Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experimental and Design
2.3. Biochemical Parameters Determination
2.4. Histopathological Observation
2.5. UPLC-QTOF/MS Analysis
2.6. Statistical Analysis
3. Results
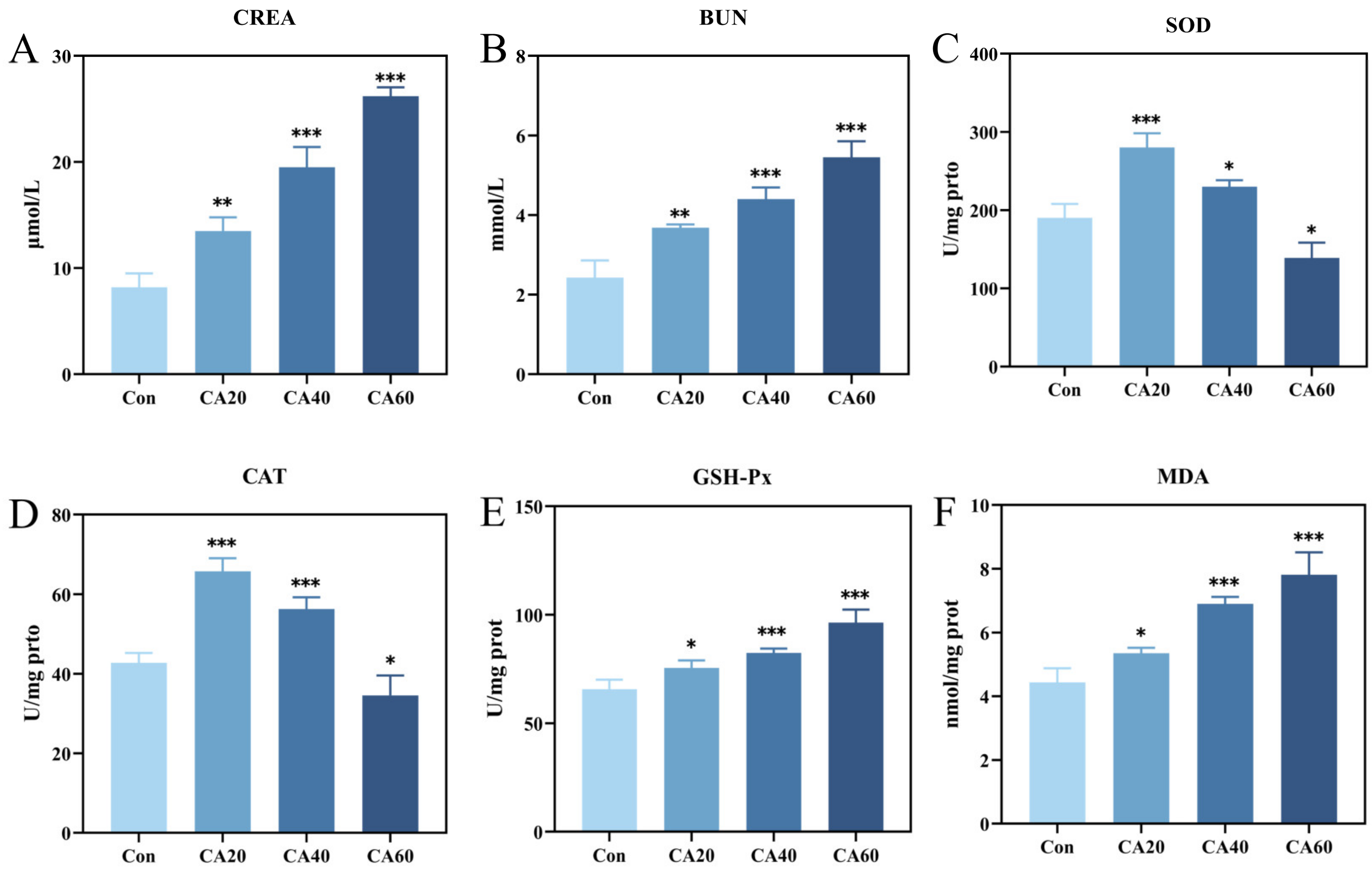
3.1. Biochemical Index Changes after Carbonate Alkalinity Exposure
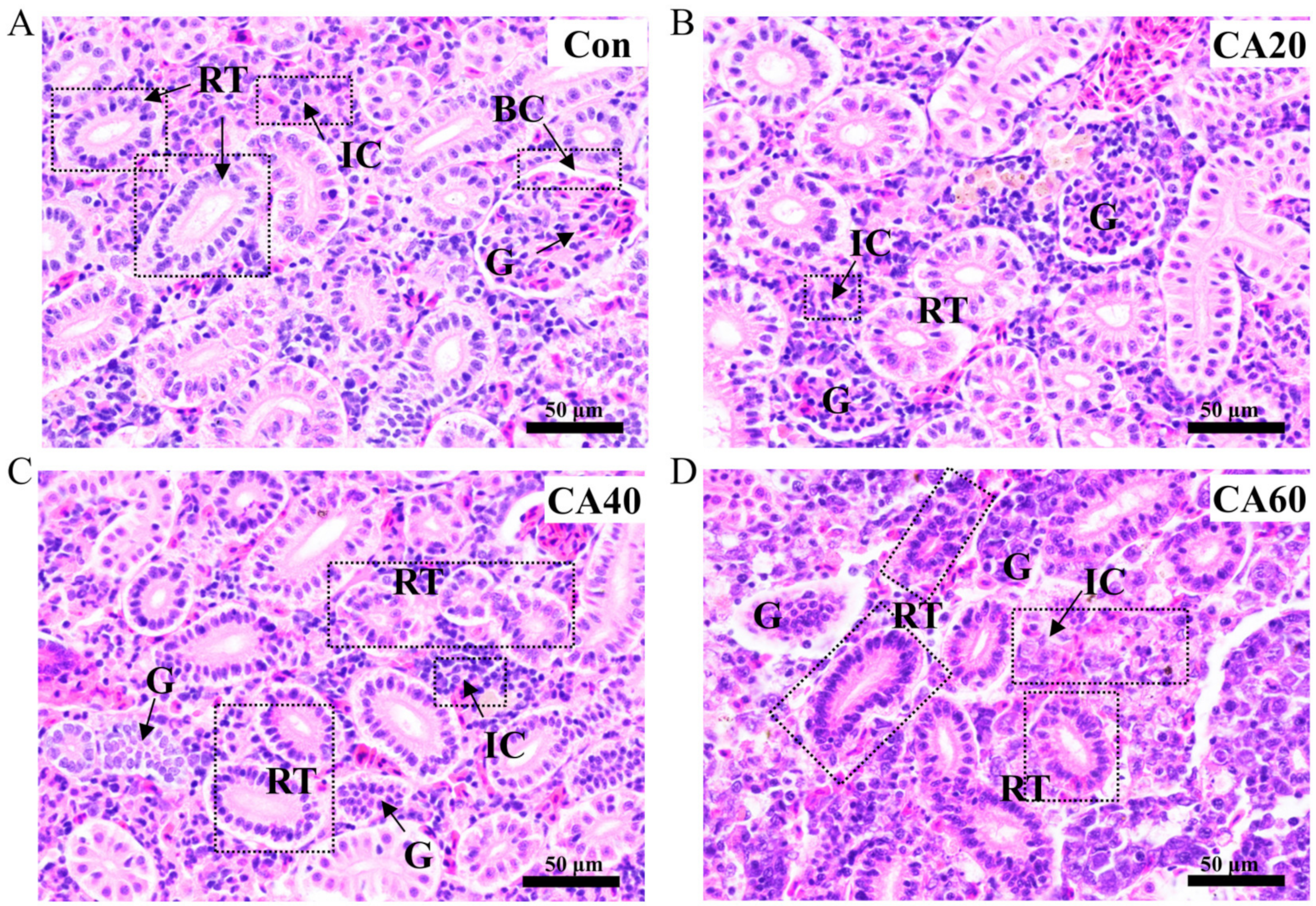
3.2. Histological Changes of the Kidney after Carbonate Alkalinity Exposure
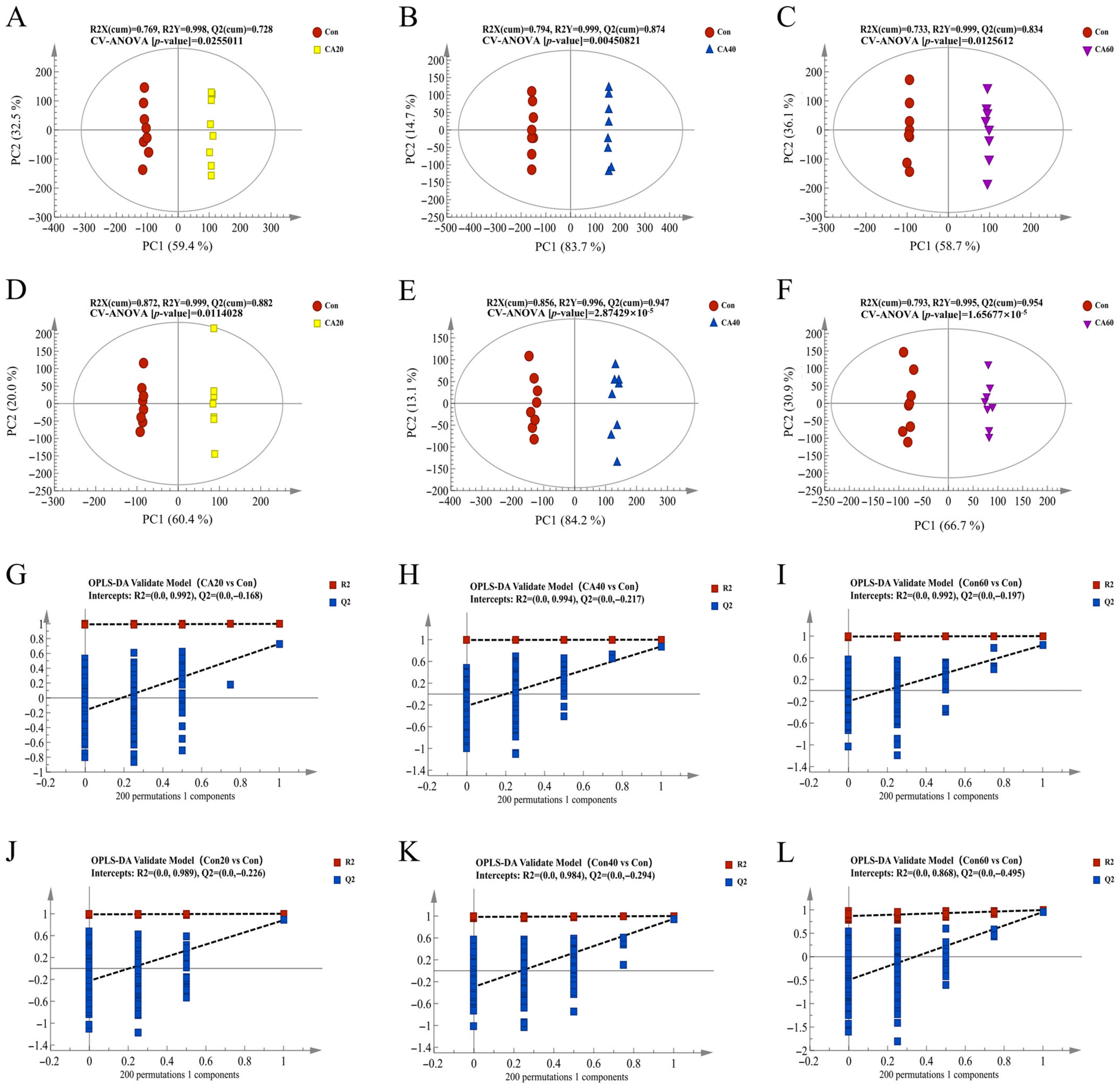
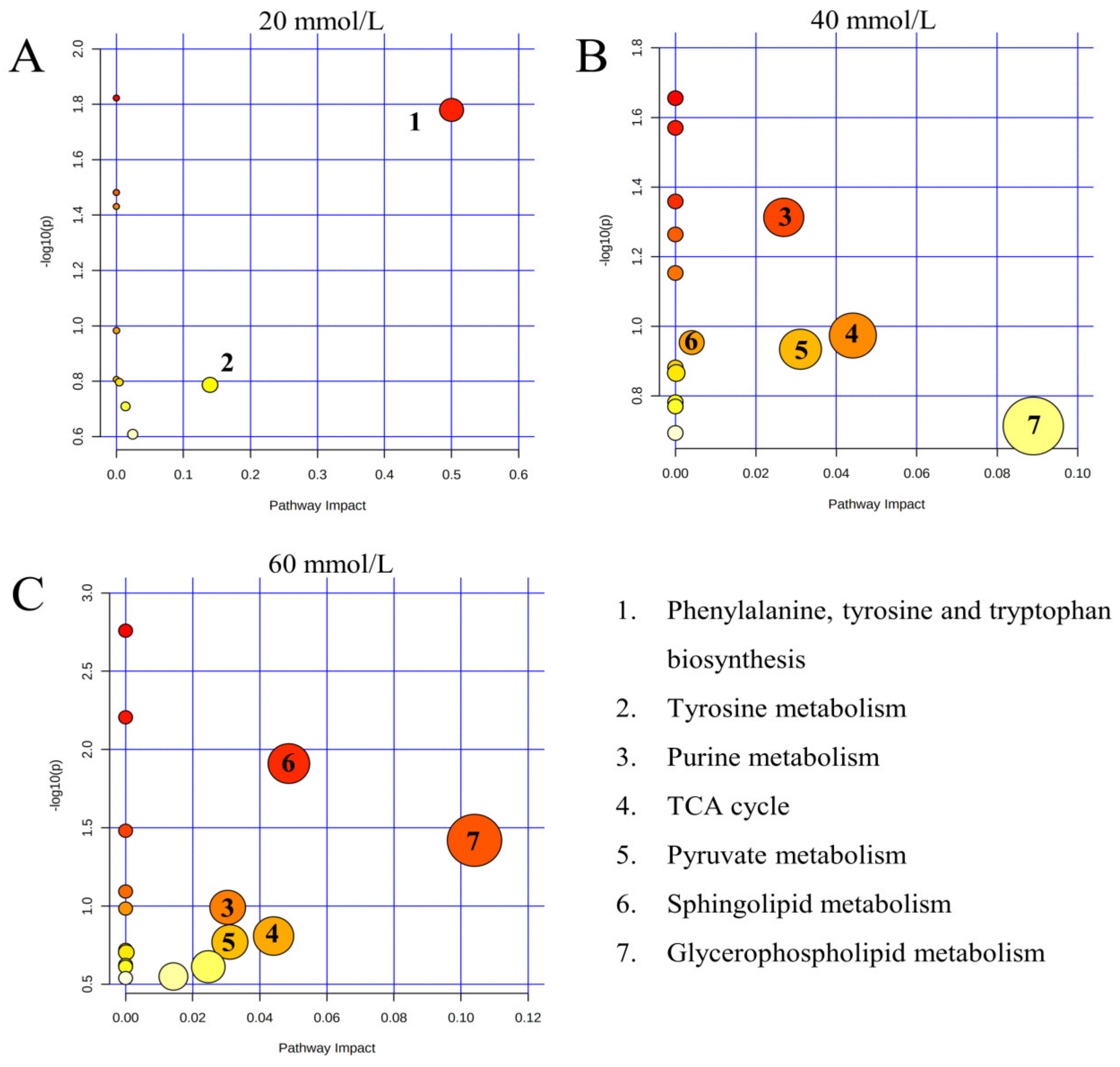
3.3. Metabolome Alterations of the Kidney after Carbonate Alkalinity Exposure
4. Discussion
4.1. Carbonate Alkaline Stress-Induced Oxidative Damage in Kidney
4.2. Effect of Carbonate Alkaline Stress on Kidney Metabolism
4.2.1. Amino Acid Metabolism
4.2.2. Lipid Metabolism
4.2.3. Purine Metabolism
4.2.4. Energy Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.; Li, C.h.; Ye, C.; Chen, H.-S.; Xu, J.; Dong, X.H.; Liu, X.S.; Li, D. Effects of aquaculture on the shallow lake aquatic ecological environment of Lake Datong, China. Environ. Sci. Eur. 2022, 34, 19. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Xu, J.; Han, L.; Yin, W. Research on the ecologicalization efficiency of mariculture industry in China and its influencing factors. Mar. Policy 2022, 137, 104935. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, D.; Zhang, Y.; Li, J.; Xu, Q.; Wang, L. Carbonate alkalinity and dietary protein levels affected growth performance, intestinal immune responses and intestinal microflora in Songpu mirror carp (Cyprinus carpio Songpu). Aquaculture 2021, 545, 737135. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, H.; Liu, Y.; Qi, X.; Sun, D.; Zhang, C.; Zhang, K.; Zhang, M.; Li, J.; Li, Y. Gill histological and transcriptomic analysis provides insights into the response of spotted sea bass (Lateolabrax maculatus) to alkalinity stress. Aquaculture 2023, 563, 738945. [Google Scholar] [CrossRef]
- Shang, X.; Geng, L.; Yang, J.; Zhang, Y.; Xu, W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotoxicol. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef]
