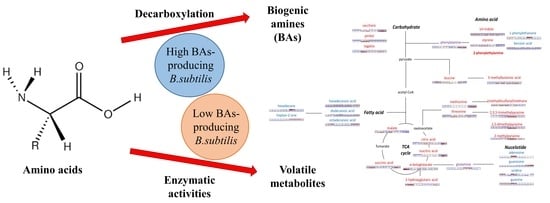
Comparative Analysis of Volatile and Non-Volatile Metabolites Derived from Bacillus subtilis Strains Producing Different Levels of Biogenic Amines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strains and Cultivation of Bacillus subtilis
2.3. Biogenic Amines Analysis
2.4. Volatile Metabolites Analysis
2.5. Non-Volatile Metabolites Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Quantitative Analysis of Biogenic Amines Production
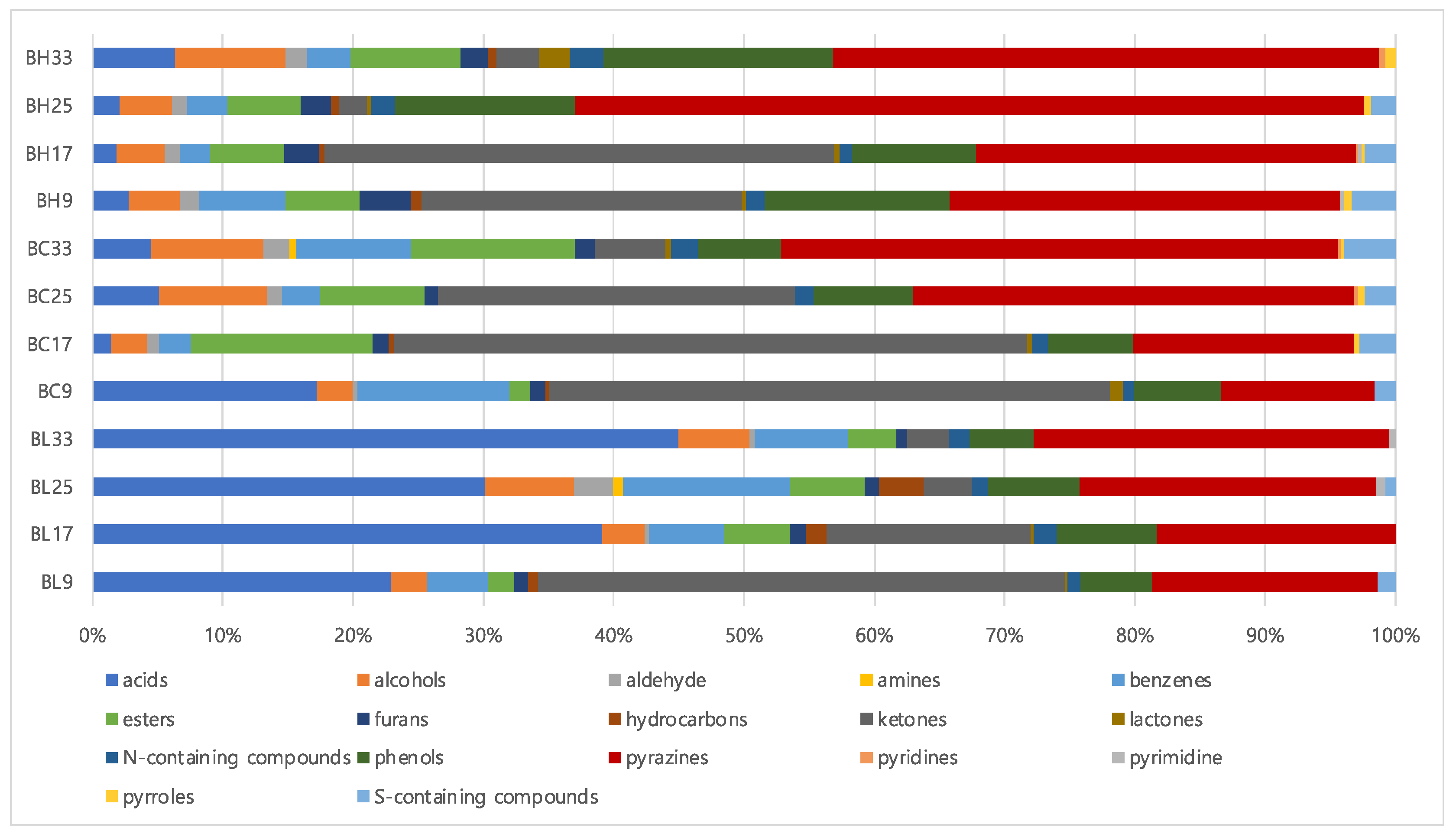
3.2. Comparison of Metabolic Profiles According to B. subtilis Strains
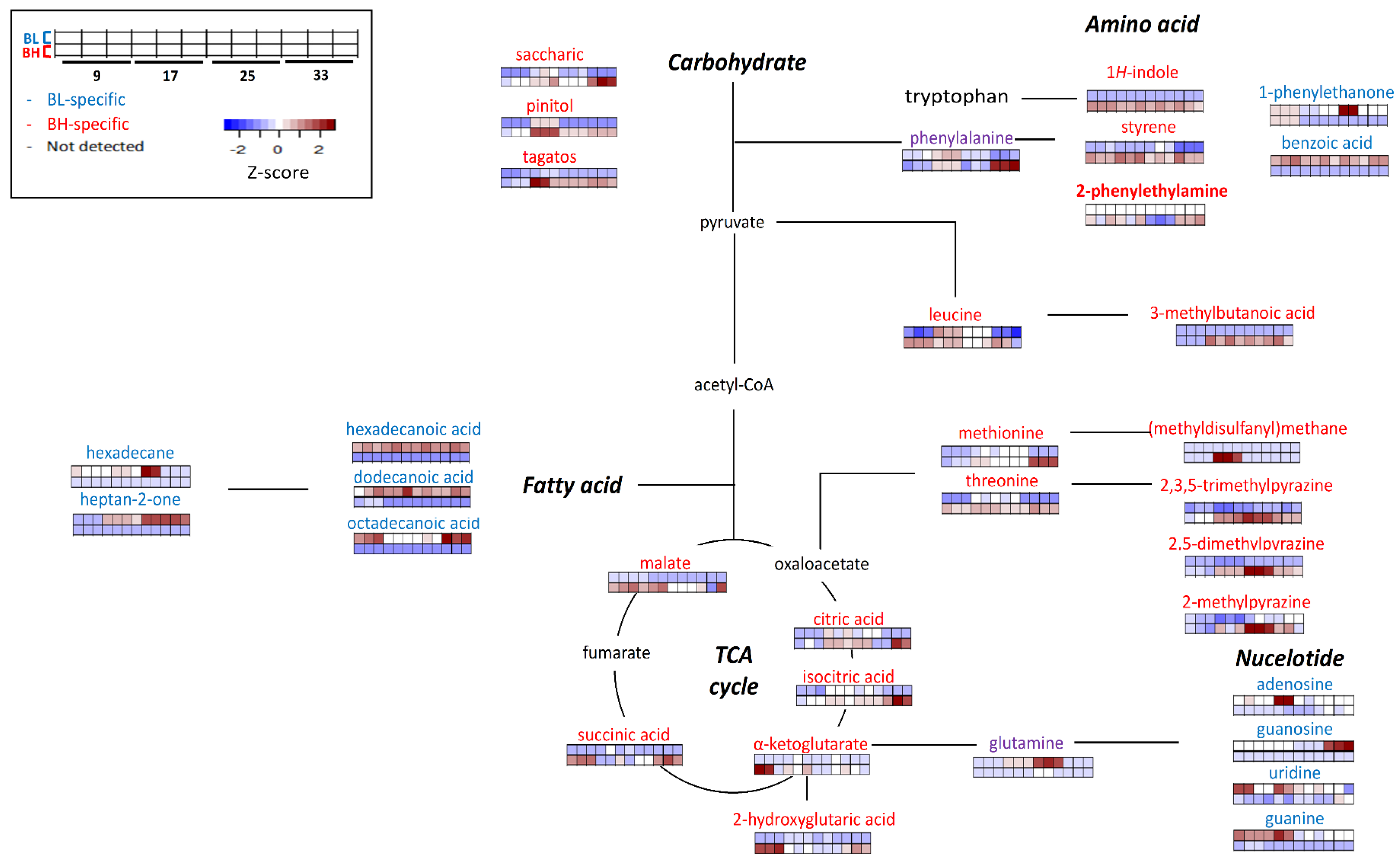
3.3. Comparative Analysis of Metabolite Formation Based on Metabolic Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Bach, B. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.-H.; Park, Y.K.; Jin, Y.H.; Lee, J.-H.; Hwang, H.-J. Bacterial Production and Control of Biogenic Amines in Asian Fermented Soybean Foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Halász, A.; Barath, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Sezer, Y.C.; Bulut, M.; Boran, G.; Alwazeer, D. The effects of hydrogen incorporation in modified atmosphere packaging on the formation of biogenic amines in cold stored rainbow trout and horse mackerel. J. Food Compost. Anal. 2022, 112, 104688. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of potent odorants in Japanese green tea (Sen-cha). J. Agric. Food Chem. 1999, 47, 5169–5172. [Google Scholar] [CrossRef]
- Perley, J.E.; Stowe, B.B. The production of tryptamine from tryptophan by Bacillus cereus (KVT). Biochem. J. 1966, 100, 169–174. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kim, Y.-H.; Kim, S.-E.; Lee, J.-H.; Park, C.-S.; Kim, H.-Y. Identification and distribution of Bacillus species in doenjang by whole-cell protein patterns and 16S rRNA gene sequence analysis. J. Microbiol. Biotechnol. 