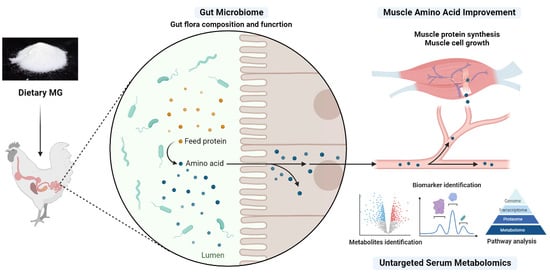
Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Management and Diets
2.2. Sample Collection
2.3. Muscle Amino Acid Determination
2.4. Serum Untargeted Metabolomics
2.4.1. Extraction of Serum Metabolites
2.4.2. Mass Spectrometry Analysis
2.5. Quantitative PCR Analysis
2.6. Gut Microbiome
2.7. Statistical Analysis
3. Results
3.1. Chicken Productive Performance
3.2. Dietary MG Alters Muscle Amino Acids Content
3.3. Chicken Serum Metabolome
3.4. Dietary MG Affects the mRNA Expression of Muscle Growth Regulation
3.5. Dietary MG Affects the Chicken Gut Microbiota
3.5.1. Changes in Chicken Microbial Diversity and Structure
3.5.2. Changes in Chicken Gut Microbiota Community and Function
3.5.3. Correlation of Chicken Gut Microbiota Composition with Amino Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zampiga, M.; Flees, J.; Meluzzi, A.; Dridi, S.; Sirri, F. Application of omics technologies for a deeper insight into quali-quantitative production traits in broiler chickens: A review. J. Anim. Sci. Biotechnol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, H.N.; Alizadeh-Ghamsari, A.H. Improved performance and small intestinal development of broiler chickens by dietary L-glutamine supplementation. J. Appl. Anim. Res. 2013, 41, 1–7. [Google Scholar] [CrossRef]
- Bartell, S.M.; Batal, A.B. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers. Poult. Sci. 2007, 86, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, L.; Wen, A.; Wang, L.; Jin, G. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340. [Google Scholar] [CrossRef]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Kimball, S.R.; Jefferson, L.S.; Davis, T.A. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E914–E921. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Guo, Q.; Wen, C.; Wang, W.; Li, Y.; Tan, B.; Li, F.; Yin, Y. Free amino acid profile and expression of genes implicated in protein metabolism in skeletal muscle of growing pigs fed low-protein diets supplemented with branched-chain amino acids. J. Agric. Food Chem. 2016, 64, 9390–9400. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. Meatabolomics: Muscle and meat metabolomics in domestic animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Q.; Wei, J.; Zhao, M.; Tang, J.; Feng, F. Mass spectrometry-based metabolomics to reveal chicken meat improvements by medium-chain monoglycerides supplementation: Taste, fresh meat quality, and composition. Food Chem. 2021, 365, 130303. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Tsvetkova, I.V.; Najdenski, H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar]
- Fortuoso, B.F.; dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, L.; Zhang, L.; Wang, T. Effect of medium-chain triglycerides on growth performance, nutrient digestibility, plasma metabolites and antioxidant capacity in weanling pigs. Anim. Nutr. 2015, 1, 12–18. [Google Scholar] [CrossRef]
- Lan, J.; Chen, G.; Cao, G.; Tang, J.; Li, Q.; Zhang, B.; Yang, C. Effects of α-glyceryl monolaurate on growth, immune function, volatile fatty acids, and gut microbiota in broiler chickens. Poult. Sci. 2021, 100, 100875. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol monolaurate ameliorated intestinal barrier and immunity in broilers by regulating intestinal inflammation, antioxidant balance, and intestinal microbiota. Front. Immunol. 2021, 12, 713485. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef]
