Efficient SABRE-SHEATH Hyperpolarization of Potent Branched-Chain-Amino-Acid Metabolic Probe [1-13C]ketoisocaproate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hyperpolarizer Setup
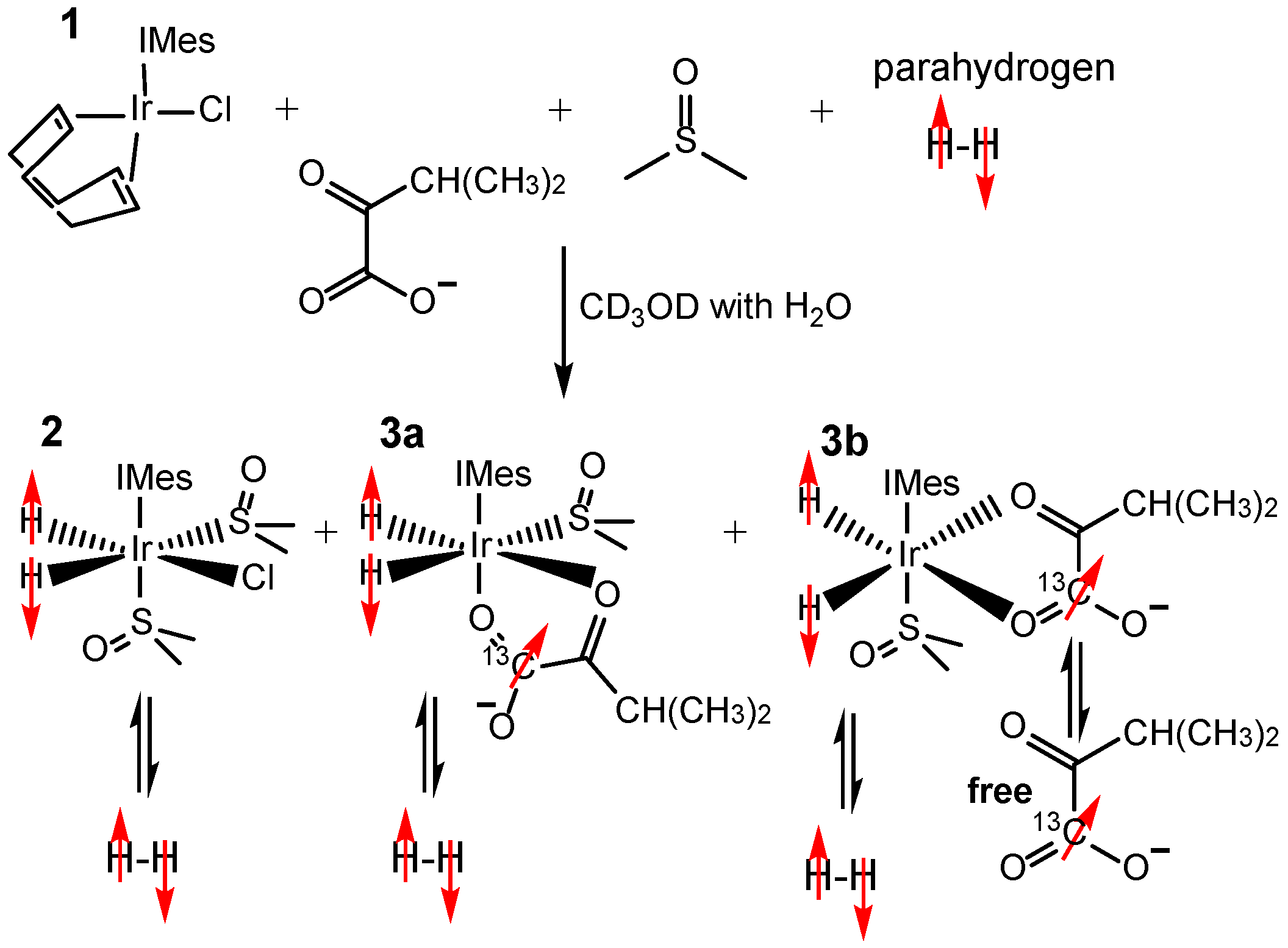
2.2. Sample Preparation for SABRE Hyperpolarization
2.3. NMR Signal Detection
2.4. 13C NMR Signal Enhancement (ε13C) and Polarization (P13C) Calculations
2.5. 13C Polarization Build-Up and T1 Relaxation Measurements
2.6. pH Modulation Study
2.7. Post-Mortem Studies Using HP [1-13C]ketoisocaproate Injection in a Euthanized Mouse
3. Results and Discussion
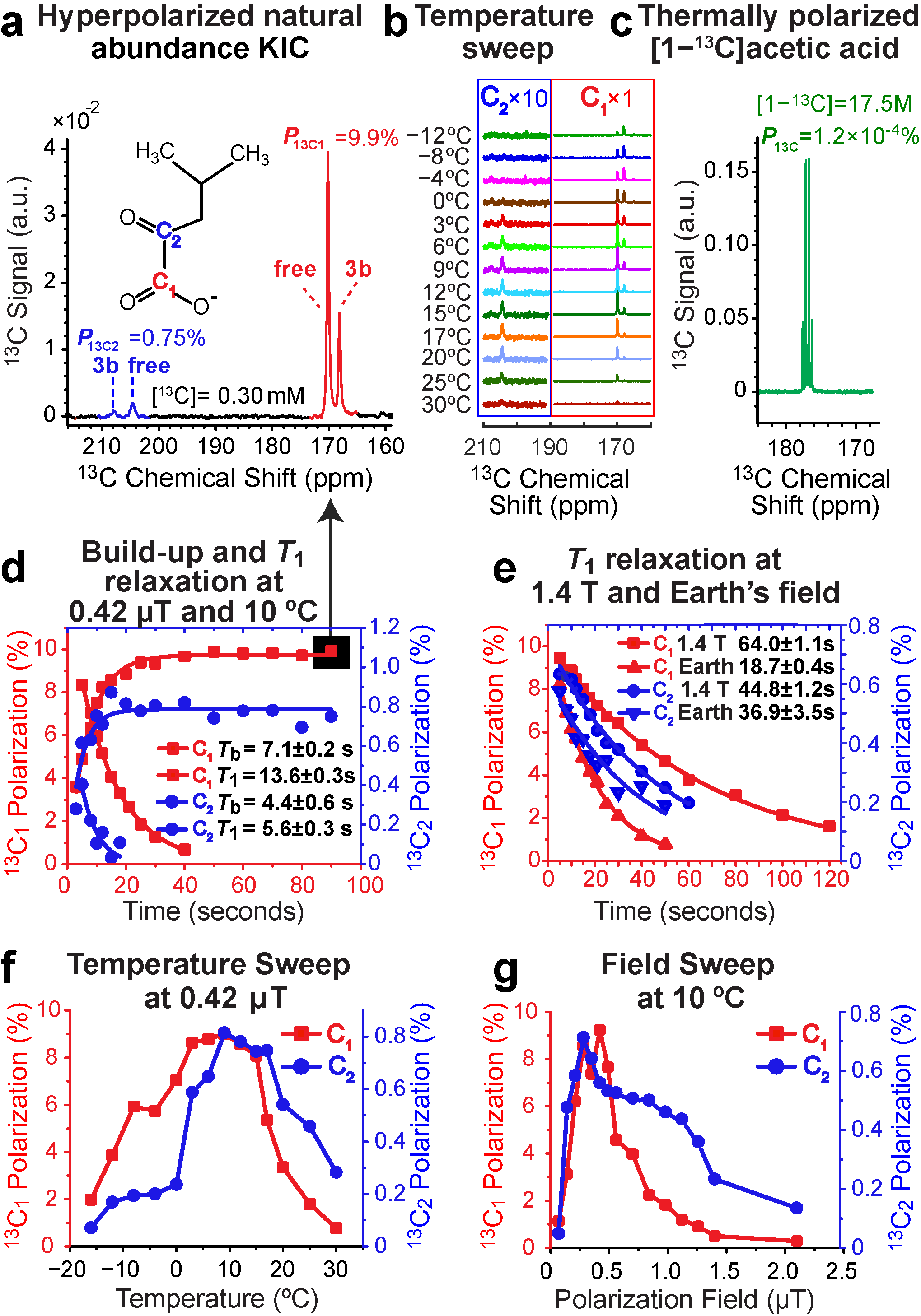
3.1. 13C SABRE-SHEATH of Natural Abundance Ketoisocaproate
3.2. 13C SABRE-SHEATH of [1-13C]ketoisocaproate
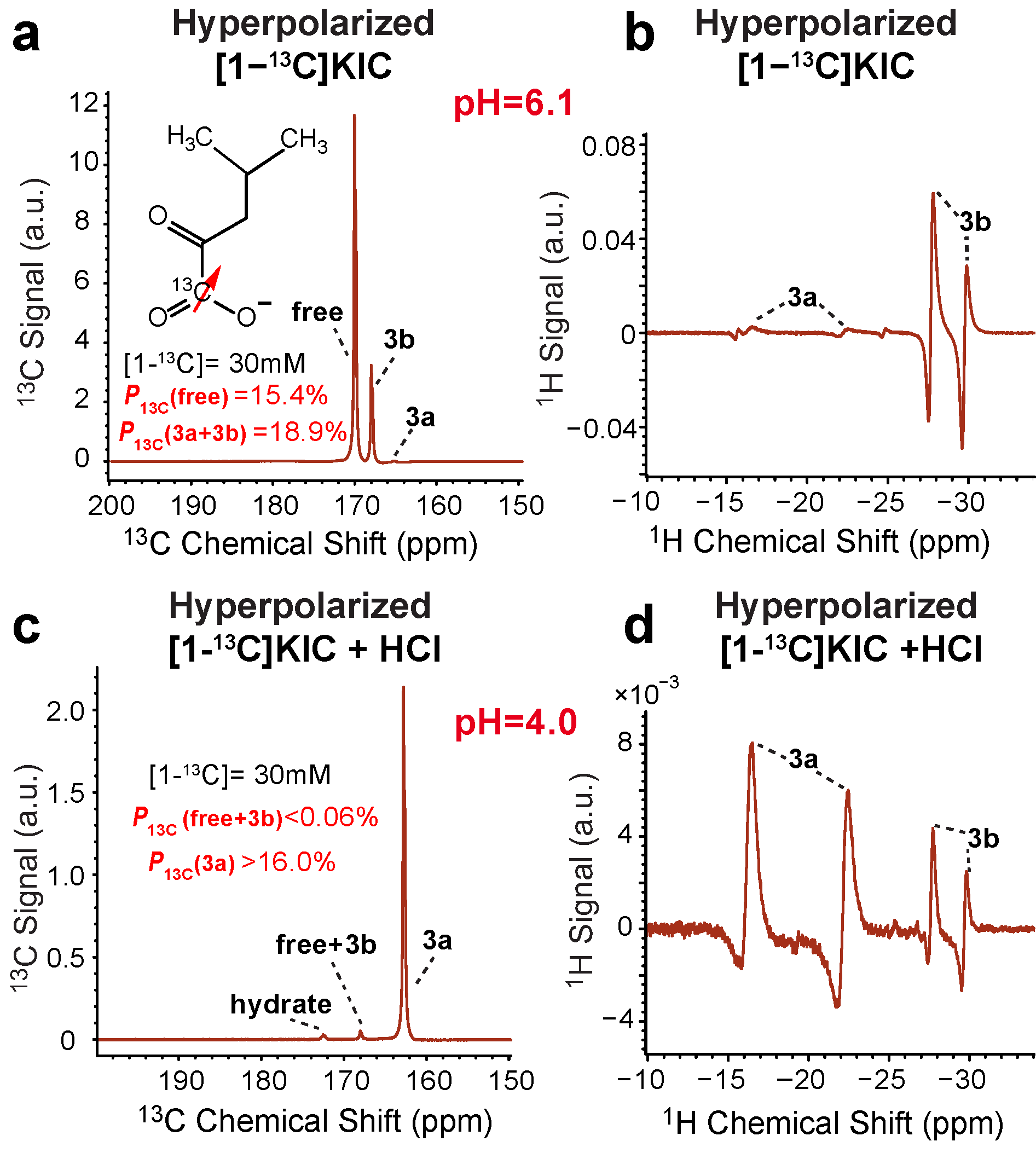
3.3. Effect of pH on 13C SABRE-SHEATH of [1-13C]ketoisocaproate
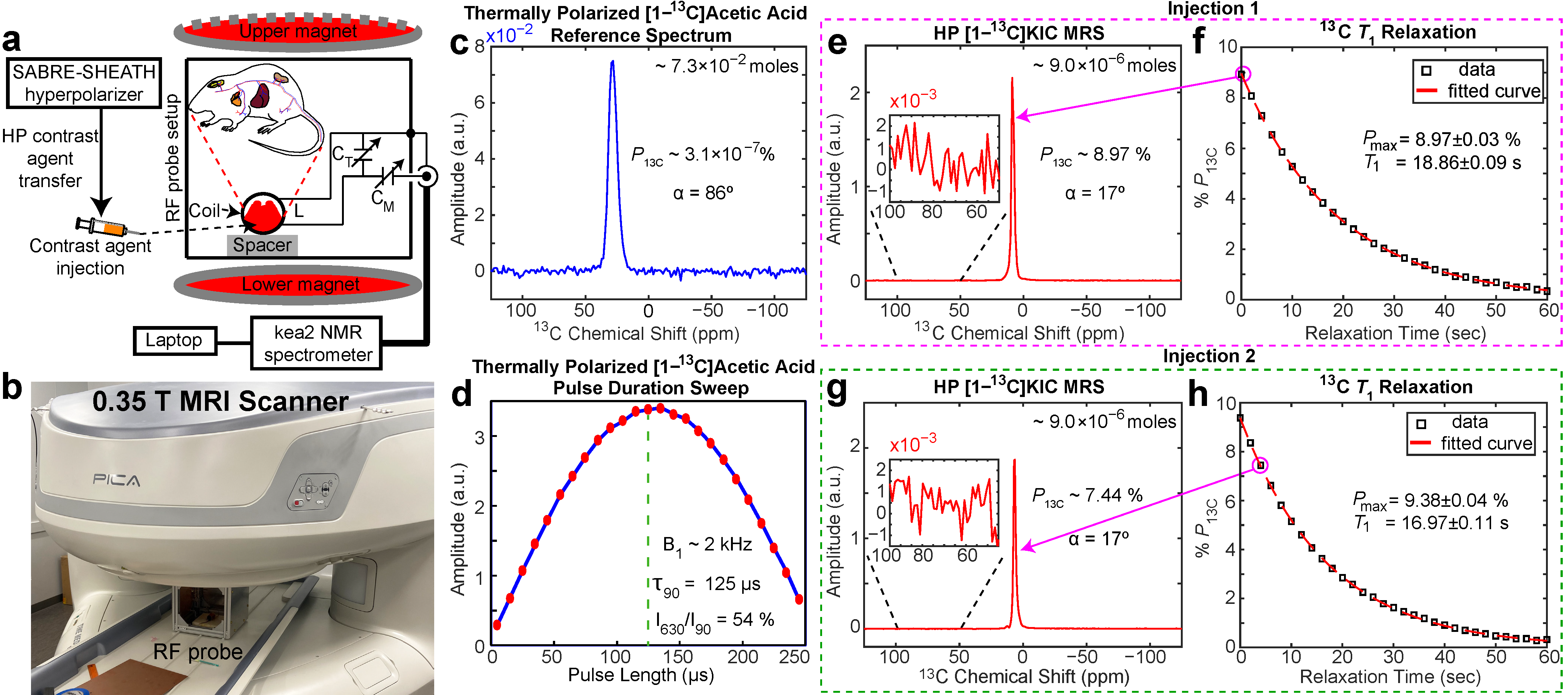
3.4. Pilot Post-Mortem 13C Dynamic Magnetic Resonance Spectroscopy Using HP [1-13C]ketoisocaproate Injection in a Euthanized Mouse
4. Biomedical Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J.; Meersmann, T.; Prisner, T.; et al. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dorrius, M.D.; Pijnappel, R.M.; Jansen-van der Weide, M.C.; Jansen, L.; Kappert, P.; Oudkerk, M.; Sijens, P.E. Determination of Choline Concentration in Breast Lesions: Quantitative Multivoxel Proton MR Spectroscopy as a Promising Noninvasive Assessment Tool to Exclude Benign Lesions. Radiology 2011, 259, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, P.; Goodson, B.M.; Chekmenev, E.Y. NMR Hyperpolarization Techniques for Biomedicine. Chem. Eur. J. 2015, 21, 3156–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtunov, K.V.; Pokochueva, E.V.; Salnikov, O.G.; Cousin, S.; Kurzbach, D.; Vuichoud, B.; Jannin, S.; Chekmenev, E.Y.; Goodson, B.M.; Barskiy, D.A.; et al. Hyperpolarized NMR: D-DNP, PHIP, and SABRE. Chem. Asian J. 2018, 13, 1857–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodson, B.M.; Kidd, B.; Hövener, J.-B.; Schröder, L.; Theis, T.; Whiting, N.; Chekmenev, E.Y. Nuclear Magnetic Resonance Spectroscopy | Hyperpolarization for Sensitivity Enhancement. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 168–181. [Google Scholar]
