zhang, H.; Weibo, J.; Xiaole, Z.; Lin, L.; Zhigui, H.; Shushen, Y.; Zongsuo, L.; Xijun, Y.; Yanfeng, H.; Yan, L. Identification and Characterization of Salvia miltiorrhizain miRNAs in Response to Replanting Disease. PLoS ONE 2016, 11, e0159905. [Google Scholar] [CrossRef]
- Shen, W.; Hu, M.; Qian, D.; Xue, H.; Gao, N.; Lin, X. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Jiang, F.; Gong, T.; Chen, J.; Chen, T.; Yang, J.; Zhu, P. Synthetic biology of plants-derived medicinal natural products. Sheng Wu Gong Cheng Xue Bao 2021, 37, 1931–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Demirer, G.S. Synthetic biology for plant genetic engineering and molecular farming. Trends Biotechnol. 2023, 41, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Kotopka, B.J.; Li, Y.; Smolke, C.D. Synthetic biology strategies toward heterologous phytochemical production. Nat. Prod. Rep. 2018, 35, 902–920. [Google Scholar] [CrossRef]
- Hu, Z.; Ren, L.; Bu, J.; Liu, X.; Li, Q.; Guo, W.; Ma, Y.; Wang, J.; Chen, T.; Wang, L.; et al. Functional Characterization of a 2OGD Involved in Abietane-Type Diterpenoids Biosynthetic Pathway in Salvia miltiorrhiza. Front. Plant Sci. 2022, 13, 947674. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Liao, P.; Zhang, T.; Zhou, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, L. Characterization, expression profiling, and functional identification of a gene encoding geranylgeranyl diphosphate synthase from Salvia miltiorrhiza. Biotechnol. Bioprocess Eng. 2010, 15, 236–245. [Google Scholar] [CrossRef]
- Cheng, Q.; Su, P.; Hu, Y.; He, Y.; Gao, W.; Huang, L. RNA interference-mediated repression of SmCPS (copalyldiphosphate synthase) expression in hairy roots of Salvia miltiorrhiza causes a decrease of tanshinones and sheds light on the functional role of SmCPS. Biotechnol. Lett. 2014, 36, 363–369. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, X.; Hu, T.; Chen, K.; Cui, G.; Su, P.; Xu, H.; Gao, W.; Jiang, T.; Huang, L. Structural and mechanistic insights into the precise product synthesis by a bifunctional miltiradiene synthase. Plant Biotechnol. J. 2023, 21, 165–175. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, D.; Guo, J.; Liu, X.; Li, Q.; Su, P.; Wang, J.; Ma, Y.; Huang, L. Functional Study and Efficient Catalytic Element Mining of CYP76AHs in Salvia Plants. Molecules 2023, 28, 4711. [Google Scholar] [CrossRef] [PubMed]
- Božić, D.; Papaefthimiou, D.; Brückner, K.; de Vos, R.C.; Tsoleridis, C.A.; Katsarou, D.; Papanikolaou, A.; Pateraki, I.; Chatzopoulou, F.M.; Dimitriadou, E.; et al. Towards Elucidating Carnosic Acid Biosynthesis in Lamiaceae: Functional Characterization of the Three First Steps of the Pathway in Salvia fruticosa and Rosmarinus officinalis. PLoS ONE 2015, 10, e0124106. [Google Scholar] [CrossRef]
- Brückner, K.; Božić, D.; Manzano, D.; Papaefthimiou, D.; Pateraki, I.; Scheler, U.; Ferrer, A.; de Vos, R.C.; Kanellis, A.K.; Tissier, A. Characterization of two genes for the biosynthesis of abietane-type diterpenes in rosemary (Rosmarinus officinalis) glandular trichomes. Phytochemistry 2014, 101, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hillwig, M.L.; Huang, L.; Cui, G.; Wang, X.; Kong, J.; Yang, B.; Peters, R.J. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett. 2009, 11, 5170–5173. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.L.; Heskes, A.M.; Hamberger, B.; Olsen, C.E.; Hallström, B.M.; Andersen-Ranberg, J.; Hamberger, B. The terpene synthase gene family in Tripterygium wilfordii harbors a labdane-type diterpene synthase among the monoterpene synthase TPS-b subfamily. Plant J. 2017, 89, 429–441. [Google Scholar] [CrossRef]
- Jin, B.; Cui, G.; Guo, J.; Tang, J.; Duan, L.; Lin, H.; Shen, Y.; Chen, T.; Zhang, H.; Huang, L. Functional Diversification of Kaurene Synthase-Like Genes in Isodon rubescens. Plant Physiol. 2017, 174, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, I.; Andersen-Ranberg, J.; Hamberger, B.; Heskes, A.M.; Martens, H.J.; Zerbe, P.; Bach, S.S.; Møller, B.L.; Bohlmann, J.; Hamberger, B. Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Plant Physiol. 2014, 164, 1222–1236. [Google Scholar] [CrossRef]