- Liu, T.; Guo, L.; Zhangying, Y.; Ruan, S.; Liu, W.; Zhang, X.; Feng, F. Dietary medium-chain 1-monoglycerides modulates the community and function of cecal microbiota of broilers. J. Sci. Food Agric. 2021, 102, 2242–2252. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Zhong, H.; Feng, F. Dietary medium chain α-monoglycerides increases body weight, feed intake, and carcass yield in broilers with muscle composition alteration. Poult. Sci. 2020, 100, 186–195. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-monolaurate-mediated attenuation of metabolic syndrome is associated with the modulation of gut microbiota in high-fat-diet-fed mice. Mol. Nutr. Food Res. 2019, 63, 1801417. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. mBio 2020, 11, e00190-20. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yin, F.; Yang, Y.; Lepp, D.; Yu, H.; Ruan, Z.; Yang, C.; Yin, Y.; Hou, Y.; Leeson, S. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 2018, 8, 4940. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, H.; Zhang, R.; Cao, G.; Li, Q.; Zhang, B.; Wang, Y.; Yang, C. Serum metabolome and gut microbiome alterations in broiler chickens supplemented with lauric acid. Poult. Sci. 2021, 100, 101315. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhao, Q.; Shao, M.; Duan, Y.; Li, D.; Lu, Y.; Tang, Y.; Feng, C. Untargeted metabolomics reveals the effect of selective breeding on the quality of chicken meat. Metabolites 2022, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Wang, H.; Nan, X.; Wang, Y.; Guo, Y.; Xiong, B. Alterations in the milk metabolome of dairy cows supplemented with different levels of calcium propionate in early lactation. Metabolites 2022, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, P.; Yu, S.; He, J.; Afedo, S.; Zou, S.; Zhang, Q.; Liu, J.; Song, L.; Xu, Y.; et al. Expression analysis of molecular chaperones Hsp70 and Hsp90 on development and metabolism of different organs and testis in cattle (Cattle & Yak). Metabolites 2022, 12, 1114. [Google Scholar]
- Cai, L.; Li, M.; Zhou, S.; Zhu, X.; Zhang, X.; Xu, Q. Trans-species fecal transplant revealed the role of the gut microbiome as a contributor to energy metabolism and development of skeletal muscle. Metabolites 2022, 12, 769. [Google Scholar] [CrossRef]
- Soriano-Santos, J. Chemical composition and nutritional content of raw poultry meat. Handb. Poult. Sci. Technol. 2010, 1, 467–491. [Google Scholar]
- Liu, T.; Tang, J.; Feng, F. Glycerol monolaurate improves performance, intestinal development, and muscle amino acids in yellow-feathered broilers via manipulating gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 10279–10291. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Galli, G.M.; Griss, L.G.; Armanini, E.H.; Silva, A.D.; Fracasso, M.; Mostardeiro, V.; Morsch, V.M.; Lopes, L.Q.; Santos, R.C.; et al. Effects of glycerol monolaurate on growth and physiology of chicks consuming diet containing fumonisin. Microb. Pathog. 2020, 147, 104261. [Google Scholar] [CrossRef]
- Saleh, A.A.; El-Gharabawy, B.; Hassan, A.; Badawi, N.; Eid, Y.; Selim, S.; Shukry, M.; Dawood, M. Effect of dietary inclusion of alpha-monolaurin on the growth performance, lipid peroxidation, and immunity response in broilers. Sustainability 2021, 13, 5231. [Google Scholar] [CrossRef]
- Chen, R.; Zhuang, S.; Chen, Y.; Cheng, Y.; Wen, C.; Zhou, Y. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018, 97, 4297–4305. [Google Scholar] [CrossRef]
- Alirezaei, M.; Gheisari, H.; Ranjbar, V.; Hajibemani, A. Betaine: A promising antioxidant agent for enhancement of broiler meat quality. Br. Poult. Sci. 2012, 53, 699–707. [Google Scholar] [CrossRef]
- Chen, R.; Wen, C.; Gu, Y.; Wang, C.; Chen, Y.; Zhuang, S.; Zhou, Y. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food Agric. 2020, 100, 2656–2663. [Google Scholar] [CrossRef]
- Liu, C.; Pan, D.; Ye, Y.; Cao, J. 1H NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chem. 2013, 141, 1281–1286. [Google Scholar] [CrossRef]
- Uenoyama, R.; Miyazaki, M.; Miyazaki, T.; Shigeno, Y.; Tokairin, Y.; Konno, H.; Yamashita, T. LC-ESI-MS/MS quantification of carnosine, anserine, and balenine in meat samples. J. Chromatogr. B 2019, 1132, 121826. [Google Scholar] [CrossRef]
