In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction
2.3.1. Essential Oil Distillation
2.3.2. Methanolic Extraction
2.4. Methanolic Extract Derivatization
2.5. Instrumentation and Chromatographic Conditions
2.5.1. GC-MS Conditions for Essential Oil Analysis
2.5.2. GC-MS Conditions for Derivatized Extracts’ Analysis
2.5.3. HPLC Conditions for Phenolics Determination
2.6. Antioxidant Activity
2.6.1. Oxygen Radical Absorbance Capacity Assay (ORAC)
2.6.2. Measurement of the DPPH Radical Scavenging Activity
2.7. Antimicrobial Activity
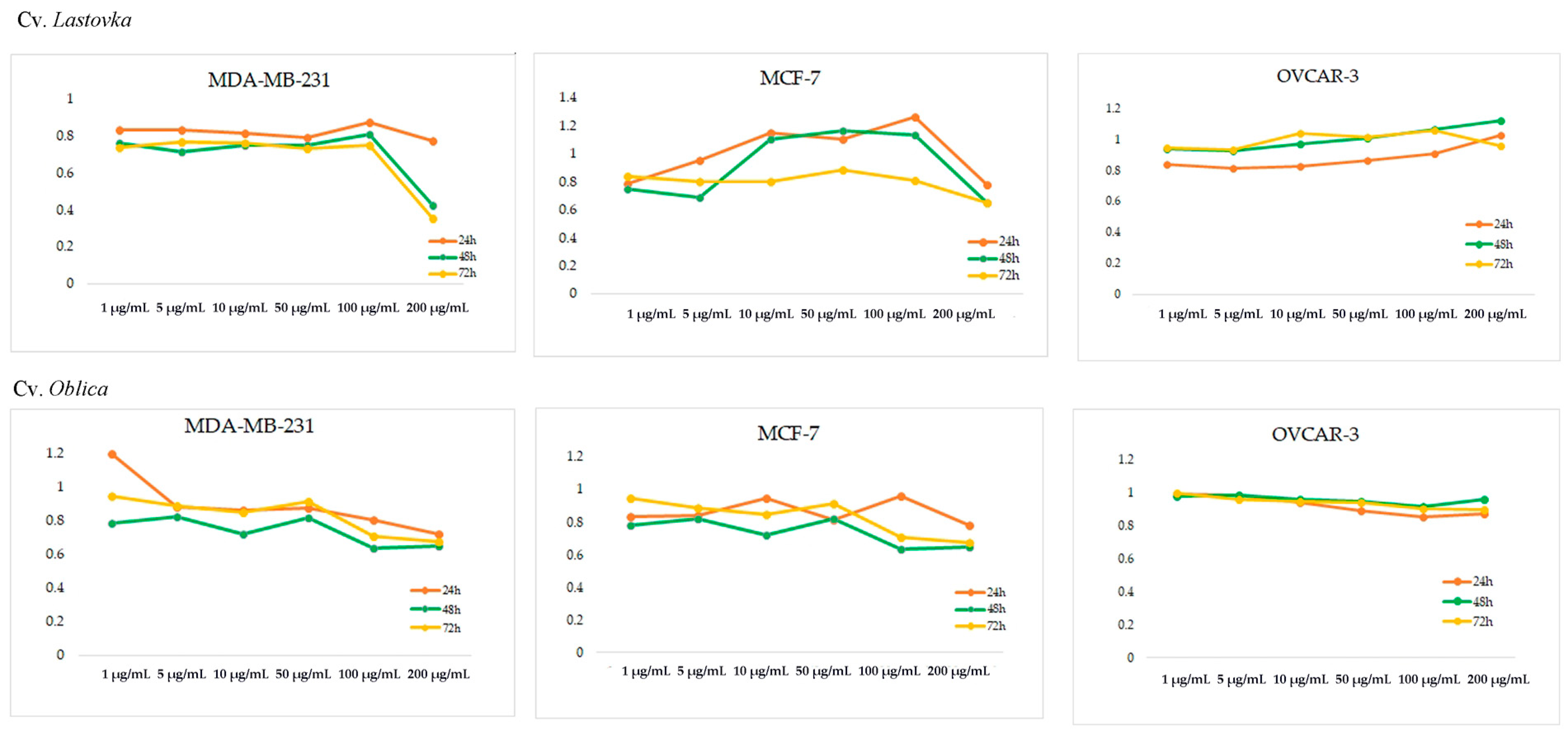
2.8. Cytotoxic Activity
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langgut, D.; Cheddadi, R.; Carrión, J.S.; Cavanagh, M.; Colombaroli, D.; Eastwood, W.J.; Greenberg, R.; Litt, T.; Mercuri, A.M.; Miebach, A.; et al. The Origin and Spread of Olive Cltivation in the Mediterranean Basin: The Fossil Pollen Evidence. Holocene 2019, 29, 902–922. [Google Scholar] [CrossRef]
- Wren, R.C.; Holmes, E.M. Potter’s Cyclopaedia of Botanical Drugs and Preparations, 2nd ed.; Potter & Clarke: London, UK, 1915; p. 111. [Google Scholar]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [PubMed]
- la Lastra, A.d.C.; Barranco, D.M.; Motilva, V.; Herrerias, M.J. Mediterrranean Diet and Health Biological Importance of Olive Oil. Curr. Pharm. Des. 2001, 7, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols Benefits of Olive Leaf (Olea europaea L) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar]
- Jukić Špika, M.; Žanetić, M.; Kraljić, K.; Soldo, B.; Ljubenkov, I.; Politeo, O.; Škevin, D. Differentiation Between Unfiltered and Filtered Oblica and Leccino cv. Virgin Olive Oils. J. Food Sci. 2019, 84, 877–885. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Phenolic Compounds in Olive Oil and Olives. Curr. Top. Nutraceutical Res. 2005, 3, 125–136. [Google Scholar]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of Major Bioactive Compounds from Olive Leaf. LWT Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation–A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive Leaf Extract as a Hypoglycemic Agent in Both Human Diabetic Subjects and in Rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Abunab, H.; Dator, W.L.; Hawamdeh, S. Effect of Olive Leaf Extract on Glucose Levels in Diabetes-Induced Rats: A Systematic Review and Meta-Analysis. J. Diabetes 2017, 9, 947–957. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of Phenolic-Rich Olive Leaf Extract on Blood Pressure, Plasma Lipids and Inflammatory Markers: A Randomised Controlled Trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Acar-Tek, N.; Ağagündüz, D. Olive leaf (Olea europaea L. Folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15. [Google Scholar] [CrossRef]
- Toulabi, T.; Delfan, B.; Rashidipour, M.; Yarahmadi, S.; Ravanshad, F.; Javanbakht, A.; Almasian, M. The Efficacy of Olive Leaf Extract on Healing Herpes Simplex Virus Labialis: A Randomized Double-Blind Study. Explore 2022, 18, 287–292. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Toulabi, T.; Yadegarinia, D.; Yarahmadi, S.; Mohammadi, R.; Keyvanfar, A. Efficacy of Olive Leaves Extract on the Outcomes of Hospitalized COVID-19 Patients: A Randomized, Triple-Blinded Clinical Trial. Explore, 2022; in press. [Google Scholar]