- Yao, Z.; Lai, Q.; Hao, Z.; Chen, L.; Lin, T.; Zhou, K.; Wang, H. Carbonic anhydrase 2-like and Na+-K+-ATPase α gene expression in medaka (Oryzias latipes) under carbonate alkalinity stress. Fish Physiol. Biochem. 2015, 41, 1491–1500. [Google Scholar] [CrossRef]
- Fang, H.; Yang, Y.Y.; Wu, X.M.; Zheng, S.Y.; Song, Y.J.; Zhang, J.; Chang, M.X. Effects and molecular regulation mechanisms of salinity stress on the health and disease resistance of Grass Carp. Front. Immunol. 2022, 13, 917497. [Google Scholar] [CrossRef]
- Ouyang, H.; Deng, N.; Xu, J.; Huang, J.; Han, C.; Liu, D.; Liu, S.; Yan, B.; Han, L.; Li, S. Effects of hyperosmotic stress on the intestinal microbiota, transcriptome, and immune function of mandarin fish (Siniperca chuatsi). Aquaculture 2023, 563, 738901. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Zhao, Z.; Luo, L.; Zhang, R.; Guo, K.; Zhang, L.; Yang, Y. Effects of alkalinity on the antioxidant capacity, nonspecific immune response and tissue structure of Chinese Mitten Crab Eriocheir sinensis. Fishes 2022, 7, 206. [Google Scholar] [CrossRef]
- Olson, J.R. Predicting combined effects of land use and climate change on river and stream salinity. Philos. Trans. R. Soc. B 2019, 374, 20180005. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [PubMed]
- Xu, J.; Ji, P.; Wang, B.; Zhao, L.; Wang, J.; Zhao, Z.; Zhang, Y.; Li, J.; Xu, P.; Sun, X. Transcriptome sequencing and analysis of wild Amur Ide (Leuciscus waleckii) inhabiting an extreme alkaline-saline lake reveals insights into stress adaptation. PLoS ONE 2013, 8, e59703. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Xu, L.; Wang, S.; Jiang, Y.; Zhao, Z.; Zhang, Y.; Li, J.; Dong, C.; Xu, P. Gene expression changes leading extreme alkaline tolerance in Amur ide (Leuciscus waleckii) inhabiting soda lake. BMC Genom. 2013, 14, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gonzalez, R.J.; Patrick, M.L.; Grosell, M.; Zhang, C.; Feng, Q.; Du, J.; Walsh, P.J.; Wood, C.M. Unusual physiology of scale-less carp, Gymnocypris przewalskii, in Lake Qinghai: A high altitude alkaline saline lake. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Martinez, C.B. Morphological changes in the kidney of a fish living in an urban stream. Environ. Toxicol. Pharmacol. 2007, 23, 185–192. [Google Scholar] [CrossRef]
