Interaction between γ-Hydroxybutyric Acid and Ethanol: A Review from Toxicokinetic and Toxicodynamic Perspectives
Abstract
:1. Introduction
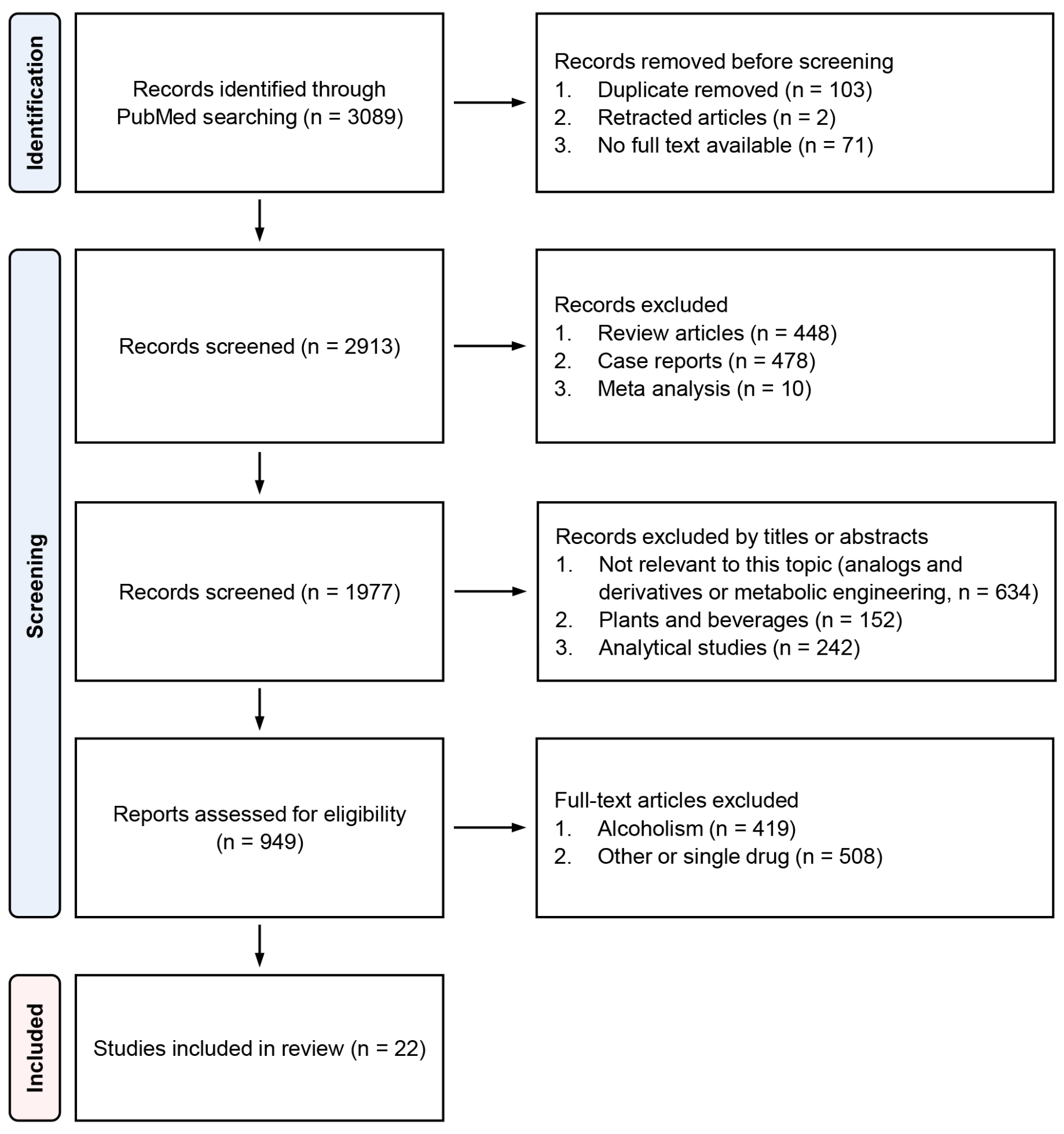
2. Search Methods and Results
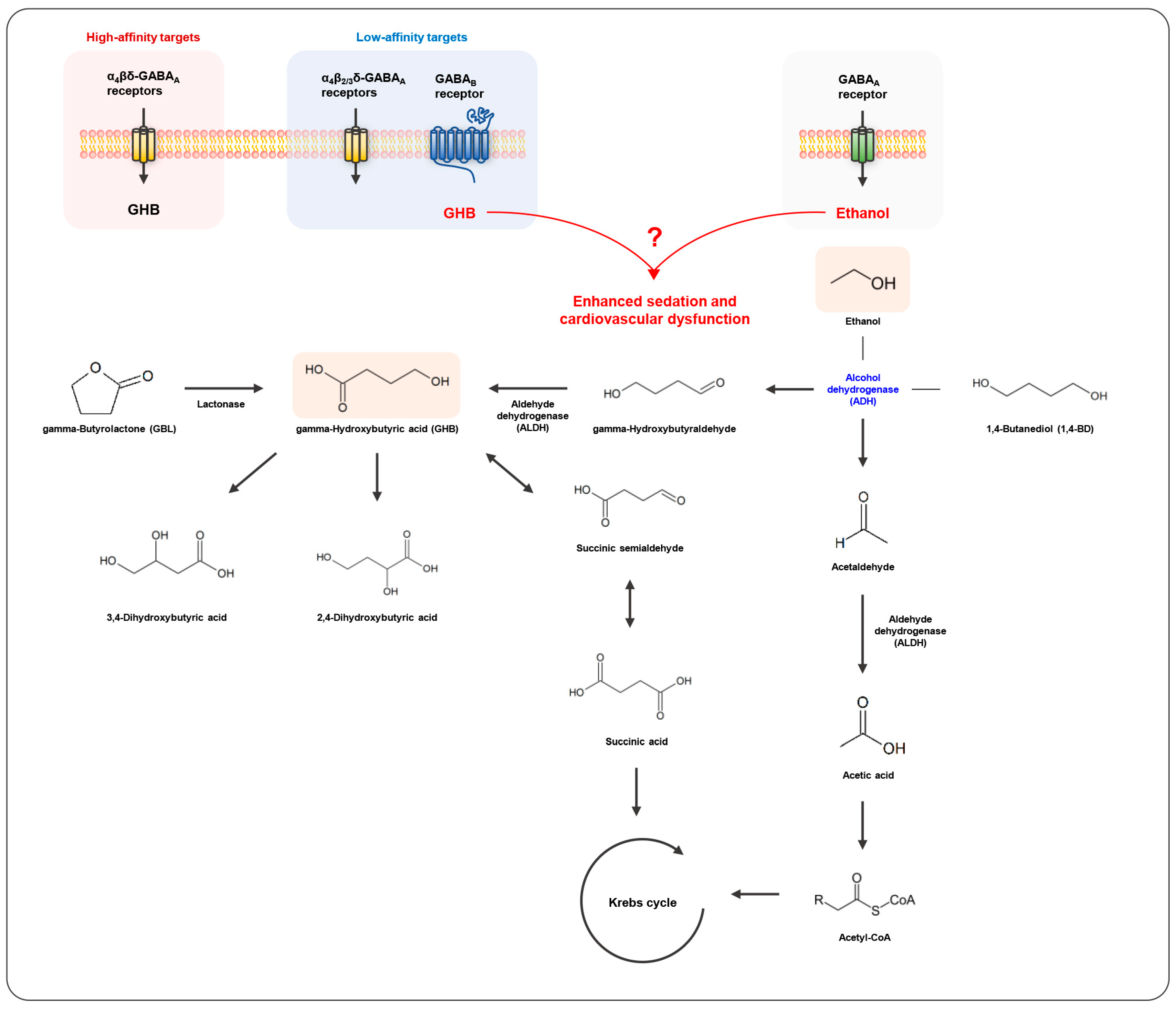
3. Pharmacology and Toxicology of GHB
4. GHB Intoxication in Crimes under the Influence of Drugs
5. Clinical Case Studies of GHB/GBL and Ethanol-Related Intoxication
6. Toxicokinetic and Toxicodynamic Interactions between GHB and Ethanol in Humans
7. Toxicokinetic and Toxicodynamic Interactions between GHB and Ethanol in Animals
| Subject | Method | Result | Ref. | |||
|---|---|---|---|---|---|---|
| Male Wistar rats | Group | Saline, GHB (GBL), EtOH, GBL/EtOH | Toxicokinetics | GHB/EtOH → Vmax (↓), VT (↑), Vdss (↑) | [11] | |
| Protocol | Toxicokinetics: EtOH (infusion to steady-state EtOH target conc. 300–3000 µg/mL) followed by GHB (a single bolus, 400 mg/kg) Toxicodynamics: 20 min after target conc. of GHB (infusion to steady state GHB target conc. 200–1400 µg/mL) or EtOH (infusion to steady-state EtOH target conc. 1000–3000 µg/mL) Sedation test (RR): EtOH (3 g/kg, i.p.), GBL (0.3 g/kg, i.p.), GBL/EtOH | Toxicodynamics | RR: GHB/EtOH (>2000 µg/mL) → synergy; GHB/EtOH (lower conc.) → additivity | |||
| SR: GHB/EtOH (<1000 µg/mL) → antagonism; GHB/EtOH (higher conc.) → additivity | ||||||
| TC: GHB/EtOH (all conc.) → antagonism | ||||||
| Sedation (RR) | GBL/EtOH > EtOH > GBL | |||||
| Male Swiss-Webster mice | Group | Vehicle, GHB, EtOH, GHB/EtOH | Locomotor activity | Vehicle, EtOH > GHB > GHB/EtOH | [18] | |
| Protocol | Behavior test: GHB (0.1–1.0 g/kg, i.g.), EtOH (2.0–5.0 g/kg, i.g.) | |||||
| RR | Vehicle, GHB, EtOH < GHB/EtOH | |||||
| FGS | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| ISP | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| HS | Vehicle < GHB, EtOH < GHB/EtOH | |||||
| Body temperature | Vehicle > GHB, EtOH > GHB/EtOH | |||||
| Male SD rats | Group | GHB, GHB/EtOH, GHB/EtOH/AZD or ARC | Toxicokinetics | GHB ≃ GHB/EtOH (oral) GHB/EtOH (i.v.) → terminal T1/2 (↑) | [19] | |
| Protocol | EtOH (2 g/kg, i.v.), GHB (0.6 g/kg, i.v. or 1.5 g/kg, i.g.), AR-C 155858 (MCT1 inhibitors, 5 mg/kg, i.v.), AZD-3965 (MCT1 inhibitors, 5 mg/kg, i.v.) | |||||
| Sedation | EtOH < GHB/EtOH/AZD-3965 ≃ GHB < GHB/EtOH | |||||
| MCT1 inhibition by AR-C 155858 | GHB/EtOH → respiratory depression (↓) GHB/EtOH → CLNR (↓) GHB → T1/2 (↑), CLNR (↓), Vss (↓) | |||||
| MCT1 inhibition by AZD-3965 | GHB conc. in brain and brain-to-plasma ratio at RRR (↓) GHB/EtOH → respiratory depression (↓) GHB/EtOH → CLR (↑), Vss/F (↑), Cmax (↓) GHB → CL/F (↑), CLNR/F (↑), Vss/F (↑), CLR (↑), AUC (↓), Cmax (↓), Tmax (↓) | |||||
| Male SD rats | Group | GHB, EtOH, GHB/EtOH, GHB/EtOH/inhibitors or antagonists | Toxicokinetics | GHB ≃ GHB/0.1–0.4% EtOH | [20] | |
| Protocol | Sedation: EtOH (2.0 g/kg, i.v.), GHB (600 mg/kg, i.v.), L-lactate (MCT inhibitor, 66 mg/kg + 302.5 mg/kg/h), Bicuculline (bic, GABAAR antagonist, 1 mg/kg), SGS742 (SGS, GABABR antagonist, 500, 1000 mg/kg), SCH50911 (SCH, GABABR antagonist, 100, 200 mg/kg) Respiratory depression/fatality and toxicokinetics: GHB (600, 1500 mg/kg, i.v.), GHB/EtOH (steady-state conc. 0.1–0.2% or 0.3–0.4%), GHB/EtOH (steady-state conc. 0.1–0.2% or 0.3–0.4%)/inhibitors or antagonists Oral toxicokinetics:GHB (1.5 g/kg, i.g.), EtOH (2.5 g/kg, i.g.) | RR | GHB/EtOH > GHB, GHB/EtOH/L-lactate | |||
| Sleep time | GHB/EtOH≃GHB/EtOH/bic > GHB > GHB/EtOH/SGS or SCH > EtOH | |||||
| GHB conc. in brain and brain-to-plasma ratio at RRR | GHB ≃ GHB/EtOH > GHB/EtOH/L-lactate | |||||
| Respiratory Depression | Frequency: | GHB/EtOH/SCH → completely prevented GHB ≃ GHB/EtOH ≃ GHB/EtOH/bic | ||||
| Tidal volume: | GHB/EtOH/SCH → completely prevented GHB > GHB/EtOH ≃ GHB/EtOH/bic | |||||
| Fatality: | GHB/EtOH > GHB/EtOH/L-lactate > GHB, GHB/EtOH/SCH | |||||
