Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Patient with pancreatic cancer;
- Surgery on the pancreas;
- Age of patients between 35 and 70.
- Critical condition on admission;
- Identification of contraindications for surgery;
- Repeat abdominal surgery
2.2. Patients and Samples
2.3. Reagents
2.4. GC–MS Analysis
2.5. Biomarkers Analysis
2.6. Statistical Analysis
3. Results
3.1. Metabolites in Healthy Volunteers and Patients before the Surgery
3.2. Changes in the Metabolomic Profile of Patients during the Perioperative Period
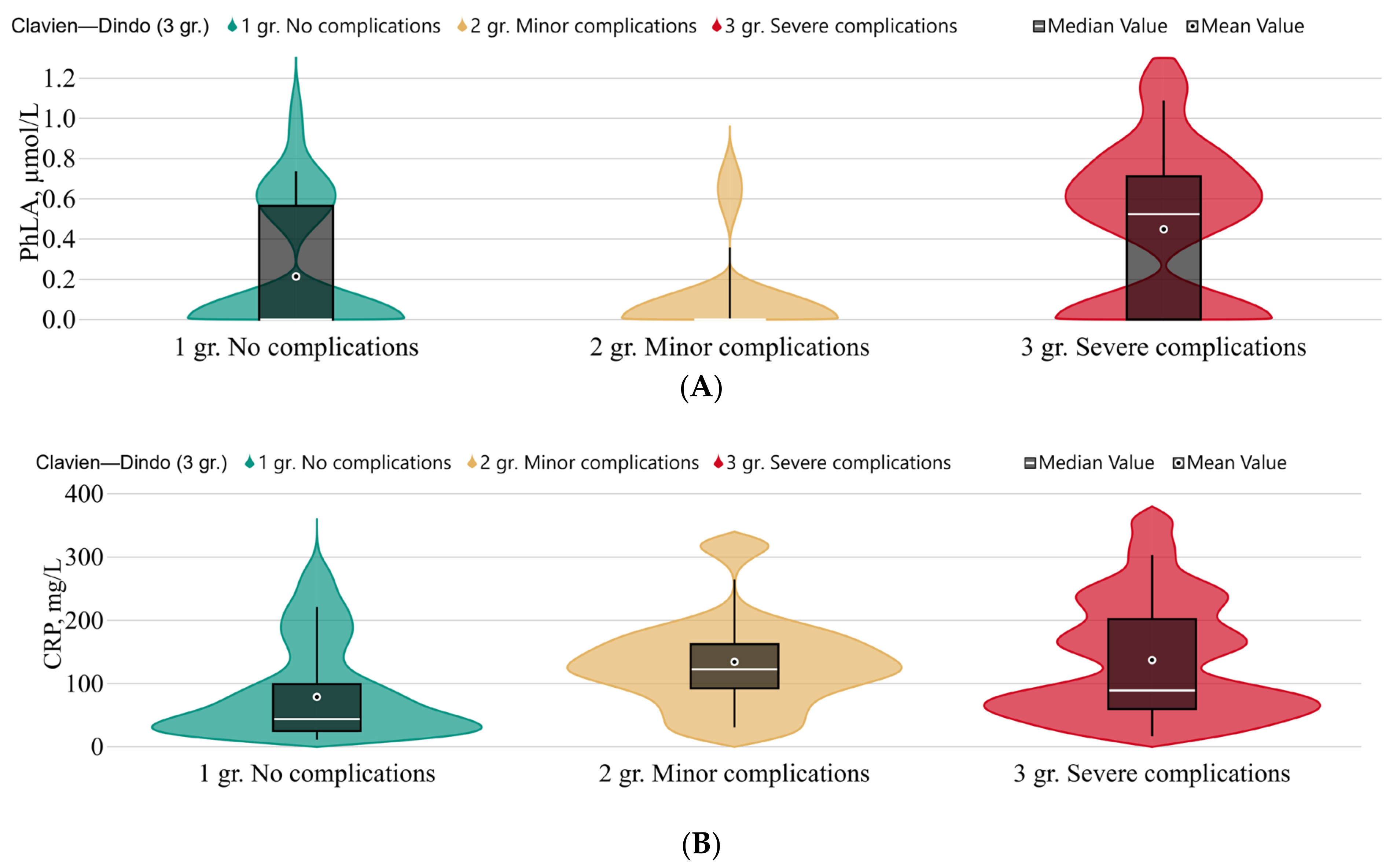
3.3. The Metabolomic Profile in Patients with a Complicated Course during the Postoperative Period
3.3.1. The Clavien–Dindo Classification
3.3.2. The Length of Stay in the ICU
3.3.3. Infectious Complications
3.3.4. Chemotherapy and Extent of Surgery
3.4. Metabolomic Profile in Drainage Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Metabolites, µmol/L | No Complications (n = 28) | Minor Complications (n = 10) | Severe Complications (n = 24) | p-Value * | |
|---|---|---|---|---|---|
| Patients before surgery | |||||
| Benzoic acid | 0.64 (<0.5; 0,78) | <0.5 (<0.5; 0.59) | 0.59 (<0.5; 0.67) | 0.123 | |
| Phenyllactic acid | <0.5 (<0.5; <0.5) | <0.5 (<0.5; <0.5) | <0.5 (<0.5; <0.5) | - | |
| p-Hydroxyphenylacetic acid | <0.5 (<0.5; 0.63) | <0.5 (<0.5; <0.5) | <0.5 (<0.5; 0.53) | - | |
| p-Hydroxyphenyllactic acid | 1.1 (0.87; 1.4) | 0.89 (0.71; 1.1) | 1.2 (0.91; 1.7) | 0.202 | |
| Σ 3 AMM | 1.9 (1.60; 2.3) | 1.7 (1.3; 2.4) | 1.9 (1.4; 2.6) | 0.854 | |
| Succinic acid | 2.9 (2.2; 3.9) | 3.2 (2.6; 4.2) | 2.7 (2.0; 3.6) | 0.582 | |
| Fumaric acid | 0.79 (0.54; 1.5) | 0.53 (<0.5; 0.71) | 0.66 (0.52; 0.91) | 0.076 | |
| C-reactive protein | 3.5 (1.8; 10) | 21 (4.4; 56) | 5.8 (1.2; 19) | 0.299 | |
| PCT | 0.05 (0.03; 0.08) | 0.08 (0.02; 0.13) | 0.05 (0.02; 0.21) | 0.583 | |
| Patients 1–3 days after the surgery | |||||
| Benzoic acid | 0.64 (<0.5; 0.76) | 0.51 (0.406; 0.724) | 0.56 (<0.5; 0.68) | 0.687 | |
| Phenyllactic acid | <0.5 (<0.5; 0.57) | <0.5 (<0.5; <0.5) | 0.52 (<0.5; 0.72) | 0.012 | |