- Wang, X.D. The Fine Structure of Zebrafish Spleen, Heart and Body Kidney and the Standardization of Sample Preparation. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2018. [Google Scholar]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion transporters and osmoregulation in the kidney of teleost fishes as a function of salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Oğuz, A. A histological study of the kidney structure of Van fish (Alburnus tarichi) acclimated to highly alkaline water and freshwater. Mar. Freshw. Behav. Physiol. 2015, 48, 135–144. [Google Scholar] [CrossRef]
- Chang, Y.M.; Tang, R.; Dou, X.J.; Tao, R.; Sun, X.W.; Liang, L.Q. Transcriptome and expression profiling analysis of Leuciscus waleckii: An exploration of the alkali-adapted mechanisms of a freshwater teleost. Mol. BioSyst. 2014, 10, 491–504. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.; Li, S.; Wei, X.; Ding, L.; Han, S.; Wang, P.; Lv, B.; Chen, Z.; Sun, Y. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Gong, B.; Bao, F.; Wang, Y.; Liu, H.; Xiao, M.; He, J. Metabonomics study on the effect of traditional Chinese medicines feed addition on growth performance and serum metabolic profile of juvenile Chinese softshell turtle (Pelodiscus sinensis Wiegmann). Aquac. Rep. 2021, 20, 100632. [Google Scholar] [CrossRef]
- Sun, Y.C.; Han, S.C.; Yao, M.Z.; Liu, H.B.; Wang, Y.M. Exploring the metabolic biomarkers and pathway changes in crucian under carbonate alkalinity exposure using high-throughput metabolomics analysis based on UPLC-ESI-QTOF-MS. RSC Adv. 2020, 10, 1552–1571. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Wu, S.; Du, N.N.; Song, Y.; Xu, W. High-throughput metabolomics enables metabolite biomarkers and metabolic mechanism discovery of fish in response to alkalinity stress. RSC Adv. 2018, 8, 14983–14990. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lai, Q.; Fang, W.; Wang, J. Study on aquaculture in different type of saline-alkali water. In Proceedings of the 2003 Forum on Fishery Science and Technology China Ocean Press, Beijing, China, 30 June 2003. [Google Scholar]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.