| Male SD rats | Group | 1,4-BD, GHB, EtOH, 1,4-BD/EtOH, GHB/EtOH | Mutual metabolic inhibition | EtOH/1,4-BD → significant; EtOH/GHB → not significant | [21] | |
| Protocol | Toxicokinetics: 1,4-BD (1.58, 6.34 mmol/kg, i.v. or oral), GHB (1.58, 1.79, 6.34 mmol/kg, i.v.), EtOH (6.34, 12.7 mmol/kg, i.v.) LRR test: 6.34 mmol/kg (i.v.) | |||||
| Oral absorption of 1,4-BD | Rapid and complete | |||||
| Total duration of LRR | 1,4-BD > 1,4-BD/EtOH > GHB/EtOH > GHB | |||||
| Rhesus monkeys | Group | EtOH, GHB/EtOH | Reinforcing effects in self-administration | EtOH ≃ GHB/EtOH | [22] | |
| Protocol | Self-administration: EtOH (50, 100, 200 mg/kg/inj, i.v.), GHB (1.0, 3.2 mg/kg/inj, i.v.) | Demand functions in self-administration | EtOH ≃ GHB/EtOH | |||
| Subject | Method | Result | Ref. | |||
|---|---|---|---|---|---|---|
| Male LE rats (behavioral study), Male SD rats cardiovascular study) | Group | Saline, 1,4-BD, EtOH, 1,4-BD/EtOH | Behavioral study (response rate, % of control) | 1,4-BD, EtOH → dose-dependently decrease, 1,4-BD/EtOH > 1,4-BD | [23] | |
| Protocol | Behavioral study (fixed-ratio 20 schedule of food presentation): EtOH (0.25–2 g/kg), 1,4-BD (0.18–0.56 g/kg) Cardiovascular study: Saline (1.0 mL, i.p. or i.v.), EtOH (2.0 g/kg, i.p.), 1,4-BD (0.18–1.0 g/kg, i.p. or i.v.), 1,4-BD (0.56 g/kg, i.v.)/EtOH (2.0 g/kg, i.p.) | |||||
| Mean arterial blood pressure | 1,4-BD > Saline 1,4-BD > 1,4-BD/EtOH | |||||
| Heart rate | 1,4-BD > Saline EtOH > Saline 1,4-BD > 1,4-BD/EtOH | |||||
| Male DBA/2JIco mice | Group | 1,4-BD, GHB, EtOH/1,4-BD, 1,4-BD/inhibitors,1,4-BD/antagonists | LRR | 4MP/1,4-BD, EtOH/1,4-BD, DS/1,4-BD < 1,4-BD NCS-382/1,4-BD ≃ 1,4-BD SCH50911/1,4-BD < 1,4-BD CGP 46381/1,4-BD < 1,4-BD | [24] | |
| Protocol | GHB and 1,4-BD (0.2–1 g/kg, i.p.), EtOH (1 g/kg, i.p.), 4-methylpyrazole (4MP, ADH inhibitor, 0.1 mg/kg, i.p.), disulfiram (DS, ALDH inhibitor, 1–30 mg/kg, i.p.), NCS-382 (GHB receptor antagonist, 0.25 g/kg, i.p.), SCH50911, CGP46381 (GABAB receptor antagonist, 0.1 g/kg, i.p.) | |||||
| Male SD rats | Group | Saline, GHB, 1,4-BD, EtOH/1,4-BD | Mean arterial blood pressure | 1,4-BD > GHB | [25] | |
| Protocol | Cardiovascular study: GHB (0.56–10 g/kg, i.g.), 1,4-BD (0.18–1.0 g/kg, i.g.), 1,4-BD (1.8 g/kg, i.g.) Mortality: EtOH (2.0 g/kg, i.p.)/1,4-BD (1.8 g/kg, i.g.) | Heart rate | 1,4-BD > GHB | |||
| Mortality | 1,4-BD > EtOH/1,4-BD | |||||
| Male SD rats | Group | Vehicle, EtOH, 1,4-BD, 1,4-BD/EtOH, GBL | Electroencephalogram activity | 1,4-BD > GBL, EtOH followed by 1,4-BD < GBL | [26] | |
| Protocol | 1,4-BD (1 g/kg, i.g.), EtOH (3 g/kg, i.g.), GBL (400 mg/kg, i.g.) | LRR | EtOH < EtOH/1,4-BD | |||
| GHB conc. in brain and liver | 1,4-BD > EtOH/1,4-BD | |||||
| EtOH conc. (blood) | EtOH ≃ EtOH/1,4-BD | |||||
| Male SD rats (in vitro) | Group | 1,4-BD, EtOH, Pyrazole, Disulfiram | Conversion rate of 1,4-BD to GHB | EtOH in brain and liver (↓) Pyrazole, Disulfiram in liver (↓) | [27] | |
| Protocol | Conversion rate of 1,4-BD to GHB in brain and liver and oxidation rate of 1,4-BD to GHB: 1,4-BD (8 mM), EtOH (10, 20 mM), pyrazole (ADH inhibitor, 1 mM), disulfiram (ALDH inhibitor, 1 mM) | |||||
| Oxidation of 1,4-BD to GHB | Competitively inhibited by EtOH | |||||
| Human livers (autopsy within 72 h after death) | Group | 1,4-BD, 1,4-BD/EtOH, 1,4-BD/AL, 1,4-BD/inhibitors | Conversion of 1,4-BD to GHB | 1,4-BD/EtOH (↓), 1,4-BD/AL (↑) | [28] | |
| Protocol | 10 human livers (5 men, 5 women, 43–79 years old) Conversion of 1,4-BD to GHB: 1,4-BD (3–80 mM), EtOH (0–2 mM), acetaldehyde (AL, ADH inhibitor, 0–2 mM) Inhibitors efficiency: 1,4-BD (0.5–5 mM) + ADH inhibitors (fomepizole, pyrazole) or ALDH inhibitors (disulfiram, cimetidine) | Inhibitors efficiency | Fomepizole: | GHB formation (↓), most potent inhibitor | ||
| Pyrazole: | GHB formation (↓) | |||||
| Disulfiram: | GHB formation (↓) | |||||