- Goodson, B.M. Nuclear magnetic resonance of laser-polarized noble gases in molecules, materials, and organisms. J. Magn. Reson. 2002, 155, 157–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugler, J.P.; Altes, T.A. Hyperpolarized 129Xe MRI of the human lung. J. Magn. Reson. Imaging 2013, 37, 313–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golman, K.; Axelsson, O.; Johannesson, H.; Mansson, S.; Olofsson, C.; Petersson, J.S. Parahydrogen-induced polarization in imaging: Subsecond C-13 angiography. Magn. Reson. Med. 2001, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Witte, C.; Schroder, L. NMR of hyperpolarised probes. NMR Biomed. 2013, 26, 788–802. [Google Scholar] [CrossRef]
- Goodson, B.M.; Whiting, N.; Coffey, A.M.; Nikolaou, P.; Shi, F.; Gust, B.M.; Gemeinhardt, M.E.; Shchepin, R.V.; Skinner, J.G.; Birchall, J.R.; et al. Hyperpolarization Methods for MRS. Emagres 2015, 4, 797–810. [Google Scholar]
- Hövener, J.-B.; Pravdivtsev, A.N.; Kidd, B.; Bowers, C.R.; Glöggler, S.; Kovtunov, K.V.; Plaumann, M.; Katz-Brull, R.; Buckenmaier, K.; Jerschow, A.; et al. Parahydrogen-based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed. 2018, 57, 11140–11162. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Ardenkjær-Larsen, J.H.; Petersson, J.S.; Månsson, S.; Leunbach, I. Molecular imaging with endogenous substances. Proc. Natl. Acad. Sci. USA 2003, 100, 10435–10439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, M.; Harrison, C.; Storey, C.; Jeffrey, F.; Sherry, A.; Malloy, C. Hyperpolarized C-13 allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc. Natl. Acad. Sci. USA 2007, 104, 19773–19777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [Green Version]
- Day, S.E.; Kettunen, M.I.; Gallagher, F.A.; Hu, D.E.; Lerche, M.; Wolber, J.; Golman, K.; Ardenkjaer-Larsen, J.H.; Brindle, K.M. Detecting tumor response to treatment using hyperpolarized C-13 magnetic resonance imaging and spectroscopy. Nat. Med. 2007, 13, 1382–1387. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Brindle, K.; Chekmenev, E.Y.; Comment, A.; Cunningham, C.H.; DeBerardinis, R.J.; Green, G.G.; Leach, M.O.; Rajan, S.S.; et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 2011, 13, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Golman, K.; in’t Zandt, R.; Thaning, M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. USA 2006, 103, 11270–11275. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.T.; Chen, H.-Y.; Gordon, J.; Mammoli, D.; Sriram, R.; Autry, A.W.; Le Page, L.M.; Chaumeil, M.M.; Shin, P.; Slater, J.; et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J. Magn. Reson. 2019, 309, 106617. [Google Scholar] [CrossRef]
