Metabolic Contributions to Pathobiology of Asthma
Abstract
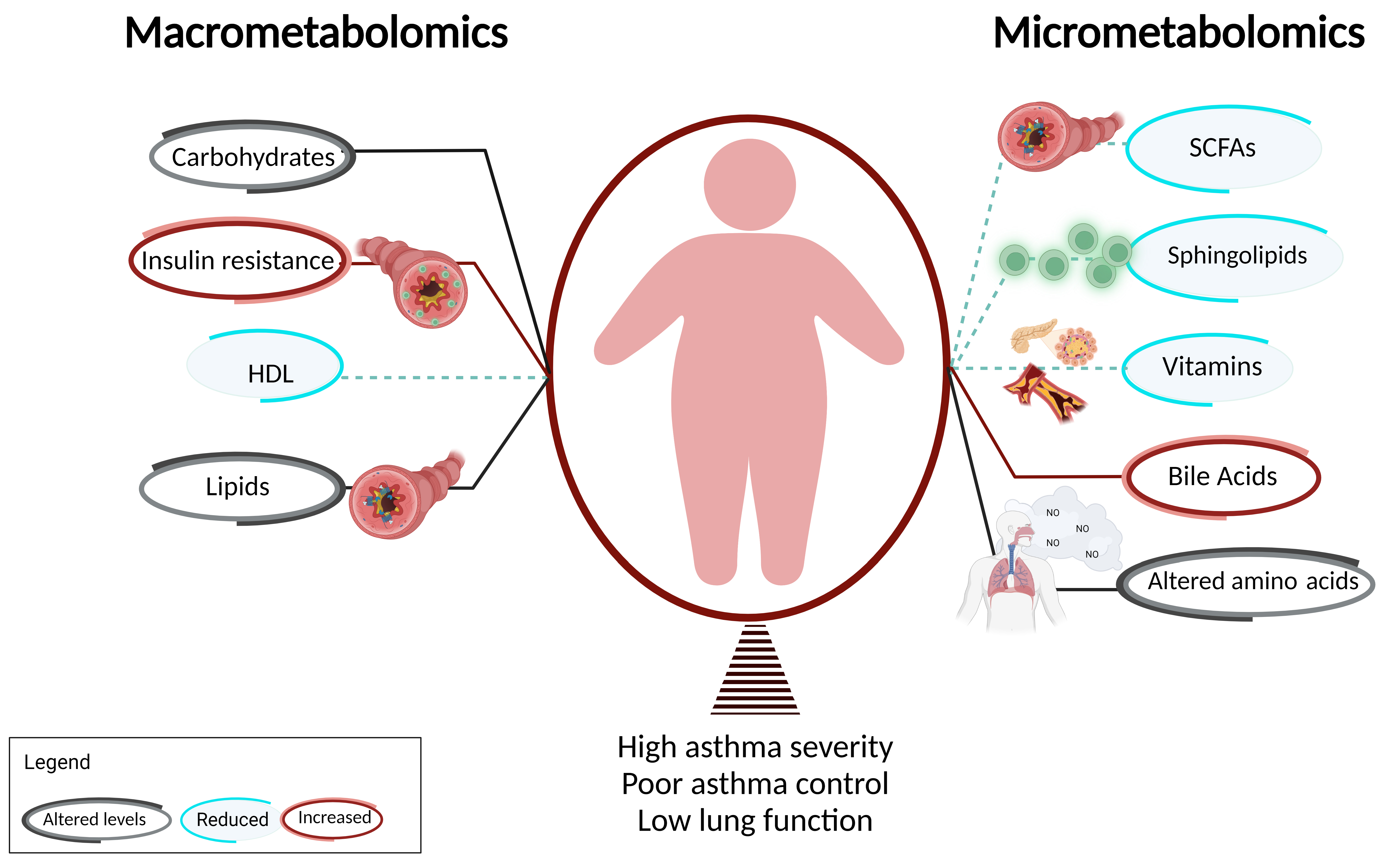
1. Introduction
2. Macrometabolic Associations in Asthma
2.1. Altered Carbohydrate Metabolism Is Associated with Disease Burden in Obesity-Related Asthma
2.2. Insulin Resistance Influences Asthma Phenotype Partly via Effects on Airway Smooth Muscle
2.3. Dyslipidemia and Dysregulation of Fatty Acids Are Associated with Obesity-Related Asthma
2.4. Dyslipidemia Influences Asthma Phenotype Partly via Its Effects on FFA Receptors
2.5. Therapies for Dyslipidemia/FFAs Are Effective in Decreasing Disease Burden of Obesity-Related Asthma
3. Micrometabolic Associations in Asthma
3.1. Sphingolipids
3.2. Short Chain Fatty Acids (SCFAs)
3.3. Amino Acids
3.4. Vitamins
3.5. Bile Acids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cleave, J.V.; Gortmaker, S.L.; Perrin, J.M. Dynamics of obesity and chronic health conditions among children and youth. JAMA 2010, 303, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Al-lergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Oriss, T.B.; Wenzel, S.E. Emerging molecular phenotypes of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L130–L140. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; King, T.S.; Lehman, E.; Stevens, A.D.; Jackson, L.P.; Stream, A.R.; Fahy, J.V. Cluster Analysis of Obesity and Asthma Phenotypes. PLoS ONE 2012, 7, e36631. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.H.; Demissie, K.; Rhoads, G.G. Asthma Development with Obesity Exposure: Observations from the Cohort of the National Health and Nutrition Evaluation Survey Epidemiologic Follow-up Study (NHEFS). J. Asthma 2005, 42, 97–99. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Jiang, Y. The Association Between Obesity and Asthma Is Stronger in Nonallergic Than Allergic Adults. Chest 2006, 130, 890–895. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, S.; Zhang, S.; Ouyang, Z.; Wang, G.; Wang, F. Research Progress of Metabolomics in Asthma. Metabolites 2021, 11, 567. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Forno, E.; Han, Y.Y.; Muzumdar, R.H.; Celedón, J.C. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J. Allergy Clin. Immunol. 2015, 136, 304–311.e308. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.; Choi, S.-H.; Shin, S.-H.; Kim, S.K.; Shim, Y.S.; Jeon, Y.H. Metabolic syndrome and lung function in Korean children and adolescents: A cross-sectional study. Sci. Rep. 2019, 9, 15646. [Google Scholar] [CrossRef] [PubMed]
- McCravy, M.; Ingram, J.L.; Que, L.G. Dysregulated Metabolism in the Pathophysiology of Non-Allergic Obese Asthma. J. Asthma Allergy 2021, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Macian, F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban ado-lescents with asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef]
- Cardet, J.C.; Ash, S.; Kusa, T.; Camargo, C.A.; Jr Israel, E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur. Respir. J. 2016, 48, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Forno, E. Asthma and diabetes: Does treatment with metformin improve asthma? Respirology 2016, 21, 1144–1145. [Google Scholar] [CrossRef] [PubMed]