| Cimetidine: | GHB formation (↓), weakest inhibitor | |||||
| Male SD rats | Group | 1,4-BD, EtOH, 1,4-BD/EtOH | EtOH conc. (blood) | EtOH ≃ 1,4-BD/EtOH | [29] | |
| Protocol | Measurement of EtOH and 1,4-BD levels, mortality rate and histochemistry (brain. Liver, kidney): 1,4-BD (1 g/kg, i.g.), EtOH (3 g/kg, i.p.) | 1,4-BD conc. (brain, liver, kidney) | 1,4-BD/EtOH > 1,4-BD | |||
| Mortality rate | 1,4-BD/EtOH > 1,4-BD | |||||
| Histological alterations | EtOH → no change; 1,4-BD → hyperemia in all organs (↑); 1,4-BD/EtOH → tissue damage (↑), fatty infiltration and necrosis in liver, extensive medullary necrosis in kidney) | |||||
8. Drug Discrimination or Responding Following GHB and Ethanol Co-Administration
9. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bay, T.; Eghorn, L.F.; Klein, A.B.; Wellendorph, P. GHB receptor targets in the CNS: Focus on high-affinity binding sites. Biochem. Pharmacol. 2014, 87, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Trombley, T.A.; Capstick, R.A.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Gamma-Hydroxybutyrate (GHB). ACS Chem. Neurosci. 2019, 11, 3850–3859. [Google Scholar] [CrossRef]
- Brunt, T.M.; van Amsterdam, J.G.; van den Brink, W. GHB, GBL and 1,4-BD addiction. Curr. Pharm. Des. 2014, 20, 4076–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, R.D. NTP summary report on the metabolism, disposition, and toxicity of 1,4-butanediol (CAS No. 110-63-4). Toxic. Rep. Ser. 1996, 54, 1–28. [Google Scholar]
- Zvosec, D.L.; Smith, S.W.; McCutcheon, J.R.; Spillane, J.; Hall, B.J.; Peacock, E.A. Adverse events, including death, associated with the use of 1,4-butanediol. N. Engl. J. Med. 2001, 344, 87–94. [Google Scholar] [CrossRef]
- Busardò, F.P.; Varì, M.R.; di Trana, A.; Malaca, S.; Carlier, J.; di Luca, N.M. Drug-facilitated sexual assaults (DFSA): A serious underestimated issue. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10577–10587. [Google Scholar] [CrossRef]
- Miró, Ò.; Waring, W.S.; Dargan, P.I.; Wood, D.M.; Dines, A.M.; Yates, C.; Giraudon, I.; Moughty, A.; O’Connor, N.; Heyerdahl, F.; et al. Variation of drugs involved in acute drug toxicity presentations based on age and sex: An epidemiological approach based on European emergency departments. Clin. Toxicol. 2021, 59, 896–904. [Google Scholar] [CrossRef]
- Singh, A.K. Alcohol Interaction with Cocaine, Methamphetamine, Opioids, Nicotine, Cannabis, and γ-Hydroxybutyric Acid. Biomedicines 2019, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef] [Green Version]
- Hendler, R.A.; Ramchandani, V.A.; Gilman, J.; Hommer, D.W. Stimulant and sedative effects of alcohol. Curr. Top. Behav. Neurosci. 2013, 13, 489–509. [Google Scholar] [CrossRef]
- Van Sassenbroeck, D.K.; De Paepe, P.; Belpaire, F.M.; Buylaert, W.A. Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. Toxicol. Sci. 2003, 73, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miró, Ò.; Galicia, M.; Dargan, P.; Dines, A.M.; Giraudon, I.; Heyerdahl, F.; Hovda, K.E.; Yates, C.; Wood, D.M.; Liakoni, E.; et al. Intoxication by gamma hydroxybutyrate and related analogues: Clinical characteristics and comparison between pure intoxication and that combined with other substances of abuse. Toxicol. Lett. 2017, 277, 84–91. [Google Scholar] [CrossRef]
- Galicia, M.; Dargan, P.I.; Dines, A.M.; Yates, C.; Heyerdahl, F.; Hovda, K.E.; Giraudon, I.; Wood, D.M.; Miró, Ò. Clinical relevance of ethanol coingestion in patients with GHB/GBL intoxication. Toxicol. Lett. 2019, 314, 37–42. [Google Scholar] [CrossRef]
- Liechti, M.E.; Kunz, I.; Greminger, P.; Speich, R.; Kupferschmidt, H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006, 81, 323–326. [Google Scholar] [CrossRef]