| p-Hydroxyphenylacetic acid | <0.5 (<0.5; 0.95) | <0.5 (<0.5; 0.51) | <0.5 (<0.5; 1.2) | 0.548 | |
| p-Hydroxyphenyllactic acid | 1.3 (0.95; 1.6) | 1.2 (0.98; 1.4) | 1.4 (0.98; 2.1) | 0.377 | |
| Σ 3 AMM | 2.3 (1.9; 2.9) | 1.9 (1.5; 2.5) | 2.7 (1.8;4.2) | 0.130 | |
| Succinic acid | 2.6 (1.7; 3,1) | 2.1 (1.3; 3.4) | 2.4 (1.9; 2.6) | 0.595 | |
| Fumaric acid | 0.55 (<0.5; 0.66) | <0.5 (<0.5; 0.51) | 0.59 (0.50; 0.67) | 0.031 | |
| C-reactive protein | 44 (24; 102) | 123 (85; 166) | 89 (59; 218) | 0.029 | |
| PCT | 0.10 (0.05; 0.30) | 0.31 (0.21; 0.44) | 0.16 (0.1; 0.40) | 0.042 | |
| General | |||||
| Sex (M) | 12 (42.9%) | 4 (40.0%) | 13 (54.2%) | 0.643 | |
| Age | 63 (56;71.5) | 59.5 (53;68) | 57.5 (53.5;69.5) | 0.582 | |
| ASA | 2 | 16 (57.1%) | 5 (50%) | 14 (58.3%) | 0.120 |
| 3 | 12 (42.9%) | 5 (50%) | 6 (25%) | ||
| 4 | 0 (0%) | 0 (0%) | 4 (16.7%) | ||
| Stages of cancer | 0 | 2 (7.1%) | 1 (10%) | 2 (8.3%) | 0.051 |
| 1 | 14 (50%) | 3 (30%) | 11 (45.8%) | ||
| 2 | 11 (39.3%) | 2 (20%) | 10 (41.7%) | ||
| 3 | 1 (3.6%) | 4 (40%) | 0 (0%) | ||
| 4 | 0 (0%) | 0 (0%) | 1 (4.2%) | ||
| Tumor localization | Head of the pancreas | 14 (50%) | 6 (60%) | 8 (33.3%) | 0.280 |
| Body/tail of the pancreas | 10 (35.7%) | 1 (10%) | 8 (33.3%) | ||
| Major duodenal papilla | 0 (0%) | 0 (0%) | 1 (4.2%) | ||
| Terminal cholidochus | 3 (10.7%) | 3 (30%) | 7 (29.2%) | ||
| total | 1 (3.6%) | 0 (0%) | 0 (0%) | ||
| Model Step (Total) | Parameter | p-Value | Adj. OR | 95% DI | |
|---|---|---|---|---|---|
| Lower Boundary | Upper Boundary | ||||
| Step 8 | p-Hydroxyphenyllactic acid | 0.012 | 12.771 | 1.753 | 93.053 |
| Constant | 0.020 | 0.052 | |||
| Variables not included in the equation | Sex | 0.271 | 2.086 | 0.563 | 7.722 |
| Tumor stage | 0.249 | 0.588 | 0.238 | 1.451 | |
| Age | 0.175 | 1.042 | 0.982 | 1.106 | |
| Succinic acid | 0.275 | 0.713 | 0.389 | 1.309 | |
| ASA | 0.696 | 0.749 | 0.175 | 3.204 | |
References