- Brindle, K.M. Imaging Metabolism with Hyperpolarized 13C-Labeled Cell Substrates. J. Am. Chem. Soc. 2015, 137, 6418–6427. [Google Scholar] [CrossRef]
- Karlsson, M.; Jensen, P.R.; in’t Zandt, R.; Gisselsson, A.; Hansson, G.; Duus, J.Ø.; Meier, S.; Lerche, M.H. Imaging of branched chain amino acid metabolism in tumors with hyperpolarized 13C ketoisocaproate. Int. J. Cancer 2010, 127, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.A.; Søgaard, L.V.; Magnusson, P.O.; Lauritzen, M.H.; Laustsen, C.; Åkeson, P.; Ardenkjær-Larsen, J.H. Imaging cerebral 2-ketoisocaproate metabolism with hyperpolarized 13C magnetic resonance spectroscopic imaging. J. Cereb. Blood Flow Metab. 2012, 32, 1508–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comment, A.; Merritt, M.E. Hyperpolarized Magnetic Resonance as a Sensitive Detector of Metabolic Function. Biochemistry 2014, 53, 7333–7357. [Google Scholar] [CrossRef] [Green Version]
- Ardenkjaer-Larsen, J.H.; Leach, A.M.; Clarke, N.; Urbahn, J.; Anderson, D.; Skloss, T.W. Dynamic Nuclear Polarization Polarizer for Sterile Use Intent. NMR Biomed. 2011, 24, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H. On the present and future of dissolution-DNP. J. Magn. Reson. 2016, 264, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardenkjaer-Larsen, J.H.; Bowen, S.; Petersen, J.R.; Rybalko, O.; Vinding, M.S.; Ullisch, M.; Nielsen, N.C. Cryogen-free dissolution dynamic nuclear polarization polarizer operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med. 2019, 81, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tickner, B.J.; Ahwal, F.; Whitwood, A.C.; Duckett, S.B. Reversible Hyperpolarization of Ketoisocaproate Using Sulfoxide-containing Polarization Transfer Catalysts. ChemPhysChem 2021, 22, 13–17. [Google Scholar] [CrossRef]
- Theis, T.; Truong, M.L.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.L.; Theis, T.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. 15N Hyperpolarization By Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; Lopez-Serrano, J.; Williamson, D.C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef] [Green Version]