- Thai, D.; Dyer, J.E.; Benowitz, N.L.; Haller, C.A. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J. Clin. Psychopharmacol. 2006, 26, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Haller, C.; Thai, D.; Jacob, P., 3rd; Dyer, J.E. GHB urine concentrations after single-dose administration in humans. J. Anal. Toxicol. 2006, 30, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Pross, N.; Patat, A.; Vivet, P.; Bidaut, M.; Fauchoux, N. Pharmacodynamic interactions of a solid formulation of sodium oxybate and ethanol in healthy volunteers. Br. J. Clin. Pharmacol. 2015, 80, 480–492. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.D.; Biddlestone, L.; Coop, A.; Beardsley, P.M. Effects of combining ethanol (EtOH) with gamma-hydroxybutyrate (GHB) on the discriminative stimulus, locomotor, and motor-impairing functions of GHB in mice. Psychopharmacology 2006, 185, 112–122. [Google Scholar] [CrossRef]
- Rodriguez-Cruz, V.; Morris, M.E. γ-Hydroxybutyric Acid-Ethanol Drug-Drug Interaction: Reversal of Toxicity with Monocarboxylate Transporter 1 Inhibitors. J. Pharmacol. Exp. Ther. 2021, 378, 42–50. [Google Scholar] [CrossRef]
- Morse, B.L.; Morris, M.E. Toxicokinetics/Toxicodynamics of γ-hydroxybutyrate-ethanol intoxication: Evaluation of potential treatment strategies. J. Pharmacol. Exp. Ther. 2013, 346, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Fung, H.L.; Tsou, P.S.; Bulitta, J.B.; Tran, D.C.; Page, N.A.; Soda, D.; Mi Fung, S. Pharmacokinetics of 1,4-butanediol in rats: Bioactivation to gamma-hydroxybutyric acid, interaction with ethanol, and oral bioavailability. AAPS J. 2008, 10, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winger, G.; Galuska, C.M.; Hursh, S.R. Modification of ethanol’s reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology 2007, 193, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Gerak, L.R.; Hicks, A.R.; Winsauer, P.J.; Varner, K.J. Interaction between 1,4-butanediol and ethanol on operant responding and the cardiovascular system. Eur. J. Pharmacol. 2004, 506, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Carai, M.A.; Colombo, G.; Reali, R.; Serra, S.; Mocci, I.; Castelli, M.P.; Cignarella, G.; Gessa, G.L. Central effects of 1,4-butanediol are mediated by GABA(B) receptors via its conversion into gamma-hydroxybutyric acid. Eur. J. Pharmacol. 2002, 441, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.R.; Varner, K.J. Cardiovascular responses elicited by intragastric administration of BDL and GHB. J. Recept. Signal Transduct. Res. 2008, 28, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Poldrugo, F.; Snead, O.C., 3rd. 1,4 Butanediol, gamma-hydroxybutyric acid and ethanol: Relationships and interactions. Neuropharmacology 1984, 23, 109–113. [Google Scholar] [CrossRef]
- Poldrugo, F.; Snead, O.C., 3rd. 1,4-Butanediol and ethanol compete for degradation in rat brain and liver in vitro. Alcohol 1986, 3, 367–370. [Google Scholar] [CrossRef]
- Lenz, D.; Jübner, M.; Bender, K.; Wintermeyer, A.; Beike, J.; Rothschild, M.A.; Käferstein, H. Inhibition of 1,4-butanediol metabolism in human liver in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 647–654. [Google Scholar] [CrossRef]
- Poldrugo, F.; Barker, S.; Basa, M.; Mallardi, F.; Snead, O.C. Ethanol potentiates the toxic effects of 1,4-butanediol. Alcohol. Clin. Exp. Res. 1985, 9, 493–497. [Google Scholar] [CrossRef]
- Metcalf, B.R.; Stahl, J.M.; Allen, J.D.; Woolfolk, D.R.; Soto, P.L. Discrimination of gamma-hydroxybutyrate and ethanol administered separately and as a mixture in rats. Pharmacol. Biochem. Behav. 2001, 70, 31–41. [Google Scholar] [CrossRef]
- Lamb, R.J.; Munn, J.; Duiker, N.J.; Coop, A.; Wu, H.; Koek, W.; France, C.P. Interactions of gamma-hydroxy butyrate with ethanol and NCS 382. Eur. J. Pharmacol. 2003, 470, 157–162. [Google Scholar] [CrossRef]
- Baker, L.E.; Pynnonen, D.; Poling, A. Influence of reinforcer type and route of administration on gamma-hydroxybutyrate discrimination in rats. Psychopharmacology 2004, 174, 220–227. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Morse, B.L.; Morris, M.E. γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology. AAPS J. 2021, 23, 22. [Google Scholar] [CrossRef]