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Le, A.-T.; Huang, B.; Hnoosh, D.; Saeed, H.; Dineen, S.P.; Hosein, P.J.; Durbin, E.B.; Kudrimoti, M.; McGrath, P.C.; Tzeng, C.-W.D. Effect of complications on oncologic outcomes after pancreaticoduodenectomy for pancreatic cancer. J. Surg. Res. 2017, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mintziras, I.; Wächter, S.; Manoharan, J.; Kanngiesser, V.; Maurer, E.; Bartsch, D.K. Postoperative morbidity following pancreatic cancer surgery is significantly associated with worse overall patient survival; Systematic review and meta-analysis. Surg. Oncol. 2021, 38, 101573. [Google Scholar] [CrossRef]
- Braga, M. Perioperative immunonutrition and gut function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.; Zhbannikov, P.; Ganert, A.; Larionov, S.; Lyuboshevskiy, P. Accuracy of predictors of perioperative cardiovascular complications in onco-surgery. Russ. J. Anesthesiol. Reanimatol. 2023, 3, 37–44. [Google Scholar] [CrossRef]
- Trembach, N.V.; Magomedov, M.A.; Krasnov, V.G.; Chernienko, L.Y.; Shevyrev, S.N.; Popov, A.S.; Tyutyunova, E.V.; Vatutin, S.N.; Dmitriev, A.A.; Fisher, V.V.; et al. The Effect of ACE Inhibitors/ARBs Withdrawal on the Risk of Postoperative Complications in Abdominal Surgery. Gen. Reanimatol. 2023, 19, 21–30. [Google Scholar] [CrossRef]
- Danzi, F.; Pacchiana, R.; Mafficini, A.; Scupoli, M.T.; Scarpa, A.; Donadelli, M.; Fiore, A. To metabolomics and beyond: A technological portfolio to investigate cancer metabolism. Signal Transduct. Target. Ther. 2023, 8, 1–22. [Google Scholar] [CrossRef]
- Cheung, P.K.; Ma, M.H.; Tse, H.F.; Yeung, K.F.; Tsang, H.F.; Chu, M.K.M.; Kan, C.M.; Cho, W.C.S.; Ng, L.B.W.; Chan, L.W.C.; et al. The applications of metabolomics in the molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 2019, 19, 785–793. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J.; Wang, H.; Lu, H. Metabolomics identified new biomarkers for the precise diagnosis of pancreatic cancer and associated tissue metastasis. Pharmacol. Res. 2020, 156, 104805. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, W. Succinate in the cancer–immune cycle. Cancer Lett. 2017, 390, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Xu, S.; Zhang, S. The role of amino acid metabolism alterations in pancreatic cancer: From mechanism to application. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188893. [Google Scholar] [CrossRef]