- Yeh, Y.T.; Cho, Y.Y.; Hsieh, S.C.; Chiang, A.N. Chinese Olive Extract Ameliorates Hepatic Lipid Accumulation in Vitro and in Vivo by Regulating Lipid Metabolism. Sci. Rep. 2018, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vij, G.; Chopra, K. Possible Role of Oxidative Stress and Immunological Activation in Mouse Model of Chronic Fatigue Syndrome and its Attenuation by Olive Extract. J. Neuroimmunol. 2010, 226, 3–7. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Zhong, R.; Wan, F.; Chen, L.; Liu, L.; Yi, B.; Zhang, H. Olive Fruit Extracts Supplement Improve Antioxidant Capacity via Altering Colonic Microbiota Composition in Mice. Front. Nutr. 2021, 8, 645099. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud Extracts as New Phytochemical Source for Herbal Preparations—Quality Control and Standardization by Analytical Fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health, 1st ed.; Rao, A.V., Leticia, G.R., Eds.; IntechOpen: London, UK, 2015; Chapter 8; pp. 187–218. [Google Scholar]
- Hoefler, C.; Fleurentin, J.; Mortier, F.; Pelt, J.M.; Guillemain, J. Comparative choleretic and hepatoprotective properties of young sprouts and total plant extracts of rosmarinus officinalis in rats. J. Ethnopharmacol. 1987, 19, 133–143. [Google Scholar] [CrossRef]
- Brigo, B. L’uomo, la Fitoterapia, la Gemmoterapia. 211 Sindromi Cliniche Trattate con Fitocomplessi e Gemmoderivati, 3rd ed.; Tecniche Nuove: Milano, Italy, 2009; p. 768. [Google Scholar]
- Kovalikova, Z.; Lnenicka, J.; Andrys, R. The Influence of Locality on Phenolic Profile and Antioxidant Capacity of Bud Extracts. Foods 2021, 10, 1608. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Bradford, J.M. Changes in Oleuropein Levels During Differentiation and Development of Floral Buds in ‘Arbequina’ Olives. Sci. Hortic. 2006, 110, 274–278. [Google Scholar] [CrossRef]
- Taamalli, A.; Abaza, L.; Arráez Román, D.; Segura Carretero, A.; Fernández Gutiérrez, A.; Zarrouk, M.; Ben Youssef Nabil, B. Characterisation of Phenolic Compounds by HPLC–TOF/IT/MS in Buds and Open Flowers of ‘Chemlali’ Olive Cultivar. Phytochem. Anal. 2013, 24, 504–512. [Google Scholar] [CrossRef]
- Popović, M.; Jukić Špika, M.; Veršić Bratinčević, M.; Ninčević, T.; Matešković, A.; Mandušić, M.; Rošin, J.; Nazlić, M.; Dunkić, V.; Vitanović, E. Essential Oil Volatile Fingerprint Differentiates Croatian cv. Oblica from Other Olea europaea L. Cultivars. Molecules 2021, 26, 3533. [Google Scholar] [CrossRef] [PubMed]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Zarate, E.; Boyle, V.; Rupprecht, U.; Green, S.; Villas-Boas, S.G.; Baker, P.; Pinu, F.R. Fully Automated Trimethylsilyl (TMS) Derivatisation Protocol for Metabolite Profiling by GC-MS. Metabolites 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas Chromatography Mass Spectrometry-Based Metabolite Profiling in Plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Frleta, R.; Popović, M.; Smital, T.; Šimat, V. Comparison of Growth and Chemical Profile of Diatom Skeletonema grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases. Mar. Drugs 2022, 20, 697. [Google Scholar] [CrossRef]
- Nazlić, M.; Kremer, D.; Grubešić, R.J.; Soldo, B.; Vuko, E.; Stabentheiner, E.; Ballian, D.; Bogunić, F.; Dunkić, V. Endemic Veronica saturejoides Vis. ssp. Saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds. Plants 2020, 9, 1646. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of Resveratrol with Other Phenolics and Activity Against Food-Borne Pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.d.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Srisawat, T.; Chumkaew, P.; Heed-Chim, W.; Sukpondma, Y.; Kanokwiroon, K. Phytochemical Screening and Cytotoxicity of Crude Extracts of Vatica diospyroides Symington Type LS. Trop. J. Pharm. Res. 2013, 12, 71–76. [Google Scholar] [CrossRef]