- Castelli, M.P.; Ferraro, L.; Mocci, I.; Carta, F.; Carai, M.A.; Antonelli, T.; Tanganelli, S.; Cignarella, G.; Gessa, G.L. Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of gamma-hydroxybutyric acid. J. Neurochem. 2003, 87, 722–732. [Google Scholar] [CrossRef]
- Gobaille, S.; Hechler, V.; Andriamampandry, C.; Kemmel, V.; Maitre, M. gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo. J. Pharmacol. Exp. Ther. 1999, 290, 303–309. [Google Scholar]
- Hechler, V.; Gobaille, S.; Bourguignon, J.J.; Maitre, M. Extracellular events induced by gamma-hydroxybutyrate in striatum: A microdialysis study. J. Neurochem. 1991, 56, 938–944. [Google Scholar] [CrossRef]
- Cash, C.D. Gamma-hydroxybutyrate: An overview of the pros and cons for it being a neurotransmitter and/or a useful therapeutic agent. Neurosci. Biobehav. Rev. 1994, 18, 291–304. [Google Scholar] [CrossRef]
- Duenas, M.R. Tobacco Smoke and Atherosclerosis Progression. JAMA 1998, 280, 32–33. [Google Scholar] [CrossRef]
- Chin, R.L.; Sporer, K.A.; Cullison, B.; Dyer, J.E.; Wu, T.D. Clinical Course of γ-Hydroxybutyrate Overdose. Ann. Emerg. Med. 1998, 31, 716–722. [Google Scholar] [CrossRef]
- Mamelak, M. Gammahydroxybutyrate: An endogenous regulator of energy metabolism. Neurosci. Biobehav. Rev. 1989, 13, 187–198. [Google Scholar] [CrossRef]
- Kemmel, V.; Klein, C.; Dembélé, D.; Jost, B.; Taleb, O.; Aunis, D.; Mensah-Nyagan, A.G.; Maitre, M. A single acute pharmacological dose of γ-hydroxybutyrate modifies multiple gene expression patterns in rat hippocampus and frontal cortex. Physiol. Genom. 2010, 41, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Saltini, S.; Bartiromo, M.; Zaffe, D.; Renzo Botticelli, A.; Ferrari, A.; Bertolini, A.; Genedani, S. Effect of gamma-hydroxybutyrate in two rat models of focal cerebral damage. Brain Res. 2003, 986, 181–190. [Google Scholar] [CrossRef]
- Wendt, G.; Kemmel, V.; Patte-Mensah, C.; Uring-Lambert, B.; Eckert, A.; Schmitt, M.J.; Mensah-Nyagan, A.G. Gamma-hydroxybutyrate, acting through an anti-apoptotic mechanism, protects native and amyloid-precursor-protein-transfected neuroblastoma cells against oxidative stress-induced death. Neuroscience 2014, 263, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, O.; Montplaisir, J.; Lamarre, M.; Bedard, M.A. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: Further considerations on REM sleep-triggering mechanisms. Sleep 1990, 13, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Prog. Neurobiol. 2009, 89, 193–219. [Google Scholar] [CrossRef]
- Carter, L.P.; Wu, H.; Chen, W.; Matthews, M.M.; Mehta, A.K.; Hernandez, R.J.; Thomson, J.A.; Ticku, M.K.; Coop, A.; Koek, W.; et al. Novel gamma-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J. Pharmacol. Exp. Ther. 2005, 313, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Connelly, W.M.; Errington, A.C.; Crunelli, V. γ-Hydroxybutyric acid (GHB) is not an agonist of extrasynaptic GABAA receptors. PLoS ONE 2013, 8, e79062. [Google Scholar] [CrossRef]
- Scrima, L.; Hartman, P.G.; Johnson, F.H., Jr.; Thomas, E.E.; Hiller, F.C. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: A double-blind study. Sleep 1990, 13, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Gallimberti, L.; Spella, M.R.; Soncini, C.A.; Gessa, G.L. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol 2000, 20, 257–262. [Google Scholar] [CrossRef]
- United National Office of Drugs and Crime. World Drug Report 2021. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 20 December 2021).
- Supreme Prosecutor’s Office, Republic of Korea. 2020 Narcotic Crime White Paper. pp. 162–174. Available online: https://www.spo.go.kr/preview/skin/doc.html?fn=0ac5769a-3e62-4808-9336-9895cb28b407.pdf&rs=/preview/result/board/1204/ (accessed on 10 June 2021).