2010, 20, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, J.H.; Choi, A.; Hwang, H.-J.; Mah, J.-H. Validation of an HPLC analytical method for determination of biogenic amines in agricultural products and monitoring of biogenic amines in Korean fermented agricultural products. Toxicol. Res. 2015, 31, 299–305. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, S.Y.; Kim, Y.-S. Effects of pH and osmotic changes on the metabolic expressions of Bacillus subtilis strain 168 in metabolite pathways including leucine metabolism. Metabolites 2022, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Youn, G.S.; Choi, J.; Kim, C.-H.; Kim, B.Y.; Yang, S.-J.; Lee, J.H.; Park, T.-S.; Kim, B.K.; Kim, Y.B.; et al. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin. Transl. Med. 2021, 11, e634. [Google Scholar] [CrossRef]
- Chou, H.T.; Kwon, D.-H.; Hegazy, M.; Lu, C.-D. Transcriptome analysis of agmatine and putrescine catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2008, 190, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Pagot, Y.; Belin, J.; Husson, F.; Spinnler, H. Metabolism of phenylalanine and biosynthesis of styrene in Penicillium camemberti. J. Dairy Res. 2007, 74, 180–185. [Google Scholar] [CrossRef]
- Baek, H.H. Compilation of volatile flavor compounds in Cheonggukjang and Doenjang. Food Sci. Ind. 2017, 50, 24–49. [Google Scholar]
- Lapadatescu, C.; Giniès, C.; Le Quéré, J.; Bonnarme, P. Novel scheme for biosynthesis of aryl metabolites from L-phenylalanine in the fungus Bjerkandera adusta. Appl. Environ. Microbiol. 2000, 66, 1517–1522. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, H.-K.; Kwon, D.-Y.; Kim, Y.-S. Study of volatile organic acids in freeze-dried Cheonggukjang formed during fermentation using SPME and stable-isotope dilution assay (SIDA). Food Chem. 2007, 105, 1276–1280. [Google Scholar] [CrossRef]