- Nathan, B.M.; Moran, A. Metabolic complications of obesity in childhood and adolescence: More than just diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 21–29. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diab. Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawwa, B.A.; Al-Huniti, N.H.; DeMattia, L.; Gershan, W. Asthma and insulin resistance in morbidly obese children and adolescents. J. Asthma 2007, 44, 469–473. [Google Scholar] [CrossRef]
- Cottrell, L.; Neal, W.A.; Ice, C.; Perez, M.K.; Piedimonte, G. Metabolic abnormalities in children with asthma. Am. J. Respir. Crit. Care Med. 2011, 183, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Arshi, M.; Cardinal, J.; Hill, R.J.; Davies, P.S.; Wainwright, C. Asthma and insulin resistance in children. Respirology 2010, 15, 779–784. [Google Scholar] [CrossRef]
- Forno, E. A Potential New Treatment Option for Asthma in the Setting of Obesity or Insulin Resistance? Am. J. Respir. Crit. Care Med. 2021, 203, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H.S.; Min, H.K.; Lee, S.W. Association between insulin resistance and lung function trajectory over 4 years in South Korea: Community-based prospective cohort. BMC Pulm. Med. 2021, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.T.; Arky, R.A. Inhaled insulin for diabetes mellitus. N. Engl. J. Med. 2007, 356, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Bhalani, K.; Hall, C.B.; Isasi, C.R. Association of pulmonary function with adiposity and metabolic abnormalities in urban minority adolescents. Ann. Amer. Thor. Soc. 2014, 11, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, N.; Karampatakis, T.; Galli-Tsinopoulou, A.; Kotanidou, E.P.; Tsergouli, K.; Eboriadou-Petikopoulou, M.; Haidopoulou, K. Impaired glucose metabolism and bronchial hyperrespon-siveness in obese prepubertal asthmatic children. Pediatr. Pulmonol. 2017, 52, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, D.; Gosens, R.; Ris, J.M.; Zaagsma, J.; Meurs, H.; Nelemans, S.A. Insulin induces airway smooth muscle contraction. Br. J. Pharmacol. 2007, 150, 136–142. [Google Scholar] [CrossRef]
- Orfanos, S.; Jude, J.; Deeney, B.T.; Cao, G.; Rastogi, D.; Van Zee, M.; Pushkarsky, I.; Munoz, H.E.; Damoiseaux, R.; Di Carlo, D.; et al. Obesity increases airway smooth muscle responses to contractile agonists. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L673–L681. [Google Scholar] [CrossRef]
- Wu, T.D.; Keet, C.A.; Fawzy, A.; Segal, J.B.; Brigham, E.P.; McCormack, M.C. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-based Cohort Study. Ann. Am. Thorac. Soc. 2019, 16, 1527–1533. [Google Scholar] [CrossRef]
- Foer, D.; Beeler, P.E.; Cui, J.; Karlson, E.W.; Bates, D.W.; Cahill, K.N. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am. J. Respir. Crit. Care Med. 2021, 203, 831–840. [Google Scholar] [CrossRef]
- Rastogi, D. Evidence Builds for a Role of Metformin in Asthma Management. Ann. Am. Thorac. Soc. 2019, 16, 1497–1499. [Google Scholar] [CrossRef]
- Proskocil, B.J.; Calco, G.N.; Nie, Z. Insulin acutely increases agonist-induced airway smooth muscle contraction in humans and rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L545–L556. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Jacoby, D.B.; Fryer, A.D. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 251–261. [Google Scholar] [CrossRef]