- Su, P.; Guan, H.; Zhao, Y.; Tong, Y.; Xu, M.; Zhang, Y.; Hu, T.; Yang, J.; Cheng, Q.; Gao, L.; et al. Identification and functional characterization of diterpene synthases for triptolide biosynthesis from Tripterygium wilfordii. Plant J. 2018, 93, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Sugai, Y.; Ueno, Y.; Hayashi, K.; Oogami, S.; Toyomasu, T.; Matsumoto, S.; Natsume, M.; Nozaki, H.; Kawaide, H. Enzymatic (13)C labeling and multidimensional NMR analysis of miltiradiene synthesized by bifunctional diterpene cyclase in Selaginella moellendorffii. J. Biol. Chem. 2011, 286, 42840–42847. [Google Scholar] [CrossRef]
- Zerbe, P.; Chiang, A.; Dullat, H.; O’Neil-Johnson, M.; Starks, C.; Hamberger, B.; Bohlmann, J. Diterpene synthases of the biosynthetic system of medicinally active diterpenoids in Marrubium vulgare. Plant J. 2014, 79, 914–927. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, J.; Tong, Y.; Su, P.; Li, X.; Liu, Y.; Liu, N.; Wu, X.; Zhang, Y.; Wang, J.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96. [Google Scholar] [CrossRef]
- Contento, A.L.; Bassham, D.C. Structure and function of endosomes in plant cells. J. Cell Sci. 2012, 125, 3511–3518. [Google Scholar] [CrossRef]
- Galili, G.; Sengupta-Gopalan, C.; Ceriotti, A. The endoplasmic reticulum of plant cells and its role in protein maturation and biogenesis of oil bodies. Plant Mol. Biol. 1998, 38, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The Rise and Rise of Nicotiana benthamiana: A Plant for All Reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef]
- Xia, P.; Hu, W.; Liang, T.; Yang, D.; Liang, Z. An attempt to establish an Agrobacterium-mediated transient expression system in medicinal plants. Protoplasma 2020, 257, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Špánik, I.; Machyňáková, A. Recent applications of gas chromatography with high-resolution mass spectrometry. J. Sep. Sci. 2018, 41, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef]
- Zi, J.; Peters, R.J. Characterization of CYP76AH4 clarifies phenolic diterpenoid biosynthesis in the Lamiaceae. Org. Biomol. Chem. 2013, 11, 7650–7652. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.J.; Hillwig, M.L.; Shen, Y.; Yang, L.; Wang, Y.; Zhang, X.; Liu, W.; Peters, R.J.; Chen, X.; et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc. Natl. Acad. Sci. USA 2013, 110, 12108–12113. [Google Scholar] [CrossRef]
- Guo, J.; Ma, X.; Cai, Y.; Ma, Y.; Zhan, Z.; Zhou, Y.J.; Liu, W.; Guan, M.; Yang, J.; Cui, G.; et al. Cytochrome P450 promiscuity leads to a bifurcating biosynthetic pathway for tanshinones. New Phytol. 2016, 210, 525–534. [Google Scholar] [CrossRef]
- Yang, R.; Du, Z.; Qiu, T.; Sun, J.; Shen, Y.; Huang, L. Discovery and Functional Characterization of a Diverse Diterpene Synthase Family in the Medicinal Herb Isodon lophanthoides Var. gerardiana. Plant Cell Physiol. 2021, 62, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, S. Overview of Medicinally Important Diterpenoids Derived from Plastids. Mini Rev. Med. Chem. 2017, 17, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mutanda, I.; Wang, K.; Yang, L.; Wang, J.; Wang, Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat. Commun. 2019, 10, 4850. [Google Scholar] [CrossRef] [PubMed]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, C.; Guo, X.; Du, Z.; Cheng, Y.; Wang, Z.; He, X. Enhancing fluxes through the mevalonate pathway in Saccharomyces cerevisiae by engineering the HMGR and β-alanine metabolism. Microb. Biotechnol. 2022, 15, 2292–2306. [Google Scholar] [CrossRef]
- Fitzpatrick, A.H.; Bhandari, J.; Crowell, D.N. Farnesol kinase is involved in farnesol metabolism, ABA signaling and flower development in Arabidopsis. Plant J. 2011, 66, 1078–1088. [Google Scholar] [CrossRef]