- Busardò, F.P.; Jones, A.W. Interpreting γ-hydroxybutyrate concentrations for clinical and forensic purposes. Clin. Toxicol. 2019, 57, 149–163. [Google Scholar] [CrossRef]
- Busardò, F.P.; Bertol, E.; Vaiano, F.; Baglio, G.; Montana, A.; Barbera, N.; Zaami, S.; Romano, G. Post mortem concentrations of endogenous gamma hydroxybutyric acid (GHB) and in vitro formation in stored blood and urine samples. Forensic Sci. Int. 2014, 243, 144–148. [Google Scholar] [CrossRef]
- Årnes, M.; Bachs, L.; Sammarai, M.A.; Jones, A.W.; Høiseth, G. Rate of elimination of γ-hydroxybutyrate from blood determined by analysis of two consecutive samples from apprehended drivers in Norway. Forensic Sci. Int. 2020, 314, 110374. [Google Scholar] [CrossRef]
- Liechti, M.E.; Quednow, B.B.; Liakoni, E.; Dornbierer, D.; von Rotz, R.; Gachet, M.S.; Gertsch, J.; Seifritz, E.; Bosch, O.G. Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects. Br. J. Clin. Pharmacol. 2016, 81, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Schröck, A.; Hari, Y.; König, S.; Auwärter, V.; Schürch, S.; Weinmann, W. Pharmacokinetics of GHB and detection window in serum and urine after single uptake of a low dose of GBL—An experiment with two volunteers. Drug Test. Anal. 2014, 6, 363–366. [Google Scholar] [CrossRef]
- Brailsford, A.D.; Cowan, D.A.; Kicman, A.T. Urinary γ-hydroxybutyrate concentrations in 1126 female subjects. J. Anal. Toxicol. 2010, 34, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, F.; Serpelloni, G.; Furlanetto, S.; Palumbo, D.; Mari, F.; Fioravanti, A.; Bertol, E. Determination of endogenous concentration of γ-hydroxybutyric acid (GHB) in hair through an ad hoc GC-MS analysis: A study on a wide population and influence of gender and age. J. Pharm. Biomed. Anal. 2016, 118, 161–166. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.; Seo, Y.; Lee, S. Metabolic Alterations Associated with γ-Hydroxybutyric Acid and the Potential of Metabolites as Biomarkers of Its Exposure. Metabolites 2021, 11, 101. [Google Scholar] [CrossRef]
- Steuer, A.E.; Raeber, J.; Simbuerger, F.; Dornbierer, D.A.; Bosch, O.G.; Quednow, B.B.; Seifritz, E.; Kraemer, T. Towards Extending the Detection Window of Gamma-Hydroxybutyric Acid-An Untargeted Metabolomics Study in Serum and Urine Following Controlled Administration in Healthy Men. Metabolites 2021, 11, 166. [Google Scholar] [CrossRef]
- Tanaka, E. Toxicological interactions involving psychiatric drugs and alcohol: An update. J. Clin. Pharm. Ther. 2003, 28, 81–95. [Google Scholar] [CrossRef]
- Ameeta Rani, V.; Nadiger, H.A.; Marcus, S.R.; Chandrakala, M.V.; Sadasivudu, B. Acute and short term effects of ethanol on the metabolism of glutamic acid and GABA in rat brain. Neurochem. Res. 1985, 10, 297–306. [Google Scholar] [CrossRef]
- Piepponen, T.P.; Kiianmaa, K.; Ahtee, L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol. Biochem. Behav. 2002, 74, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.; Lee, M.S.; Kim, M.; Park, M.; Han, S.; Han, S.; Lee, H.S.; Lee, S. Urinary Profile of Endogenous Gamma-Hydroxybutyric Acid and its Biomarker Metabolites in Healthy Korean Females: Determination of Age-Dependent and Intra-Individual Variability and Identification of Metabolites Correlated With Gamma-Hydroxybutyric Acid. Front. Pharmacol. 2022, 13, 853971. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.S.; Kim, M.; Ko, B.J.; Lee, H.S.; Lee, S. Derivatization-assisted LC-MS/MS method for simultaneous quantification of endogenous gamma-hydroxybutyric acid and its metabolic precursors and products in human urine. Anal. Chim. Acta 2022, 1194, 339401. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Nutt, D.J. Gamma hydroxy butyrate abuse and dependency. J. Psychopharmacol. 2005, 19, 195–204. [Google Scholar] [CrossRef]
- Kintz, P.; Goullé, J.P.; Cirimele, V.; Ludes, B. Window of detection of gamma-hydroxybutyrate in blood and saliva. Clin. Chem. 2001, 47, 2033–2034. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.F.; Matschinsky, F.M. Ethanol metabolism: The good, the bad, and the ugly. Med. Hypotheses 2020, 140, 109638. [Google Scholar] [CrossRef]
- Galicia, M.; Nogue, S.; Miró, O. Liquid ecstasy intoxication: Clinical features of 505 consecutive emergency department patients. Emerg. Med. J. 2011, 28, 462–466. [Google Scholar] [CrossRef]
- Knudsen, K.; Jonsson, U.; Abrahamsson, J. Twenty-three deaths with gamma-hydroxybutyrate overdose in western Sweden between 2000 and 2007. Acta Anaesthesiol. Scand. 2010, 54, 987–992. [Google Scholar] [CrossRef]
- Krul, J.; Girbes, A.R. γ-Hydroxybutyrate: Experience of 9 years of γ-hydroxybutyrate (GHB)-related incidents during rave parties in The Netherlands. Clin. Toxicol. 2011, 49, 311–315. [Google Scholar] [CrossRef]
- Munir, V.L.; Hutton, J.E.; Harney, J.P.; Buykx, P.; Weiland, T.J.; Dent, A.W. Gamma-hydroxybutyrate: A 30 month emergency department review. Emerg. Med. Australas. 2008, 20, 521–530. [Google Scholar] [CrossRef]
- Van Sassenbroeck, D.K.; De Neve, N.; De Paepe, P.; Belpaire, F.M.; Verstraete, A.G.; Calle, P.A.; Buylaert, W.A. Abrupt awakening phenomenon associated with gamma-hydroxybutyrate use: A case series. Clin. Toxicol. 2007, 45, 533–538. [Google Scholar] [CrossRef]
- Zvosec, D.L.; Smith, S.W.; Porrata, T.; Strobl, A.Q.; Dyer, J.E. Case series of 226 γ-hydroxybutyrate-associated deaths: Lethal toxicity and trauma. Am. J. Emerg. Med. 2011, 29, 319–332. [Google Scholar] [CrossRef]
- Wood, D.M.; Brailsford, A.D.; Dargan, P.I. Acute toxicity and withdrawal syndromes related to γ-hydroxybutyrate (GHB) and its analogues γ-butyrolactone (GBL) and 1,4-butanediol (1,4-BD). Drug Test. Anal. 2011, 3, 417–425. [Google Scholar] [CrossRef]