- Schaafsma, D.; McNeill, K.D.; Stelmack, G.L.; Gosens, R.; Baarsma, H.; Dekkers, B.G.J.; Frohwerk, E.; Penninks, J.-M.; Sharma, P.; Ens, K.M.; et al. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am. J. Physiol. Cell Physiol. 2007, 293, C429–C439. [Google Scholar] [CrossRef] [PubMed]
- Gosens, R.; Nelemans, S.A.; Hiemstra, M.; Grootte Bromhaar, M.M.; Meurs, H.; Zaagsma, J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur. J. Pharmacol. 2003, 481, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Gopireddy, R.R.; Wu, Y.; Wu, L.; Tao, X.; Shao, J.; Wang, W.; Li, L.; Jovanovic, A.; Xu, B.; et al. Hyperinsulinemia promotes heterologous desensitization of beta2 adrenergic receptor in airway smooth muscle in obesity. FASEB J. 2020, 34, 3996–4008. [Google Scholar] [CrossRef]
- Singh, S.; Bodas, M.; Bhatraju, N.K.; Pattnaik, B.; Gheware, A.; Parameswaran, P.K.; Thompson, M.; Freeman, M.; Mabalirajan, U.; Gosens, R.; et al. Hyperinsulinemia adversely affects lung structure and function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L837–L845. [Google Scholar] [CrossRef]
- Dixon, A.E.; Subramanian, M.; DeSarno, M.; Black, K.; Lane, L.; Holguin, F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir. Res. 2015, 16, 143. [Google Scholar] [CrossRef]
- Andre, D.M.; Calixto, M.C.; Sollon, C.; Alexandre, E.C.; Tavares, E.B.G.; Naime, A.C.A.; Anhê, G.F.; Antunes, E. High-fat diet-induced obesity impairs insulin signaling in lungs of aller-gen-challenged mice: Improvement by resveratrol. Sci. Rep. 2017, 7, 17296. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Linderholm, A.; Haczku, A.; Kenyon, N. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharmacol. Ther. 2017, 180, 139–143. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
- Park, C.S.; Bang, B.-R.; Kwon, H.-S.; Moon, K.-A.; Kim, T.-B.; Lee, K.-Y.; Moon, H.-B.; Cho, Y.S. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012, 84, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Jin, Q.; Guo, H.; Han, X.; Xu, L.; Lu, S.; Wu, C. Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPKalpha Activity. Front. Pharmacol. 2022, 13, 780148. [Google Scholar] [CrossRef] [PubMed]
- Esquivel Zuniga, R.; DeBoer, M.D. Prediabetes in Adolescents: Prevalence, Management and Diabetes Prevention Strategies. Diabetes Metab. Syndr. Obes. 2021, 14, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Bensignor, M.O.; Wolf, J.M.; Rudser, K.D.; Kelly, A.S.; Arslanian, S. Glucagon-like peptide-1 receptor agonist prescribing patterns in adolescents with type 2 diabetes. Diabetes Obes. Metab. 2022, 24, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matoba, A.; Shibata, S.; Masaki, E.; Emala, C.W., Sr. Obesity-induced asthma: Role of free fatty acid receptors. Jpn. Dent. Sci. Rev. 2019, 55, 103–107. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Mizuta, K.; Zhang, Y.; Mizuta, F.; Hoshijima, H.; Shiga, T.; Masaki, E.; Charles, W.E., Sr. Novel identification of the free fatty acid receptor FFAR1 that promotes contraction in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L970–L982. [Google Scholar] [CrossRef]