- Klamt, S.; Stelling, J. Two approaches for metabolic pathway analysis? Trends Biotechnol. 2003, 21, 64–69. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The mTOR pathway in the control of protein synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Tesseraud, S.; Everaert, N.; Ezzine, S.B.-O.; Collin, A.; Métayer-Coustard, S.; Berri, C. Manipulating tissue metabolism by amino acids. World’s Poult. Sci. J. 2011, 67, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.A.; Mayhew, D.L.; Hornberger, T.A. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell. Signal. 2011, 23, 1896–1906. [Google Scholar] [CrossRef] [Green Version]
- Tesseraud, S.; Bigot, K.; Taouis, M. Amino acid availability regulates S6K1 and protein synthesis in avian insulin-insensitive QM7 myoblasts. FEBS Lett. 2003, 540, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem. Biophys. Res. Commun. 2004, 313, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesseraud, S.; Bouvarel, I.; Collin, A.; Audouin, E.; Crochet, S.; Seiliez, I.; Leterrier, C. Daily variations in dietary lysine content alter the expression of genes related to proteolysis in chicken pectoralis major muscle. J. Nutr. 2009, 139, 38–43. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Zhang, C.L.; Olson, E.N. MEF2: A calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 2002, 27, 40–47. [Google Scholar] [CrossRef]
- Black, B.L.; Olson, E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998, 14, 167–196. [Google Scholar] [CrossRef]
- Wen, C.; Jiang, X.; Ding, L.; Wang, T.; Zhou, Y. Effects of dietary methionine on breast muscle growth, myogenic gene expression and IGF-I signaling in fast-and slow-growing broilers. Sci. Rep. 2017, 7, 1924. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, Q.; Liu, J.; Liu, Y.; Xu, Y.; Zhang, R.; Yu, Y.; Wang, Y.; Yang, C. Integrating serum metabolome and gut microbiome to evaluate the benefits of lauric acid on lipopolysaccharide- challenged broilers. Front. Immunol. 2021, 12, 759323. [Google Scholar] [CrossRef]
- Sakamoto, M.; Lapidus, A.L.; Han, J.; Trong, S.; Haynes, M.; Reddy, T.; Mikhailova, N.; Huntemann, M.; Pati, A.; Ivanova, N.N. High quality draft genome sequence of Bacteroides barnesiae type strain BL2T (DSM 18169T) from chicken caecum. Stand. Genom. Sci. 2015, 10, 48. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed. Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]





| CON | MG | p Value | |
|---|---|---|---|
| Average daily gain (ADG), g | 35.36 ± 0.31 | 36.41 ± 0.30 *** | <0.001 |
| Average feed intake (FI), g | 6028.08 ± 8.43 | 6138.19 ± 8.75 ** | 0.006 |
| Body weight (BW), g | 2515.20 ± 20.30 | 2589.46 ± 21.59 *** | <0.001 |
| Feed conversion rate (FCR) | 2.399 ± 0.021 | 2.373 ± 0.020 | 0.084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Ruan, S.; Mo, Q.; Zhao, M.; Wang, J.; Ye, Z.; Chen, L.; Feng, F. Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides. Metabolites 2023, 13, 208. https://doi.org/10.3390/metabo13020208
Liu T, Ruan S, Mo Q, Zhao M, Wang J, Ye Z, Chen L, Feng F. Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides. Metabolites. 2023; 13(2):208. https://doi.org/10.3390/metabo13020208
Chicago/Turabian StyleLiu, Tao, Shengyue Ruan, Qiufen Mo, Minjie Zhao, Jing Wang, Zhangying Ye, Li Chen, and Fengqin Feng. 2023. "Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides" Metabolites 13, no. 2: 208. https://doi.org/10.3390/metabo13020208
APA StyleLiu, T., Ruan, S., Mo, Q., Zhao, M., Wang, J., Ye, Z., Chen, L., & Feng, F. (2023). Integrated Serum Metabolome and Gut Microbiome to Decipher Chicken Amino Acid Improvements Induced by Medium-Chain Monoglycerides. Metabolites, 13(2), 208. https://doi.org/10.3390/metabo13020208