- Devlin, R.J.; Henry, J.A. Clinical review: Major consequences of illicit drug consumption. Crit. Care 2008, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Snead, O.C., 3rd; Gibson, K.M. Gamma-hydroxybutyric acid. N. Engl. J. Med. 2005, 352, 2721–2732. [Google Scholar] [CrossRef]
- Tegeris, J.S.; Balster, R.L. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam. Appl. Toxicol. 1994, 22, 240–250. [Google Scholar] [CrossRef]
- Lingenhoehl, K.; Brom, R.; Heid, J.; Beck, P.; Froestl, W.; Kaupmann, K.; Bettler, B.; Mosbacher, J. Gamma-hydroxybutyrate is a weak agonist at recombinant GABA(B) receptors. Neuropharmacology 1999, 38, 1667–1673. [Google Scholar] [CrossRef]
- Mathivet, P.; Bernasconi, R.; De Barry, J.; Marescaux, C.; Bittiger, H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur. J. Pharmacol. 1997, 321, 67–75. [Google Scholar] [CrossRef]
- Nicholson, K.L.; Balster, R.L. GHB: A new and novel drug of abuse. Drug Alcohol Depend. 2001, 63, 1–22. [Google Scholar] [CrossRef]
- Wang, Q.; Darling, I.M.; Morris, M.E. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J. Pharmacol. Exp. Ther. 2006, 318, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Morris, M.E. Flavonoids modulate monocarboxylate transporter-1-mediated transport of gamma-hydroxybutyrate in vitro and in vivo. Drug Metab. Dispos. 2007, 35, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Morris, M.E. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): Characterization of SMCT-mediated uptake and inhibition. Drug Metab. Dispos. 2009, 37, 1404–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.E.; Hu, K.; Wang, Q. Renal clearance of gamma-hydroxybutyric acid in rats: Increasing renal elimination as a detoxification strategy. J. Pharmacol. Exp. Ther. 2005, 313, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Beleslin, D.B.; Djokanović, N.; Jovanović Mićić, D.; Samardzić, R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol 1997, 14, 167–173. [Google Scholar] [CrossRef]
- Carai, M.A.; Colombo, G.; Brunetti, G.; Melis, S.; Serra, S.; Vacca, G.; Mastinu, S.; Pistuddi, A.M.; Solinas, C.; Cignarella, G.; et al. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur. J. Pharmacol. 2001, 428, 315–321. [Google Scholar] [CrossRef]
- Liljequist, S.; Engel, J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 1982, 78, 71–75. [Google Scholar] [CrossRef]
- Morse, B.L.; Vijay, N.; Morris, M.E. γ-Hydroxybutyrate (GHB)-induced respiratory depression: Combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol. Pharmacol. 2012, 82, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Morse, B.L.; Vijay, N.; Morris, M.E. Mechanistic modeling of monocarboxylate transporter-mediated toxicokinetic/toxicodynamic interactions between γ-hydroxybutyrate and L-lactate. AAPS J. 2014, 16, 756–770. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Morris, M.E. Effects of L-lactate and D-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab. Dispos. 2008, 36, 2244–2251. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Q.; Morris, M.E. Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: Potential involvement of monocarboxylate transporters. AAPS J. 2008, 10, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Colpaert, F.C.; Janssen, P.A. Agonist and antagonist effects of prototype opiate drugs in fentanyl dose-dose discrimination. Psychopharmacology 1986, 90, 222–228. [Google Scholar] [CrossRef]
- Goudie, A.J.; Leathley, M.J. Actions of ritanserin, a 5-HT(2/1C) antagonist, in benzodiazepine-dependent rats. Behav. Pharmacol. 1993, 4, 247–255. [Google Scholar] [CrossRef]
- Holtzman, S.G. Caffeine as a model drug of abuse. Trends Pharmacol. Sci. 1990, 11, 355–356. [Google Scholar] [CrossRef]
- Overton, D.A. Multiple drug training as a method for increasing the specificity of the drug discrimination procedure. J. Pharmacol. Exp. Ther. 1982, 221, 166–172. [Google Scholar]
- Goodwin, A.K.; Brown, P.R.; Jansen, E.E.; Jakobs, C.; Gibson, K.M.; Weerts, E.M. Behavioral effects and pharmacokinetics of gamma-hydroxybutyrate (GHB) precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in baboons. Psychopharmacology 2009, 204, 465–476. [Google Scholar] [CrossRef] [Green Version]
| Subject | Method | Result | Ref. | ||
|---|---|---|---|---|---|
| Patients (ED) | Subject Period Country Group | 710 of 5629 patients October 2013–September 2014 10 European countries A (GHB or GBL) B ([GHB or GBL]/other drugs) | Patient’s characteristics | Mean age (31 years), male (n = 592), female (n = 118) Group A (n = 201), Group B (n = 509) Other drugs consumed with GHB/GBL: EtOH (50%) > amphetamine derivatives (36%) > cocaine (12%) > cannabis (8%) | [12] |
| Common clinical features (Group A + B, % of patients) | Altered behavior (39%), reduced consciousness (34%), anxiety (14%) | ||||
| Specific symptoms (Group A vs. Group B, % of patients) | Vomiting (3% vs. 15%), cardiovascular symptoms (1.5% vs. 5.3%) | ||||
| Patients (ED) | Subject Period Country Group | 609 of 17,371 patients October 2013–December 2016 14 European countries A (GHB or GBL) B ([GHB or GBL]/EtOH) | Patient’s characteristics | Mean age (32 years), male (n = 493), female (n = 116) Group A (n = 183), Group B (n = 426) | [13] |
| Common clinical features (Group A + B, % of patients) | Decreased consciousness (56.1%), agitation or aggressive behavior (33.6%) | ||||
| Specific symptoms (Group A vs. Group B, % of patients) | Decreased consciousness (49.1% vs. 58.9%), bradycardia (23.5% vs. 15.7%) | ||||