- Yoon, K.-H.; Chang, Y.-I.; Lee, G.-H. Characteristic aroma compounds of cooked and fermented soybean (Chungkook-Jang) inoculated with various Bacilli. J. Sci. Food Agric. 2013, 93, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Y.; Tong, J.; Xu, Y. An alkylpyrazine synthesis mechanism involving l-threonine-3-dehydrogenase describes the production of 2, 5-dimethylpyrazine and 2, 3, 5-trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019, 85, 1807. [Google Scholar] [CrossRef] [PubMed]
- Sablé, S.; Cottenceau, G. Current knowledge of soft cheeses flavor and related compounds. J. Agric. Food Chem. 1999, 47, 4822–4836. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.D.; Allagheny, N.; Kipping, G.; Ames, J.M. Formation of volatile compounds during Bacillus subtilis fermentation of soya beans. J. Sci. Food Agric. 1997, 74, 132–140. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Buey, R.M.; Revuelta, J.L. Increased production of inosine and guanosine by means of metabolic engineering of the purine pathway in Ashbya gossypii. Microb. Cell Fact. 2015, 14, 58–64. [Google Scholar] [CrossRef][Green Version]


| BAs a | Strain b | Results of the Quantitative Analysis of BAs (Mean ± SD) (mg/L) | |||
|---|---|---|---|---|---|
| 9 h c | 17 h | 25 h | 33 h | ||
| PHE | BL | N.D. d | N.D. | N.D. | N.D. |
| BH | 4.66 ± 0.51 ab | 4.70 ± 0.40 ab | 3.40 ± 0.06 a | 5.12 ± 0.17 b | |
| p-value e | 3.97 × 10−3 | 2.41 × 10−3 | 8.73 × 10−5 | 3.89 × 10−4 | |
| PUT | BL | N.D. a | N.D. a | 0.74 ± 0.07 b | 0.76 ± 0.05 b |
| BH | N.D. a | 2.23 ± 0.15 b | 2.61 ± 0.10 b | 1.60 ± 0.31 ab | |
| p-value | - | 1.51 × 10−3 | 1.15 × 10−5 | 3.98 × 10−2 | |
| CAD | BL | N.D. | N.D. | N.D. | N.D. |
| BH | N.D. | N.D. | N.D. | N.D. | |
| p-value | - | - | - | - | |
| HIS | BL | 1.71 ± 0.12 c | 1.66 ± 0.05 c | 1.27 ± 0.05 b | 0.96 ± 0.11 a |