- Barskiy, D.A.; Shchepin, R.V.; Tanner, C.P.N.; Colell, J.F.P.; Goodson, B.M.; Theis, T.; Warren, W.S.; Chekmenev, E.Y. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohman, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iali, W.; Roy, S.S.; Tickner, B.J.; Ahwal, F.; Kennerley, A.J.; Duckett, S.B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chem. Int. Ed. 2019, 58, 10271–10275. [Google Scholar] [CrossRef] [Green Version]
- Tickner, B.J.; Semenova, O.; Iali, W.; Rayner, P.J.; Whitwood, A.C.; Duckett, S.B. Optimisation of pyruvate hyperpolarisation using SABRE by tuning the active magnetisation transfer catalyst. Catal. Sci. Technol. 2020, 10, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.B.; de Maissin, H.; Adelabu, I.; Nantogma, S.; Ettedgui, J.; TomHon, P.; Goodson, B.M.; Theis, T.; Chekmenev, E.Y. Catalyst-Free Aqueous Hyperpolarized [1-13C]Pyruvate Obtained by Re-Dissolution Signal Amplification by Reversible Exchange. ACS Sensors 2022, 7, 3430–3439. [Google Scholar] [CrossRef]
- Joalland, B.; Nantogma, S.; Chowdhury, M.R.H.; Nikolaou, P.; Chekmenev , E.Y. Magnetic Shielding of Parahydrogen Hyperpolarization Experiments for the Masses. Magn. Reson. Chem. 2021, 59, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Adelabu, I.; TomHon, P.; Kabir, M.S.H.; Nantogma, S.; Abdulmojeed, M.; Mandzhieva, I.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Theis, T.; et al. Order-Unity 13C Nuclear Polarization of [1-13C]Pyruvate in Seconds and the Interplay of Water and SABRE Enhancement. ChemPhysChem 2022, 23, 131–136. [Google Scholar] [CrossRef]
- Adelabu, I.; Ettedgui, J.; Joshi, S.M.; Nantogma, S.; Chowdhury, M.R.H.; McBride, S.; Theis, T.; Sabbasani, V.R.; Chandrasekhar, M.; Sail, D.; et al. Rapid 13C Hyperpolarization of the TCA Cycle Intermediate α-Ketoglutarate via SABRE-SHEATH. Anal. Chem. 2022, 94, 13422–13431. [Google Scholar] [CrossRef]
- Shchepin, R.V.; Jaigirdar, L.; Chekmenev, E.Y. Spin-Lattice Relaxation of Hyperpolarized Metronidazole in Signal Amplification by Reversible Exchange in Micro-Tesla Fields. J. Phys. Chem. C 2018, 122, 4984–4996. [Google Scholar] [CrossRef]
- Vazquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. The search for new hydrogenation catalyst motifs based on N-heterocyclic carbene ligands. Inorg. Chim. Acta 2006, 359, 2786–2797. [Google Scholar] [CrossRef]
- Rayner, P.J.; Duckett, S.B. Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew. Chem. Int. Ed. 2018, 57, 6742–6753. [Google Scholar] [CrossRef] [PubMed]
- Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; He, P.; Groome, K.A.; Best, Q.A.; Shi, F.; Goodson, B.M.; Shchepin, R.V.; Coffey, A.M.; et al. The feasibility of formation and kinetics of NMR Signal Amplification by Reversible Exchange (SABRE) at high magnetic field (9.4 T). J. Am. Chem. Soc. 2014, 136, 3322–3325. [Google Scholar] [CrossRef]
- Nantogma, S.; Joalland, B.; Wilkens, K.; Chekmenev, E.Y. Clinical-Scale Production of Nearly Pure (>98.5%) Parahydrogen and Quantification by Benchtop NMR Spectroscopy. Anal. Chem. 2021, 93, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Coffey, A.M.; Colon, R.D.; Chekmenev, E.Y.; Waddell, K.W. A pulsed injection parahydrogen generator and techniques for quantifying enrichment. J. Magn. Reson. 2012, 214, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, M.L.; Shi, F.; He, P.; Yuan, B.; Plunkett, K.N.; Coffey, A.M.; Shchepin, R.V.; Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; et al. Irreversible Catalyst Activation Enables Hyperpolarization and Water Solubility for NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. B 2014, 18, 13882–13889. [Google Scholar] [CrossRef] [Green Version]
- Shchepin, R.V.; Birchall, J.R.; Chukanov, N.V.; Kovtunov, K.V.; Koptyug, I.V.; Theis, T.; Warren, W.S.; Gelovani, J.G.; Goodson, B.M.; Shokouhi, S.; et al. Hyperpolarizing Concentrated Metronidazole 15NO2 Group Over Six Chemical Bonds with More Than 15% Polarization and 20 Minute Lifetime. Chem. Eur. J. 2019, 25, 8829–8836. [Google Scholar] [CrossRef]
- Shchepin, R.V.; Jaigirdar, L.; Theis, T.; Warren, W.S.; Goodson, B.M.; Chekmenev, E.Y. Spin Relays Enable Efficient Long-Range Heteronuclear Signal Amplification by Reversible Exchange. J. Phys. Chem. C 2017, 121, 28425–28434. [Google Scholar] [CrossRef]
- Salnikov, O.G.; Chukanov, N.V.; Svyatova, A.; Trofimov, I.A.; Kabir, M.S.H.; Gelovani, J.G.; Kovtunov, K.V.; Koptyug, I.V.; Chekmenev, E.Y. 15N NMR Hyperpolarization of Radiosensitizing Antibiotic Nimorazole via Reversible Parahydrogen Exchange in Microtesla Magnetic Fields. Angew. Chem. Int. Ed. 2021, 60, 2406–2413. [Google Scholar] [CrossRef]
- Debarba, L.K.; Mulka, A.; Lima, J.B.M.; Didyuk, O.; Fakhoury, P.; Koshko, L.; Awada, A.A.; Zhang, K.; Klueh, U.; Sadagurski, M. Acarbose protects from central and peripheral metabolic imbalance induced by benzene exposure. Brain Behav. Immun. 2020, 89, 87–99. [Google Scholar] [CrossRef]