- Tumas, J.; Baskirova, I.; Petrenas, T.; Norkuniene, J.; Strupas, K.; Sileikis, A. Towards a Personalized Approach in Pancreatic Cancer Diagnostics Through Plasma Amino Acid Analysis. Anticancer Res. 2019, 39, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.; Pautova, A.; Grekova, M.; Yadgarov, M.; Grin, O.; Eremenko, A.; Babaev, M. Microbiota Metabolism Failure as a Risk Factor for Postoperative Complications after Aortic Prosthetics. Biomedicines 2023, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Chernevskaya, E.; Zuev, E.; Odintsova, V.; Meglei, A.; Beloborodova, N. Gut Microbiota as Early Predictor of Infectious Complications before Cardiac Surgery: A Prospective Pilot Study. J. Pers. Med. 2021, 11, 1113. [Google Scholar] [CrossRef]
- De Cassai, A.; Boscolo, A.; Tonetti, T.; Ban, I.; Ori, C. Assignment of ASA-physical status re-lates to anesthesiologists’ experience: A survey-based national-study. Korean J. Anesthesiol. 2019, 72, 53–59. [Google Scholar] [CrossRef]
- Wang, W.; Babu, S.R.; Wang, L.; Chen, Y.; Tian, B.; He, H. Use of Clavien-Dindo classification in evaluating complications following pancreaticoduodenectomy in 1,056 cases: A retrospective analysis from one single institution. Oncol. Lett. 2018, 16, 2023–2029. [Google Scholar] [CrossRef]
- Pautova, A.K.; Burnakova, N.A.; Beloborodova, N.B.; Revelsky, A.I. Simultaneous determination of aromatic, short-chain fatty and dicarboxylic acids in blood serum and cerebrospinal fluid by gas chromatography-mass spectrometry. J. Anal. Chem. 2023, 79, 1–13. [Google Scholar] [CrossRef]
- Papa, V.; Schepis, T.; Coppola, G.; Chiappetta, M.F.; Del Vecchio, L.E.; Rozera, T.; Quero, G.; Gasbarrini, A.; Alfieri, S.; Papa, A. The Role of Microbiota in Pancreatic Cancer. Cancers 2023, 15, 3143. [Google Scholar] [CrossRef]