-Z. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics. Sci. Total Environ. 2018, 640, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. I Med. Dosw. (Online) 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, S.; Wang, Q.; Wang, D.; Wu, Q.; Xie, S.; Zou, J. Effects of partial replacement of dietary flour meal with seaweed polysaccharides on the resistance to ammonia stress in the intestine of hybrid snakehead (Channa maculatus♀× Channa argus♂). Fish Shellfish. Immunol. 2022, 127, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, C.; Qi, M.; Liu, Q.; Hu, Z. The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix). Biology 2022, 11, 1580. [Google Scholar] [CrossRef]
- Wu, L.; Lv, X.; Zhang, Y.; Xin, Q.; Zou, Y.; Li, X. Tartrazine exposure results in histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in juvenile crucian carp (Carassius carassius). Aquat. Toxicol. 2021, 241, 105998. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Lin, T.; Shi, J.; Zhou, K.; Wang, H.; Qi, H.; Lai, Q. Effects of carbonate alkalinity stress on SOD, ACP, and AKP activities in the liver and kidney of juvenile Gymnocypris przewalskii. J. Fish. Sci. China 2013, 20, 1212–1218. [Google Scholar] [CrossRef]
- Katikaneni, A.; Jelcic, M.; Gerlach, G.F.; Ma, Y.; Overholtzer, M.; Niethammer, P. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 2020, 22, 1049–1055. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhang, P.; Liu, J.; Wang, B.; Bu, X.; Wei, Q.; Liu, S.; Li, Y. Effects of dietary supplementation with Bacillus subtilis on immune, antioxidant, and histopathological parameters of Carassius auratus gibelio juveniles exposed to acute saline-alkaline conditions. Aquac. Int. 2022, 30, 2295–2310. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Yang, J.; Geng, L.W.; Wang, Y.; JH, F.; Li, C.Y. Effect of NaHCO3 alkalinity on oxidative stress of Luciobarbus Capito. Period. Ocean. Univ. China 2021, 51, 32–39. [Google Scholar]