| BH | 1.80 ± 0.11 a | 2.38 ± 0.28 ab | 1.49 ± 0.08 a | 3.08 ± 0.34 b | |
| p-value | 3.94 × 10−1 | 4.19 × 10−2 | 1.97 × 10−2 | 4.89 × 10−3 | |
| TYR | BL | N.D. | N.D. | N.D. | N.D. |
| BH | N.D. | N.D. | N.D. | N.D. | |
| p-value | - | - | - | - | |
| SPD | BL | 4.68 ± 0.07 b | 4.23 ± 0.86 b | 5.15 ± 0.14 b | 2.31 ± 0.11 a |
| BH | 5.30 ± 0.38 ab | 6.21 ± 0.27 b | 3.81 ± 0.16 a | 6.24 ± 0.85 b | |
| p-value | 1.05 × 10−1 | 1.14 × 10−2 | 4.94 × 10−4 | 1.39 × 10−2 | |
| Total | BL | 6.40 ± 0.15 b | 6.31 ± 0.48 b | 7.15 ± 0.20 b | 4.03 ± 0.09 a |
| BH | 11.76 ± 0.22 ab | 15.52 ± 0.30 bc | 11.31 ± 0.32 a | 16.04 ± 0.95 c | |
| p-value | 4.19 × 10−6 | 9.26 × 10−6 | 4.45 × 10−5 | 2.63 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kwon, S.-H.; Song, S.; Lee, D.-Y.; Park, M.K.; Kim, Y.-S. Comparative Analysis of Volatile and Non-Volatile Metabolites Derived from Bacillus subtilis Strains Producing Different Levels of Biogenic Amines. Metabolites 2023, 13, 219. https://doi.org/10.3390/metabo13020219
Lee K, Kwon S-H, Song S, Lee D-Y, Park MK, Kim Y-S. Comparative Analysis of Volatile and Non-Volatile Metabolites Derived from Bacillus subtilis Strains Producing Different Levels of Biogenic Amines. Metabolites. 2023; 13(2):219. https://doi.org/10.3390/metabo13020219
Chicago/Turabian StyleLee, Kyuwon, Seo-Hee Kwon, Sumin Song, Do-Yup Lee, Min Kyung Park, and Young-Suk Kim. 2023. "Comparative Analysis of Volatile and Non-Volatile Metabolites Derived from Bacillus subtilis Strains Producing Different Levels of Biogenic Amines" Metabolites 13, no. 2: 219. https://doi.org/10.3390/metabo13020219
APA StyleLee, K., Kwon, S.-H., Song, S., Lee, D.-Y., Park, M. K., & Kim, Y.-S. (2023). Comparative Analysis of Volatile and Non-Volatile Metabolites Derived from Bacillus subtilis Strains Producing Different Levels of Biogenic Amines. Metabolites, 13(2), 219. https://doi.org/10.3390/metabo13020219