- Popović, M.; Maravić, A.; Čikeš Čulić, V.; Đulović, A.; Burčul, F.; Blažević, I. Biological Effects of Glucosinolate Degradation Products from Horseradish: A Horse That Wins the Race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.B.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Cozzolino, S. Evolution of Sexual Mimicry in the Orchid Subtribe Orchidinae: The Role of Preadaptations in the Attraction of Male Bees as Pollinators. BMC Evol. Biol. 2008, 8, 27. [Google Scholar] [CrossRef]
- Son, H.-D.; Yun, S.A.; Kim, S.-C.; Im, H.-T. Hydrocarbon Patterns in Cleisostoma scolopendrifolium (Orchidaceae) as a Key Mechanism for Pollination. Korean J. Plant Taxon. 2020, 50, 148–153. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z. Genetic and Biochemical Aspects of Floral Scents in Roses. Int. J. Mol. Sci. 2022, 23, 8014. [Google Scholar] [CrossRef]
- Setzer, W.N.; Noletto, J.A.; Vincent, M.A. 1,3,5-Trimethoxybenzene and 2,4,6-Trimethoxystyrene are the Major Components in the Leaf Oil of Eugenia confusa from Abaco Island, Bahamas. Nat. Prod. Commun. 2006, 1, 43–45. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Composition. In Essential Oil Safety, 2nd ed.; Tisserand, R., Young, R., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2014; pp. 5–22. [Google Scholar]
- Reis, S.L.; Mantello, A.G.; Macedo, J.M.; Gelfuso, E.A.; Da Silva, C.P.; Fachin, A.L.; Cardoso, A.M.; Beleboni, R.O. Typical Monoterpenes as Insecticides and Repellents Against Stored Grain Pests. Molecules 2016, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Jurišić Grubešić, R.; Nazlić, M.; Miletić, T.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Dunkić, V. Antioxidant Capacity of Free Volatile Compounds From Olea europaea L. Cv. Oblica Leaves Depending on the Vegetation Stage. Antioxidants 2021, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant aActivity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Cecchi, L.; Guerrini, L.; Bellumori, M.; Balli, D.; Xie, P.; Parenti, A.; Mulinacci, N. Optimization of the Production Process of Dried Unripe Olives (Olea europaea L.) as a Nutraceutical Ingredient Naturally Rich in Phenolic Compounds. LWT 2020, 129, 109569. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Czerwinska, E.M.; Kiss, K.A. Oleacein. Translation From Mediterranean Diet to Potential Antiatherosclerotic Drug. Curr. Pharm. Des. 2015, 21, 1205–1212. [Google Scholar] [CrossRef]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive Phenolic Compounds: Metabolic and Transcriptional Profiling During Fruit Development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Dastkar, E.; Soleimani, A.; Jafary, H.; de Dios Alche, J.; Bahari, A.; Zeinalabedini, M.; Salami, S.A. Differential Expression of Genes in Olive Leaves and Buds of ON-versus OFF-Crop Trees. Sci. Rep. 2020, 10, 15762. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Jandacek, R.J. Linoleic acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary Linoleic Acid and Human Health: Focus on Cardiovascular and Cardiometabolic Effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Rekik, O.; Ben Mansour, A.; Bouaziz, M. Evaluation of Phenolic Composition and Antioxidant Activity Changes in Olive Flowers During Development Using HPLC/DAD and LC-MS/MS. Electrophoresis 2018, 39, 1663–1672. [Google Scholar] [CrossRef]