- di Sebastiano, P.; Festa, L.; De Bonis, A.; Ciuffreda, A.; Valvano, M.R.; Andriulli, A.; di Mola, F.F. A modified fast-track program for pancreatic surgery: A prospective single-center experience. Langenbeck’s Arch. Surg. 2010, 396, 345–351. [Google Scholar] [CrossRef]
- Casado, M.C.M.; Sánchez, F.P.; Sastre, F.R.; Cruchaga, P.M.; Cienfuegos, F.J. Experience of a cephalic pancreatoduodenectomy fast-track program. Cir. Esp. 2010, 87, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Dieuleveux, V.; Guéguen, M. Antimicrobial Effects of d-3-Phenyllactic Acid on Listeria monocytogenes in TSB-YE Medium, Milk, and Cheese. J. Food Prot. 1998, 61, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Ohhira, I.; Kuwaki, S.; Morita, H.; Suzuki, T.; Tomita, S.; Hisamatsu, S.; Sonoki, S.; Shinoda, S. Identification of 3-Phenyllactic Acid As a Possible Antibacterial Substance Produced by Enterococcus faecalis TH10. Biocontrol Sci. 2004, 9, 77–81. [Google Scholar] [CrossRef]
- Cho, S.; Yang, X.; Won, K.-J.; Leone, V.A.; Chang, E.B.; Guzman, G.; Ko, Y.; Bae, O.-N.; Lee, H.; Jeong, H. Phenylpropionic acid produced by gut microbiota alleviates acetaminophen-induced hepatotoxicity. Gut Microbes 2023, 15, 2231590. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.V.; Sarshor, Y.N.; Bedova, A.Y.; Chernevskaya, E.A.; Pautova, A.K. Involvement of Aromatic Metabolites in the Pathogenesis of Septic Shock. Shock 2018, 50, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, Q.; Wang, C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta-analysis. Int. J. Surg. 2015, 22, 38–45. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M.; et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
- Conlon, K.C.; Labow, D.; Leung, D.; Smith, A.; Jarnagin, W.; Coit, D.G.; Merchant, N.; Brennan, M.F. Prospective Randomized Clinical Trial of the Value of Intraperitoneal Drainage After Pancreatic Resection. Ann. Surg. 2001, 234, 487–494. [Google Scholar] [CrossRef]
- Chang, J.H.; Stackhouse, K.; Dahdaleh, F.; Hossain, M.S.; Naples, R.; Wehrle, C.; Augustin, T.; Simon, R.; Joyce, D.; Walsh, R.M.; et al. Postoperative Day 1 Drain Amylase After Pancreatoduodenectomy: Optimal Level to Predict Pancreatic Fistula. J. Gastrointest. Surg. 2023, 27, 2676–2683. [Google Scholar] [CrossRef]