- Matoba, A.; Matsuyama, N.; Shibata, S.; Masaki, E.; Emala, C.W.; Mizuta, K., Sr. The free fatty acid receptor 1 promotes airway smooth muscle cell proliferation through MEK/ERK and PI3K/Akt signaling pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L333–L348. [Google Scholar] [CrossRef]
- Xu, S.; Schwab, A.; Karmacharya, N.; Cao, G.; Woo, J.; Kim, N.; An, S.S.; Panettieri, R.A., Jr.; Jude, J.A. FFAR1 activation attenuates histamine-induced myosin light chain phosphorylation and cortical tension development in human airway smooth muscle cells. Respir. Res. 2020, 21, 317. [Google Scholar] [CrossRef]
- Worgall, T.S. Sphingolipids and Asthma. Adv. Exp. Med. Biol. 2022, 1372, 145–155. [Google Scholar]
- Perzanowski, M.S.; Ono, J.G.; Acosta, L.M.; Kim, B.I.; Divjan, A.; Miller, R.; Rundle, A.; Worgall, S.; Worgall, T.S. Distinct Serum Sphin-golipid Profiles among School-aged Children with Exercise-induced Wheeze and Asthma Persistence. Am. J. Respir. Crit. Care Med. 2017, 195, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Rago, D.; Pedersen, C.T.; Huang, M.; Kelly, R.S.; Gürdeniz, G.; Brustad, N.; Knihtilä, H.; Lee-Sarwar, K.A.; Morin, A.; Rasmussen, M.A.; et al. Characteristics and Mechanisms of a Sphingolipid-associated Childhood Asthma Endotype. Am. J. Respir. Crit. Care Med. 2021, 203, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Bourke, J.E. Solving the Riddle: Targeting the Imbalance of Sphingolipids in Asthma to Oppose Airway Hyperre-sponsiveness. Am. J. Respir. Cell Mol. Biol. 2020, 63, 555–557. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S.; Lee, Y.K.; Mazmanian, S.K.; et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Me-tabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Luthers, C.R.; Dunn, T.M.; Snow, A.L. ORMDL3 and Asthma: Linking Sphingolipid Regulation to Altered T Cell Function. Front. Immunol. 2020, 11, 597945. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Zhang, L.; Dong, F.; Zhang, X.; Yao, L.; Chang, C. Serum sphingolipid profile in asthma. J. Leukoc. Biol. 2021, 110, 53–59. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Theiler, A.; Bärnthaler, T.; Platzer, W.; Richtig, G.; Peinhaupt, M.; Rittchen, S.; Kargl, J.; Ulven, T.; Marsh, L.M.; Marsche, G.; et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J. Allergy Clin. Immunol. 2019, 144, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, ra152–ra307. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Arévalo, A.; Stiemsma, L.; Dimitriu, P.; Chico, M.E.; Loor, S.; Vaca, M.; Boutin, R.C.T.; Morien, E.; Jin, M.; et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 2017, 142, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 2017, 140, 63–75. [Google Scholar] [CrossRef]
- Kraj, L.; Krawiec, M.; Koter, M.; Grabon, W.; Kraj, G.; Cholojczyk, M.; Kulus, M.; Baranczyk-Kuzma, A. Altered L-arginine me-tabolism in children with controlled asthma. Allergy Asthma Proc. 2014, 35, 80–83. [Google Scholar] [CrossRef]
- Scott, J.A.; Grasemann, H. Arginine metabolism in asthma. Immunol. Allergy Clin. N. Am. 2014, 34, 767–775. [Google Scholar] [CrossRef]
- Bulau, P.; Zakrzewicz, D.; Kitowska, K.; Leiper, J.; Gunther, A.; Grimminger, F.; Eickelberg, O. Analysis of methylarginine metabo-lism in the cardiovascular system identifies the lung as a major source of ADMA. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L18–L24. [Google Scholar] [CrossRef]