- Mohamed, A.A.R.; El-Houseiny, W.; Abd Elhakeem, E.M.; Ebraheim, L.L.; Ahmed, A.I.; Abd El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.-M.; Xu, W.B.; Chen, D.Y.; Li, B.W.; Cheng, Y.X.; Guo, X.L.; Dong, W.R.; Shu, M.A. The effects of salinities stress on histopathological changes, serum biochemical index, non-specific immune and transcriptome analysis in red swamp crayfish Procambarus clarkii. Sci. Total Environ. 2022, 840, 156502. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, C.; Li, Z.; Duanzhi, D.; Liu, Y.; Ran, F.; Ding, J.; Yan, W.; Jia, C.; Zhang, Z. Short-term exposure to 5‰ and 15‰ salinity causes the dynamic changes of the NKA gene, enzyme activities and morphological characteristics in fish tissues of Gymnocypris przewalskii. Aquac. Res. 2022, 53, 6389–6398. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Wu, X.; Yin, Z.; Niu, X.; Wang, G. Dietary α-lipoic acid can alleviate the bioaccumulation, oxidative stress, cell apoptosis, and inflammation induced by lead (Pb) in Channa argus. Fish Shellfish. Immunol. 2021, 119, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kwon, Y.K.; Nam, M.; Vaidya, B.; Kim, S.R.; Lee, S.; Kwon, J.; Kim, D.; Hwang, G.S. Integrated profiling of global metabolomic and transcriptomic responses to viral hemorrhagic septicemia virus infection in olive flounder. Fish Shellfish. Immunol. 2017, 71, 220–229. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, J.L.; Zhao, Y.; Cao, X.Y. Study on the timing sequence of two pathway of Oreochromis niloticus ammonia metabolism under the stress of carbonate alkalinity. Freshw. Fish. 2018, 48, 25–32. [Google Scholar]
- Wilkie, M.P.; Wood, C.M. The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 113, 665–673. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Dong, Y.W.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. Threonine deficiency decreased intestinal immunity and aggravated inflammation associated with NF-κB and target of rapamycin signalling pathways in juvenile grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Br. J. Nutr. 2017, 118, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Liang, X.F.; Liu, L. Indirect effect of different dietary protein to energy ratio of bait fish mori diets on growth performance, body composition, nitrogen metabolism and relative AMPK & mTOR pathway gene expression of Chinese perch. Aquac. Rep. 2020, 16, 100276. [Google Scholar]