- Ziaei, S.; Halaby, R. Immunosuppressive, anti-inflammatory and anti-cancer properties of triptolide: A mini review. Avicenna J. Phytomedicine 2016, 6, 149–164. [Google Scholar]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Dev. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, J.K.; Park, S.U. Yeast extract improved biosynthesis of astragalosides in hairy root cultures of Astragalus membranaceus. Prep. Biochem. Biotechnol. 2021, 51, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Xu, C.; Xu, M.; Li, F.S.; Wada, N.; Mitchell, A.J.; Han, X.L.; Wen, M.L.; Fujita, M.; Weng, J.K. Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat. Commun. 2019, 10, 3206. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.L.; Kjaerulff, L.; Heck, Q.K.; Forman, V.; Staerk, D.; Møller, B.L.; Andersen-Ranberg, J. Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide. Nat. Commun. 2022, 13, 5011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, H.; Zhang, Z.; Verstrepen, K.J.; Wang, Q.; Dai, Z. Metabolic engineering of Yarrowia lipolytica for terpenoids production: Advances and perspectives. Crit. Rev. Biotechnol. 2022, 42, 618–633. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; Enfissi, E.M.A.; Fraser, P.D. Metabolic changes in leaves of N. tabacum and N. benthamiana during plant development. J. Plant Physiol. 2021, 265, 153486. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, T.; Vavitsas, K.; Andersen-Ranberg, J.; Nielsen, A.Z.; Olsen, C.E.; Hamberger, B.; Jensen, P.E. Heterologous expression of the isopimaric acid pathway in Nicotiana benthamiana and the effect of N-terminal modifications of the involved cytochrome P450 enzyme. J. Biol. Eng. 2015, 9, 24. [Google Scholar] [CrossRef]
- Liu, C. Reconstitution of Metabolic Pathway in Nicotiana benthamiana. Methods Mol. Biol. 2022, 2396, 29–33. [Google Scholar] [CrossRef]
- Heinig, U.; Gutensohn, M.; Dudareva, N.; Aharoni, A. The challenges of cellular compartmentalization in plant metabolic engineering. Curr. Opin. Biotechnol. 2013, 24, 239–246. [Google Scholar] [CrossRef]
- Neve, E.P.; Ingelman-Sundberg, M. Cytochrome P450 proteins: Retention and distribution from the endoplasmic reticulum. Curr. Opin. Drug Discov. Dev. 2010, 13, 78–85. [Google Scholar]
- Chang, W.C.; Song, H.; Liu, H.W.; Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013, 17, 571–579. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Li, W.; Zhu, W.; Ren, Z.; Wang, Z.; Li, L.; Jia, L.; Zhu, S.; Ma, Z. Genome-Wide Identification and Comparative Analysis of the 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase (HMGR) Gene Family in Gossypium. Molecules 2018, 23, 193. [Google Scholar] [CrossRef]
- Wen, L.; Cao, J.; Li, W.; Guo, Y. Changes in volatile profile and related gene expression during senescence of tobacco leaves. J. Sci. Food Agric. 2023, 103, 6540–6552. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Bach, T.J. Farnesol-induced cell death and stimulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in tobacco cv bright yellow-2 cells. Plant Physiol. 2000, 123, 1257–1268. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Lin, C.; Huang, Y.; Su, T.; Guo, J.; Yang, L. Miltiradiene Production by Cytoplasmic Metabolic Engineering in Nicotiana benthamiana. Metabolites 2023, 13, 1188. https://doi.org/10.3390/metabo13121188
Ren X, Lin C, Huang Y, Su T, Guo J, Yang L. Miltiradiene Production by Cytoplasmic Metabolic Engineering in Nicotiana benthamiana. Metabolites. 2023; 13(12):1188. https://doi.org/10.3390/metabo13121188
Chicago/Turabian StyleRen, Xiangxiang, Chuhang Lin, Yanbo Huang, Tao Su, Juan Guo, and Lei Yang. 2023. "Miltiradiene Production by Cytoplasmic Metabolic Engineering in Nicotiana benthamiana" Metabolites 13, no. 12: 1188. https://doi.org/10.3390/metabo13121188
APA StyleRen, X., Lin, C., Huang, Y., Su, T., Guo, J., & Yang, L. (2023). Miltiradiene Production by Cytoplasmic Metabolic Engineering in Nicotiana benthamiana. Metabolites, 13(12), 1188. https://doi.org/10.3390/metabo13121188