- Joalland, B.; Chekmenev, E.Y. Scanning Nuclear Spin Level Anticrossings by Constant-Adiabaticity Magnetic Field Sweeping of Parahydrogen-Induced 13C Polarization. J. Phys. Chem. Lett. 2022, 13, 1925–1930. [Google Scholar] [CrossRef]
- Coffey, A.M.; Truong, M.L.; Chekmenev, E.Y. Low-field MRI can be more sensitive than high-field MRI. J. Magn. Reson. 2013, 237, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Joalland, B.; Meersman, C.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Nikolaou, P.; Kovtunov, K.V.; Salnikov, O.G.; Koptyug, I.V.; et al. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem. 2021, 93, 8476–8483. [Google Scholar] [CrossRef] [PubMed]
- TomHon, P.; Abdulmojeed, M.; Adelabu, I.; Nantogma, S.; Kabir, M.S.H.; Lehmkuhl, S.; Chekmenev, E.Y.; Theis, T. Temperature Cycling Enables Efficient 13C SABRE-SHEATH Hyperpolarization and Imaging of [1-13C]-Pyruvate. J. Am. Chem. Soc. 2022, 144, 282–287. [Google Scholar] [CrossRef]
- Gemeinhardt, M.E.; Limbach, M.N.; Gebhardt, T.R.; Eriksson, C.W.; Eriksson, S.L.; Lindale, J.R.; Goodson, E.A.; Warren, W.S.; Chekmenev, E.Y.; Goodson, B.M. “Direct” 13C Hyperpolarization of 13C-Acetate by MicroTesla NMR Signal Amplification by Reversible Exchange (SABRE). Angew. Chem. Int. Ed. 2020, 59, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Coffey, A.M.; Feldman, M.A.; Shchepin, R.V.; Barskiy, D.A.; Truong, M.L.; Pham, W.; Chekmenev, E.Y. High-resolution hyperpolarized in vivo metabolic 13C spectroscopy at low magnetic field (48.7 mT) following murine tail-vein injection. J. Magn. Reson. 2017, 281, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef]
- Mewis, R.E.; Fekete, M.; Green, G.G.R.; Whitwood, A.C.; Duckett, S.B. Deactivation of signal amplification by reversible exchange catalysis, progress towards in vivo application. Chem. Comm. 2015, 51, 9857–9859. [Google Scholar] [CrossRef]
- Kidd, B.E.; Gesiorski, J.L.; Gemeinhardt, M.E.; Shchepin, R.V.; Kovtunov, K.V.; Koptyug, I.V.; Chekmenev, E.Y.; Goodson, B.M. Facile Removal of Homogeneous SABRE Catalysts for Purifying Hyperpolarized Metronidazole, a Potential Hypoxia Sensor. J. Phys. Chem. C 2018, 122, 16848–16852. [Google Scholar] [CrossRef] [PubMed]