- Yang, P.; Wang, W.; Chi, S.; Mai, K.; Song, F.; Wang, L. Effects of dietary lysine on regulating GH-IGF system, intermediate metabolism and immune response in largemouth bass (Micropterus salmoides). Aquac. Rep. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, J.; Tang, B.; Yu, F.; Lu, Y.; Hou, G.; Chen, J.; Du, Z. Transcriptome and metabolome analyses of the immune response to light stress in the hybrid grouper (Epinephelus lanceolatus♂×Epinephelus fuscoguttatus♀). Animal 2022, 16, 100448. [Google Scholar] [CrossRef]
- Kuo, C.H.; Ballantyne, R.; Huang, P.L.; Ding, S.; Hong, M.C.; Lin, T.Y.; Wu, F.C.; Xu, Z.Y.; Chiu, K.; Chen, B. Sarcodia suae modulates the immunity and disease resistance of white shrimp Litopenaeus vannamei against Vibrio alginolyticus via the purine metabolism and phenylalanine metabolism. Fish Shellfish. Immunol. 2022, 127, 766–777. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino assets: How amino acids support immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Yang, B.K.; Zhang, C.N.; Xu, S.X.; Sun, P. Effects of polystyrene microplastics acute exposure in the liver of swordtail fish (Xiphophorus helleri) revealed by LC-MS metabolomics. Sci. Total Environ. 2022, 850, 157772. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xiang, Q.Q.; Lian, L.H.; Chen, Z.Y.; Luo, X.; Ding, C.Z.; Chen, L.Q. Metabolic profiling of nanosilver toxicity in the gills of common carp. Ecotoxicol. Environ. Saf. 2021, 222, 112548. [Google Scholar] [CrossRef]
- Lee, M.C.; Park, J.C.; Lee, J.S. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 2018, 200, 83–92. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Ayisi, C.L.; Cao, X. Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia. Aquac. Fish. 2020, 7, 389–395. [Google Scholar] [CrossRef]