| Patients (ED) | Subject Duration Country Group | 48 patients (65 episodes) January 2001–December 2003 European countries A (GHB or GBL) B ([GHB or GBL]/EtOH | Patient’s characteristics | Mean age (24 years), male (n = 31), female (n = 17) Group B (48% of episodes) | [14] |
| Common clinical features (Group A + B, % of episodes) | Bradycardia (38%), hypotension (6%), hypothermia (48%) | ||||
| Specific symptoms (Group A + B vs. Group B, % of episodes) | Agitation (17% vs. 29%), vomiting (31% vs. 39%) | ||||
| Subject | Method | Results | Ref. | |||
|---|---|---|---|---|---|---|
| Healthy adults | Subject | 16 healthy adults 7 men, 9 women, 22–34 years old 7 Whites, 6 Asian/Pacific Islanders, 1 Latino, 2 multiple ethnicities | Toxicokinetics | GHB: | GHB ≃ GHB/EtOH | [15] |
| EtOH: | EtOH ≃ GHB/EtOH | |||||
| Toxicodynamics | O2sat.: | Placebo > GHB ≃ EtOH > GHB/EtOH | ||||
| Protocol | Randomized, double-blinded, crossover design 50 mg/kg GHB (Xyrem®), 0.6 g/kg EtOH | BP: | Placebo ≃ GHB > GHB/EtOH ≃ EtOH | |||
| HR: | EtOH ≃ GHB/EtOH > placebo ≃ GHB | |||||
| Skin temp.: | GHB, EtOH, GHB/EtOH > placebo | |||||
| Adverse events | GHB/EtOH (2 hypotension, 6 vomiting) | |||||
| Healthy adults | Subject | 16 healthy adults 7 men, 9 women, 22–34 years old 7 Whites, 6 Asian/Pacific Islanders, 1 Latino, 2 multiple ethnicities | Urinary GHB conc. | GHB/EtOH < GHB (0–3 h) | [16] | |
| Renal clearance | GHB ≃ GHB/EtOH | |||||
| Protocol | Randomized, double-blinded, crossover design 50 mg/kg GHB (Xyrem®), 0.6 g/kg EtOH | |||||
| Healthy adults | Subject | 24 healthy adults 12 men, 12 women, 18–43 years old 91.7% Caucasian, 4.2% Asian, 4.2% others | Toxicokinetics | No interaction between SMO.IR and EtOH | [17] | |
| Toxicodynamics | Physiological parameters: no effect at both 60 and 165 min after administration of SMO.IR/EtOH Subjective parameters (within 60 min after administration of SMO.IR/EtOH): alertness and stimulation (↑), sedation (↓) | |||||
| Protocol | Randomized, double-blinded, crossover design 2.25 g SMO.IR, 0.7 g/kg EtOH (male), 0.57 g/kg EtOH (female) | |||||
| Vital signs and physical examinations | All normal | |||||
| Subject | Method | Result | Ref. | ||
|---|---|---|---|---|---|
| Male Swiss-Webster mice | Group | Vehicle, GHB, EtOH, GHB/EtOH | Drug discrimination: | EtOH: <50% GHB-like discriminative stimulus effects GHB: no alteration in the GHB-like discriminative stimulus effects of EtOH | [18] |
| Protocol | Discrimination training under FR 10 schedule of sweetened condensed milk presentation: GHB (0.1 g/kg, s.c.) and water Discrimination testing: GHB (0.1 g/kg, s.c.), EtOH (1–2.5 g/kg, i.p.), NCS382 (GHB antagonist, 0.03–0.1 g/kg, i.p.) | ||||
| Male LE rats | Group | GHB trained, EtOH trained, GHB/EtOH trained | Generalization | GHB trained: GHB (225 mg/kg)/EtOH (750 mg/kg) → full generalization, higher mixture doses → >71% generalization EtOH trained: GHB (>225 mg/kg)/EtOH (>750 mg/kg) → full generalization GHB/EtOH trained: GHB (>225 mg/kg)/EtOH (>750 mg/kg) → full generalization | [30] |
| Protocol | Discrimination training under FR 10 schedule of food presentation: GHB (300 mg/kg, i.g.), EtOH (1 g/kg, i.g.), GHB (150 mg/kg, i.g.)/EtOH (500 mg/kg. i.g.) Discrimination/generalization testing: GHB (75–900 mg/kg), EtOH (250–3000 mg/kg), GHB/EtOH (a combination of one half of each of the respective doses of GHB and EtOH) | ||||
| Response rates | GHB trained: GHB/EtOH > GHB ≃ EtOH EtOH trained: GHB/EtOH ≃ EtOH > GHB GHB/EtOH trained: GHB/EtOH ≃ EtOH > GHB | ||||
| Male Lewis rats | Group | Vehicle, GHB, EtOH, GHB/EtOH, GHB/NCS382 | Drug responding | GHB: dose-relatedly decreased GHB/EtOH: greatly decreased GHB/NCS382: not antagonize the rate-decreasing effects of GHB | [31] |
| Protocol | Drug responding of rats under FR 10 schedule of sugar solution presentation: GHB (180, 300 mg/kg, i.p.), EtOH (0.1–0.8 g/kg, i.p.), NCS382 (GHB antagonist, 3.2, 32.0 mL/kg, i.p.) | ||||
| Male SD rats | Group | IP-food, IG-food, IP-water, IG-water | GHB dose-response function | IG-food ≃ IP-food > IG-water ≃ IP-water | [32] |
| Protocol | Discrimination training under FR 10 schedule of food or water presentation: GHB (300 mg/kg) and vehicle GHB dose-response function: GHB (1 mL/kg, i.p.), GHB (10 mL/kg, i.g.) Stimulus generalization test: GHB (75–300 mg/kg, i.p. and i.g.), GHB (400 mg/kg, i.g.), GBL (50–200 mg/kg, i.p.), 1,4-BD (100–400 mg/kg, i.p.), EtOH (1–3 g/kg, i.g.), GHB (150 mg/kg, i.g.)/EtOH (1–2 g/kg, i.g.) | Stimulus generalization: | GHB (i.p.): IG-water > IP-water ≃ IG-food ≃ IP-food GHB (i.g.): IP-water < IG-water ≃ IG-food ≃ IP-food GBL, 1,4-BD: fully substituted for GHB (except for in IP-Food) EtOH: partially substituted in all groups GHB/EtOH: no additive in all groups | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Kim, M.; Kim, S.; Lee, S. Interaction between γ-Hydroxybutyric Acid and Ethanol: A Review from Toxicokinetic and Toxicodynamic Perspectives. Metabolites 2023, 13, 180. https://doi.org/10.3390/metabo13020180
Jung S, Kim M, Kim S, Lee S. Interaction between γ-Hydroxybutyric Acid and Ethanol: A Review from Toxicokinetic and Toxicodynamic Perspectives. Metabolites. 2023; 13(2):180. https://doi.org/10.3390/metabo13020180
Chicago/Turabian StyleJung, Suryun, Mingyu Kim, Suji Kim, and Sooyeun Lee. 2023. "Interaction between γ-Hydroxybutyric Acid and Ethanol: A Review from Toxicokinetic and Toxicodynamic Perspectives" Metabolites 13, no. 2: 180. https://doi.org/10.3390/metabo13020180
APA StyleJung, S., Kim, M., Kim, S., & Lee, S. (2023). Interaction between γ-Hydroxybutyric Acid and Ethanol: A Review from Toxicokinetic and Toxicodynamic Perspectives. Metabolites, 13(2), 180. https://doi.org/10.3390/metabo13020180