- Ahmad, T.; Mabalirajan, U.; Ghosh, B.; Agrawal, A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am. J. Respir. Cell Mol. Biol. 2010, 42, 3–8. [Google Scholar] [CrossRef]
- Holguin, F.; Grasemann, H.; Sharma, S.; Winnica, D.; Wasil, K.; Smith, V.; Cruse, M.H.; Perez, N.; Coleman, E.; Scialla, T.J.; et al. L-Citrulline increases nitric oxide and improves control in obese asthmatics. JCI Insight 2019, 4, e131733. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Rifas-Shiman, S.L.; Carey, V.J.; et al. Fecal short-chain fatty acids in pregnancy and offspring asthma and allergic outcomes. J. Allergy Clin. Immunol. Pract. 2020, 8, 1100–1102.e13. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- de Gouw, H.W.; Verbruggen, M.B.; Twiss, I.M.; Sterk, P.J. Effect of oral L-arginine on airway hyperresponsiveness to histamine in asthma. Thorax 1999, 54, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Forno, E.; Alvarez, M.; Colon-Semidey, A.; Acosta-Perez, E.; Canino, G.; Celedón, J.C. Diet, Lung Function, and Asthma Exac-erbations in Puerto Rican Children. Pediatr. Allergy Immunol. Pulmonol. 2017, 30, 202–209. [Google Scholar] [CrossRef]
- Lautenbacher, L.A.; Jariwala, S.P.; Markowitz, M.E.; Rastogi, D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr. Pulmonol. 2016, 51, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Tobias, T.A.M.; Wood, L.G.; Rastogi, D. Carotenoids, fatty acids and disease burden in obese minority adolescents with asthma. Clin. Exp. Allergy 2019, 49, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. 5), S332–S338. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A.; Cho, Y. Obesity and asthma: Microbiome-metabolome interactions. Am. J. Respir. Cell Mol. Biol. 2016, 54, 609–617. [Google Scholar] [CrossRef]
- Penney, N.C.; Kinross, J.; Newton, R.C.; Purkayastha, S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: A systematic review. Int. J. Obes. 2015, 39, 1565–1574. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Metabolome-microbiome crosstalk and human disease. Metabolites 2020, 10, 181. [Google Scholar] [CrossRef]
- Manni, M.L.; Heinrich, V.A.; Buchan, G.J.; O’Brien, J.P.; Uvalle, C.; Cechova, V.; Koudelka, A.; Ukani, D.; Rawas-Qalaji, M.; Oury, T.D.; et al. Nitroalkene fatty acids modulate bile acid metabolism and lung function in obese asthma. Sci. Rep. 2021, 11, 17788. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshan Lal, T.; Cechinel, L.R.; Freishtat, R.; Rastogi, D. Metabolic Contributions to Pathobiology of Asthma. Metabolites 2023, 13, 212. https://doi.org/10.3390/metabo13020212
Roshan Lal T, Cechinel LR, Freishtat R, Rastogi D. Metabolic Contributions to Pathobiology of Asthma. Metabolites. 2023; 13(2):212. https://doi.org/10.3390/metabo13020212
Chicago/Turabian StyleRoshan Lal, Tamanna, Laura Reck Cechinel, Robert Freishtat, and Deepa Rastogi. 2023. "Metabolic Contributions to Pathobiology of Asthma" Metabolites 13, no. 2: 212. https://doi.org/10.3390/metabo13020212
APA StyleRoshan Lal, T., Cechinel, L. R., Freishtat, R., & Rastogi, D. (2023). Metabolic Contributions to Pathobiology of Asthma. Metabolites, 13(2), 212. https://doi.org/10.3390/metabo13020212