- Huang, M.; Dong, Y.; Zhang, Y.; Chen, Q.; Xie, J.; Xu, C.; Zhao, Q.; Li, E. Growth and lipidomic responses of juvenile pacific white shrimp Litopenaeus vannamei to low salinity. Front. Physiol. 2019, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Zhao, X.F.; Liang, L.Q.; Liew, H.J.; Chang, Y.M.; Sun, B.; Wang, S.Y.; Mi, B.H.; Zhang, L.M. Identification and analysis of long non-coding RNAs in Leuciscus waleckii adapted to highly alkaline conditions. Front. Physiol. 2021, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Loizides-Mangold, U. On the future of mass-spectrometry-based lipidomics. FEBS J. 2013, 280, 2817–2829. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Nagahara, Y.; Kawakami, K.; Sikandan, A.; Yagi, D.; Nishikawa, R.; Shinomiya, T. Sphingoid base-upregulated caspase-14 expression involves MAPK. Biol. Pharm. Bull. 2018, 41, 743–748. [Google Scholar] [CrossRef]
- Pedley, A.M.; Benkovic, S.J. A new view into the regulation of purine metabolism: The purinosome. Trends Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef]
- Salinas, I.; Esteban, M.A.; Meseguer, J. Purine nucleosides downregulate innate immune function and arrest apoptosis in teleost fish leucocytes (B168) [Z]. Am. Assoc. Immnol. 2007, LB35. [Google Scholar] [CrossRef]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef]
- Camici, M.; Garcia-Gil, M.; Pesi, R.; Allegrini, S.; Tozzi, M.G. Purine-metabolising enzymes and apoptosis in cancer. Cancers 2019, 11, 1354. [Google Scholar] [CrossRef]
- Haskó, G.; Sitkovsky, M.V.; Szabo, C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Wu, J.; Dang, H.; Wang, L.; Zhang, W.; Ding, J. Whole genome analysis and specific PCR primer development for Vibrio coralliilyticus, combined with transcription and metabolome analysis of red spotting disease in the sea urchin, Strongylocentrotus intermedius. Aquac. Rep. 2022, 22, 100957. [Google Scholar] [CrossRef]
- Qin, H.; Yu, Z.; Zhu, Z.; Lin, Y.; Xia, J.; Jia, Y. The integrated analyses of metabolomics and transcriptomics in gill of GIFT tilapia in response to long term salinity challenge. Aquac. Fish. 2022, 7, 131–139. [Google Scholar] [CrossRef]
- Yan, L.; Wang, P.; Zhao, C.; Fan, S.; Lin, H.; Guo, Y.; Ma, Z.; Qiu, L. Toxic responses of liver in Lateolabrax maculatus during hypoxia and re-oxygenation. Aquat. Toxicol. 2021, 236, 105841. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Guo, Z.; Xu, G.; Xu, P. Transcriptomic analysis reveals different responses to ammonia stress and subsequent recovery between Coilia nasus larvae and juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 230, 108710. [Google Scholar] [CrossRef]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109487. [Google Scholar] [CrossRef]