- He, S.; Xia, J.; Zhang, W.; Lai, M.; Cheng, N.; Liu, Z.; Cheng, Y. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst. Rev. 2021, 2021, CD010583. [Google Scholar] [CrossRef]
- Yu, H.-S.; Lee, N.-K.; Jeon, H.-L.; Eom, S.J.; Yoo, M.-Y.; Lim, S.-D.; Paik, H.-D. Benzoic Acid Production with Respect to Starter Culture and Incubation Temperature during Yogurt Fermentation using Response Surface Methodology. Korean J. Food Sci. Anim. Resour. 2016, 36, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jin, M.; Liu, Y.; Jin, L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 572492. [Google Scholar] [CrossRef] [PubMed]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal Microbiota in Colorectal Cancer Surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
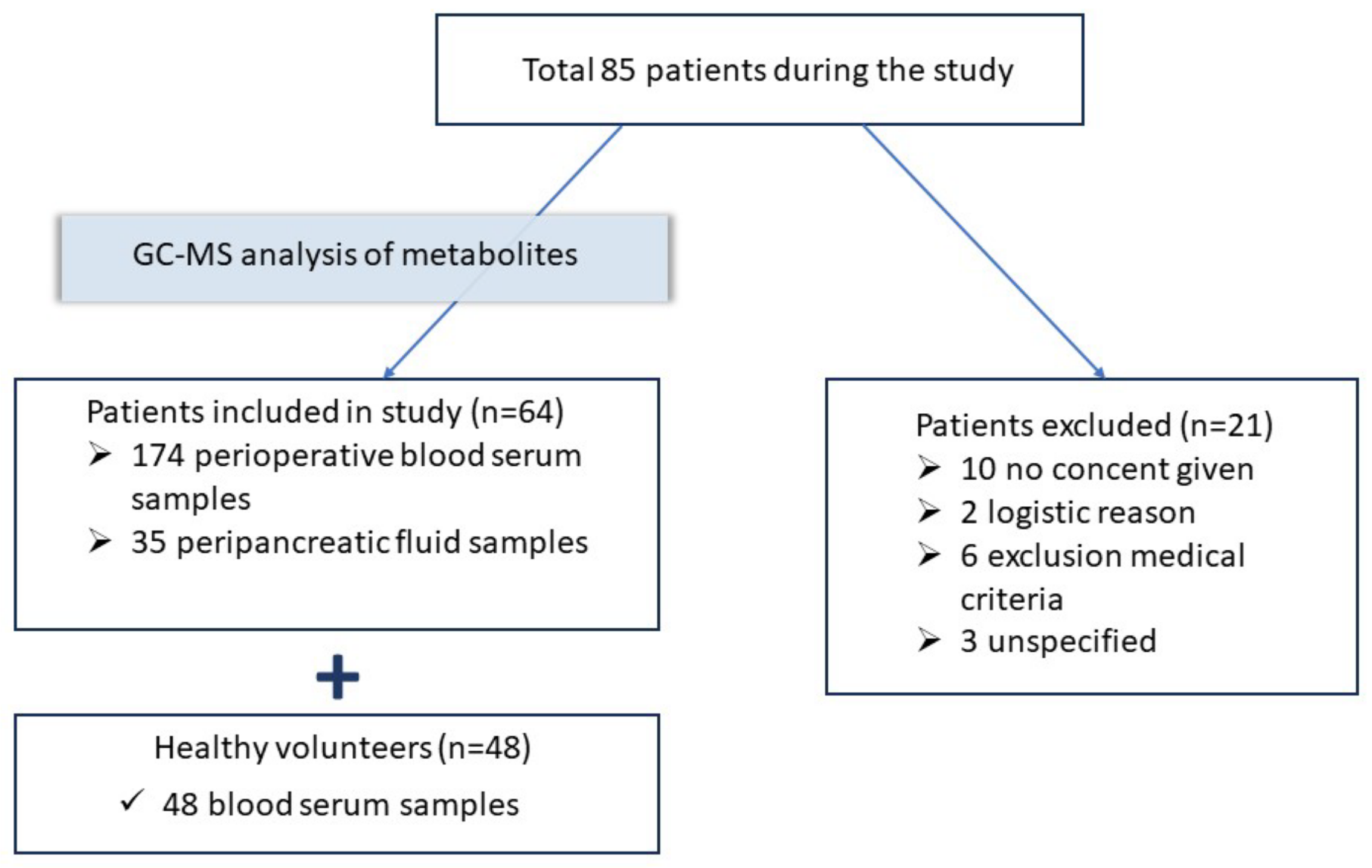
| Parameter | Healthy Volunteers (n = 48) | Patients before the Surgery (n = 64) |
|---|---|---|
| Sex, male, % | 35, 72% | 31, 48% |
| Age, years | 40 (34; 45) | 60 (54; 70) |
| Stages of cancer, n, (%) | - | 0—5, (8%); I—29, (45%), II—24, (58%), III—24, (8%), IV—1 (1%) |
| Tumor localization, n, (%) | - | Head of the pancreas—29 (46%), Body/tail of the pancreas—20 (31%), Major duodenal papilla—13 (20%), Terminal cholidochus—1 (1.5%), Total—1 (1.5%) |
| Systemic pathology, n, (%) | - | No—15 (23.5%), Cardiovascular system—29 (45%), Respiratory—1 (1.5%), Endocrine—3 (5%), Digestive—3 (5%), Polymorbidity—13 (20%) |
| American Society of Anesthesiologists (ASA), class, (%) | - | II—36, (56%), III—24, (38%), IV—4, (6%) |
| Volume of surgery, n, (%) | - | Gastropancreatoduodal resection—45 (70%), Distal resection of the pancreas—16 (25%), Duodenumpancreatectomy—3 (5%) |
| Postoperative complications, n cases, (%) | - | Acute fluid accumulation—20 (29%); Bleeding—7 (11%); Pancreatitis—23 (36%); Anastamosis failure—6 (9%); Sepsis/MOF—1 (2%); Thrombosis—2 (4%); Peritonitis—2 (4%); Pneumonia—3 (5%) |