- Barskiy, D.A.; Ke, L.A.; Li, X.; Stevenson, V.; Widarman, N.; Zhang, H.; Truxal, A.; Pines, A. Rapid Catalyst Capture Enables Metal-Free para-Hydrogen-Based Hyperpolarized Contrast Agents. J. Phys. Chem. Lett. 2018, 9, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Rayner, P.; Iali, W.; Burns, M.; Perry, V.; Duckett, S. Achieving Biocompatible SABRE: An invitro Cytotoxicity Study. ChemMedChem 2018, 13, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Coffey, A.M.; Waddell, K.W.; Chekmenev, E.Y.; Goodson, B.M. Heterogeneous Solution NMR Signal Amplification by Reversible Exchange. Angew. Chem. Int. Ed. 2014, 53, 7495–7498. [Google Scholar] [CrossRef] [PubMed]
- Kovtunov, K.V.; Kovtunova, L.M.; Gemeinhardt, M.E.; Bukhtiyarov, A.V.; Gesiorski, J.; Bukhtiyarov, V.I.; Chekmenev, E.Y.; Koptyug, I.V.; Goodson, B.M. Heterogeneous Microtesla SABRE Enhancement of 15N NMR Signals. Angew. Chem. Int. Ed. 2017, 56, 10433–10437. [Google Scholar] [CrossRef] [PubMed]
- Spannring, P.; Reile, I.; Emondts, M.; Schleker, P.P.M.; Hermkens, N.K.J.; van der Zwaluw, N.G.J.; van Weerdenburg, B.J.A.; Tinnemans, P.; Tessari, M.; Blümich, B.; et al. A New Ir-NHC Catalyst for Signal Amplification by Reversible Exchange in D2O. Chem. Eur. J. 2016, 22, 9277–9282. [Google Scholar] [CrossRef] [Green Version]
- Colell, J.F.P.; Emondts, M.; Logan, A.W.J.; Shen, K.; Bae, J.; Shchepin, R.V.; Ortiz, G.X.; Spannring, P.; Wang, Q.; Malcolmson, S.J.; et al. Direct Hyperpolarization of Nitrogen-15 in Aqueous Media with Parahydrogen in Reversible Exchange. J. Am. Chem. Soc. 2017, 139, 7761–7767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; He, P.; Best, Q.A.; Groome, K.; Truong, M.L.; Coffey, A.M.; Zimay, G.; Shchepin, R.V.; Waddell, K.W.; Chekmenev, E.Y.; et al. Aqueous NMR Signal Enhancement by Reversible Exchange in a Single Step Using Water-Soluble Catalysts. J. Phys. Chem. C 2016, 120, 12149–12156. [Google Scholar] [CrossRef] [PubMed]
- Iali, W.; Olaru, A.; Green, G.; Duckett, S. Achieving High Levels of NMR-Hyperpolarization in Aqueous Media with Minimal Catalyst Contamination Using SABRE. Chem. Eur. J. 2017, 23, 10491–10495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekete, M.; Gibard, C.; Dear, G.J.; Green, G.G.R.; Hooper, A.J.J.; Roberts, A.D.; Cisnetti, F.; Duckett, S.B. Utilisation of water soluble iridium catalysts for Signal Amplification by Reversible Exchange. Dalton Trans. 2015, 44, 7870–7880. [Google Scholar] [CrossRef]