- Biswal, A.; Srivastava, P.P.; Krishna, G.; Paul, T.; Pal, P.; Gupta, S.; Varghese, T.; Jayant, M. An Integrated biomarker approach for explaining the potency of exogenous glucose on transportation induced stress in Labeo rohita fingerlings. Sci. Rep. 2021, 11, 5713. [Google Scholar] [CrossRef]
- Ciccarone, F.; Vegliante, R.; Di Leo, L.; Ciriolo, M.R. The TCA cycle as a bridge between oncometabolism and DNA transactions in cancer. Semin. Cancer Biol. 2017, 47, 50–56. [Google Scholar] [CrossRef]
- Fu, T.; Qin, S.; He, H.; Zhang, K.; Zhang, W.; Tang, X.; Wu, W. Mechanisms of Ardisia japonica in the treatment of hepatic injury in rats based on LC-MS metabolomics. Metabolites 2022, 12, 981. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Adnan, A.M.; Abd Elnabi, H.E.; Ayman, M.F.; Soltan, M.A.; El-Haroun, E.R. Effect of dietary protease at different levels of malic acid on growth, digestive enzymes and haemato-immunological responses of Nile tilapia, fed fish meal free diets. Aquaculture 2020, 522, 735124. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Liu, Y.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Sun, Y. Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites 2023, 13, 159. https://doi.org/10.3390/metabo13020159
Ding L, Liu Y, Wei X, Geng C, Liu W, Han L, Yuan F, Wang P, Sun Y. Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites. 2023; 13(2):159. https://doi.org/10.3390/metabo13020159
Chicago/Turabian StyleDing, Lu, Yingjie Liu, Xiaofeng Wei, Chuanye Geng, Wenzhi Liu, Lin Han, Fangying Yuan, Peng Wang, and Yanchun Sun. 2023. "Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus)" Metabolites 13, no. 2: 159. https://doi.org/10.3390/metabo13020159
APA StyleDing, L., Liu, Y., Wei, X., Geng, C., Liu, W., Han, L., Yuan, F., Wang, P., & Sun, Y. (2023). Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites, 13(2), 159. https://doi.org/10.3390/metabo13020159