| Metabolites, µmol/L | Healthy Volunteers (n = 48) | Patients before the Surgery (n = 64) | p-Value *** |
|---|---|---|---|
| Benzoic acid | 0.5 (0.5; 0.6) | 0.6 (<0.5; 0.6) | - |
| Phenylpropionic acid | <0.5 (<0.5; <0.5) | <0.5 (<0.5; <0.5) | - |
| p-Hydroxyphenylacetic acid | <0.5 (<0.5; <0.5) | <0.5 (<0.5; 0.6) | - |
| p-Hydroxyphenyllactic acid | 1.3 (1.0; 1.6) | 1.1 (0.9; 1.4) | 0.002 |
| Σ 3 AMM * | 1.9 (1.5; 2.2) | 1.4 (0.9; 2.0) | <0.001 |
| Succinic acid | 4.8 (4.4; 5.9) | 2.9 (2.2; 3.9) | <0.001 |
| Fumaric acid | 1.3 (1.1; 1.5) | 0.6 (0.5; 0.9) | <0.001 |
| CRP (mg/L) | <5 ** | 4.3 (1.8; 14.8) | - |
| Metabolites, µmol/L | Point 0 | Point 1 | Point 2 | p-Value * |
|---|---|---|---|---|
| Benzoic acid | 0.59 (<0.5; 0.74) | 0.58 (<0.5; 0.72) | 0.68 (0.51; 0.78) | 0.345 |
| Phenylpropionic acid | <0.5 (<0.5; 0.5) | <0.5 (<0.5; <0.5) | <0.5 (<0.5; <0.5) | - |
| Phenyllactic acid | <0.5 (<0.5; <0.5) | <0.5 (<0.5; 0,62) | <0.5 (<0.5; 0.60) | - |
| p-Hydroxyphenylacetic acid | <0.5 (<0.5; 0.64) | <0.5 (<0.5; 0.89) | <0.5 (<0.5; 1.8) | - |
| p-Hydroxyphenyllactic acid | 1.1 (0.87; 1.5) | 1.3 (0.98; 1.9) | 1.2 (0.98; 1.7) | <0.001 |
| Σ 3 AMM | 1.9 (1.5; 2.4) | 2.4 (1.7; 3.2) | 2.2 (1.7; 3.8) | 0.005 |
| Succinic acid | 2.9 (2.2; 3.9) | 2.3 (1.7; 3.1) | 2.8 (1.7; 3.4) | 0.056 |
| Fumaric acid | 0.63 (0.50; 0.94) | 0.54 (<0.5; 0.66) | 0.49 (<0.5; 0.68) | <0.001 |
| PCT | 0.054 (0.022; 0.092) | 0.14 (0.078; 0.37) | 0.10 (0.074; 0.19) | <0.001 |
| Metabolites before Surgery, µmol/L | Patients Days in the ICU Less than 3 (n = 31) | Patients Days in the ICU 3 Days or More (n = 33) | p-Value * | |
|---|---|---|---|---|
| Benzoic acid | 0.55 (<0.5; 0.76) | 0.62 (<0.5; 0.68) | 0.614 | |
| p-Hydroxyphenyllactic acid | 0.94 (0.76; 1.2) | 1.4 (0.98; 1.6) | 0.009 | |
| Σ 3 AMM | 1.7 (1.4; 2.4) | 2.2 (1.6; 2.4) | 0.087 | |
| Succinic acid | 2.6 (2.2; 4.0) | 3.1 (2.3; 3.7) | 0.559 | |
| Fumaric acid | 0.71 (0.53; 0.94) | 0.59 (<0.5; 0.91) | 0.440 | |
| General | ||||
| Sex (M) | 12 (38.7%) | 19 (57.6%) | 0.131 | |
| Age | 59.0 (53.0; 65.0) | 65.0 (54.0; 72.0) | ||