- Hövener, J.-B.; Schwaderlapp, N.; Borowiak, R.; Lickert, T.; Duckett, S.B.; Mewis, R.E.; Adams, R.W.; Burns, M.J.; Highton, L.A.R.; Green, G.G.R.; et al. Toward Biocompatible Nuclear Hyperpolarization Using Signal Amplification by Reversible Exchange: Quantitative in Situ Spectroscopy and High-Field Imaging. Anal. Chem. 2014, 86, 1767–1774. [Google Scholar] [CrossRef]
- Billingsley, K.L.; Park, J.M.; Josan, S.; Hurd, R.; Mayer, D.; Spielman-Sun, E.; Nishimura, D.G.; Brooks, J.D.; Spielman, D. The feasibility of assessing branched-chain amino acid metabolism in cellular models of prostate cancer with hyperpolarized [1-13C]-ketoisocaproate. Magn. Reson. Imaging 2014, 32, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Suh, E.H.; Hackett, E.P.; Wynn, R.M.; Chuang, D.T.; Zhang, B.; Luo, W.; Sherry, A.D.; Park, J.M. In vivo assessment of increased oxidation of branched-chain amino acids in glioblastoma. Sci. Rep. 2019, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llombart, V.; Mansour, M.R. Therapeutic targeting of “undruggable” MYC. eBioMedicine 2022, 75, 103756. [Google Scholar] [CrossRef] [PubMed]
- Nantogma, S.; Eriksson, S.L.; Adelabu, I.; Mandzhieva, I.; Browning, A.; TomHon, P.; Warren, W.S.; Theis, T.; Goodson, B.M.; Chekmenev, E.Y. Interplay of Near-Zero-Field Dephasing, Rephasing, and Relaxation Dynamics and [1-13C]Pyruvate Polarization Transfer Efficiency in Pulsed SABRE-SHEATH. J. Phys. Chem. A 2022, 126, 9114–9123. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adelabu, I.; Chowdhury, M.R.H.; Nantogma, S.; Oladun, C.; Ahmed, F.; Stilgenbauer, L.; Sadagurski, M.; Theis, T.; Goodson, B.M.; Chekmenev, E.Y. Efficient SABRE-SHEATH Hyperpolarization of Potent Branched-Chain-Amino-Acid Metabolic Probe [1-13C]ketoisocaproate. Metabolites 2023, 13, 200. https://doi.org/10.3390/metabo13020200
Adelabu I, Chowdhury MRH, Nantogma S, Oladun C, Ahmed F, Stilgenbauer L, Sadagurski M, Theis T, Goodson BM, Chekmenev EY. Efficient SABRE-SHEATH Hyperpolarization of Potent Branched-Chain-Amino-Acid Metabolic Probe [1-13C]ketoisocaproate. Metabolites. 2023; 13(2):200. https://doi.org/10.3390/metabo13020200
Chicago/Turabian StyleAdelabu, Isaiah, Md Raduanul H. Chowdhury, Shiraz Nantogma, Clementinah Oladun, Firoz Ahmed, Lukas Stilgenbauer, Marianna Sadagurski, Thomas Theis, Boyd M. Goodson, and Eduard Y. Chekmenev. 2023. "Efficient SABRE-SHEATH Hyperpolarization of Potent Branched-Chain-Amino-Acid Metabolic Probe [1-13C]ketoisocaproate" Metabolites 13, no. 2: 200. https://doi.org/10.3390/metabo13020200