| ASA | 2 | 21 (67.7%) | 15 (45.5%) | 0.059 |
| 3 | 10 (32.3%) | 14 (42.4%) | ||
| 4 | 0 (0.0%) | 4 (12.1%) | ||
| Stages of cancer | 0 | 3 (9.7%) | 2 (6.1%) | 0.846 |
| 1 | 13 (41.9%) | 16 (48.5%) | ||
| 2 | 11 (35.5%) | 13 (39.4%) | ||
| 3 | 3 (9.7%) | 2 (6.1%) | ||
| 4 | 1 (3.2%) | 0 (0%) | ||
| Tumor localization | Head of the pancreas | 13 (41.9%) | 17 (51.5%) | 0.001 |
| Body/tail of the pancreas | 17 (54.8%) | 2 (6.1%) | ||
| Major duodenal papilla | 0 (0%) | 1 (3%) | ||
| Terminal cholidochus | 1 (3.2%) | 12 (36.4%) | ||
| Total | 0 (0%) | 1 (3%) | ||
| Metabolites, µmol/L | Serum (n = 64) | Drainage Fluid (n = 35) | p-Value * |
|---|---|---|---|
| Benzoic acid | 0.58 (<0.5; 0.72) | 23(8.8; 47) | <0.001 |
| Phenyllactic acid | <0.5 (<0.5; 0.6) | <0.5 (<0.5; 0.5) | - |
| p-Hydroxyphenylacetic acid | <0.5 (<0.5; 0.88) | <0.5 (<0.5; 0.6) | - |
| p-Hydroxyphenyllactic acid | 1.3 (0.98; 1.9) | 1.8 (1.2; 2.4) | 0.072 |
| Σ 3 AMM | 2.4 (1.7; 3.2) | 2.6 (1.8; 3.9) | 0.351 |
| Succinic acid | 2.4 (1.7; 3.1) | 5.5 (3.8; 8.9) | <0.001 |
| Fumaric acid | 0.54 (<0.5; 0.66) | 2.9 (1.6; 4.9) | <0.001 |
| Amylase, Unit/L | 68.6 (18.1; 156.5) | 1003.7 (52.3; 6337.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getsina, M.; Tsyba, N.; Polyakov, P.; Beloborodova, N.; Chernevskaya, E. Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer. Metabolites 2023, 13, 1198. https://doi.org/10.3390/metabo13121198
Getsina M, Tsyba N, Polyakov P, Beloborodova N, Chernevskaya E. Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer. Metabolites. 2023; 13(12):1198. https://doi.org/10.3390/metabo13121198
Chicago/Turabian StyleGetsina, Maria, Nikolay Tsyba, Petr Polyakov, Natalia Beloborodova, and Ekaterina Chernevskaya. 2023. "Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer" Metabolites 13, no. 12: 1198. https://doi.org/10.3390/metabo13121198
APA StyleGetsina, M., Tsyba, N., Polyakov, P., Beloborodova, N., & Chernevskaya, E. (2023). Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer. Metabolites, 13(12), 1198. https://doi.org/10.3390/metabo13121198