- Kouka, P.; Tekos, F.; Valta, K.; Mavros, P.; Veskoukis, A.S.; Angelis, A.; Skaltsounis, A.-L.; Kouretas, D. Olive Tree Blossom Polyphenolic Extracts Exert Antioxidant and Antimutagenic Activities in Vitro and in Various Cell Lines. Oncol. Rep. 2019, 42, 2814–2825. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive Tree Derivatives and Hydroxytyrosol: Their Potential Effects on Human Health and Its Use as Functional Ingredient in Meat. Foods 2021, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive Byproducts and Their Bioactive Compounds as a Valuable Source for Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract Against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts Against Oral Microorganisms. Biomed. Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Ndjateu, F.S.T.; Tsafack, R.B.N.; Nganou, B.K.; Awouafack, M.D.; Wabo, H.K.; Tene, M.; Tane, P.; Eloff, J.N. Antimicrobial and Antioxidant Activities of Extracts and Ten Compounds from Three Cameroonian medicinal plants: Dissotis perkinsiae (Melastomaceae), Adenocarpus mannii (Fabaceae) and Barteria fistulosa (Passifloraceae). S. Afr. J. Bot. 2014, 91, 37–42. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; Álvarez de Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment Against Bacterial Biofilm in Nosocomial Infections: An in Vitro and in Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Würtele, G.; Hull, E.; Spiegelhalder, B.; Bartsch, H. Olives and Olive Oil in Cancer Prevention. Eur. J. Cancer Prev. 2004, 13, 319–326. [Google Scholar] [CrossRef]
- Benot-Dominguez, R.; Tupone, M.G.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Quintiliani, M.; Cinque, B.; Forte, I.M.; Cifone, M.G.; Ippoliti, R.; et al. Olive Leaf Extract Impairs Mitochondria by Pro-Oxidant Activity in MDA-MB-231 and OVCAR-3 Cancer Cells. Biomed. Pharmacother. 2021, 134, 111139. [Google Scholar] [CrossRef]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive Oil Oleuropein has Anti-Breast Cancer Properties with Higher Efficiency on ER-Negative Cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef]
- Şenol, H.; Tulay, P.; Ergören, M.; Hanoğlu, A.; Çalış, İ.; Mocan, G. Cytotoxic Effects of Verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk Between Hydroxytyrosol, a Major Olive Oil Phenol, and HIF-1 in MCF-7 Breast Cancer Cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; Gutiérrez-Santiago, F.; Rodríguez-García, C.; Gaforio, J.J. S Synergistic Effect of Squalene and Hydroxytyrosol on Highly Invasive MDA-MB-231 Breast Cancer Cells. Nutrients 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]

| RI | Literature RI | Compound | Lastovka | Oblica |
|---|---|---|---|---|
| 867 | 865 | (Z)-3-Hexen-1-ol * | 0.67 ± 0.14 | 1.03 ± 0.14 |
| 991 | 992 | 2-Pentylfuran | 0.37 ± 0.01 | 0.65 ± 0.13 |
| 1040 | 1040 | Benzeneacetaldehyde | 2.91 ± 0.24 | 3.91 ± 1.04 |
| 1099 | 1098 | Linalool * | 0.17 ± 0.06 | 0.84 ± 0.06 |
| 1104 | 1105 | Nonanal * | 4.29 ± 0.11 | 4.73 ± 0.87 |
| 1111 | 1111 | Phenylethyl Alcohol * | 0.29 ± 0.02 | 1.30 ± 0.05 |
| 1171 | 1171 | 1-Nonanol | 0.29 ± 0.03 | 0.84 ± 0.19 |
| 1205 | 1206 | Decanal * | 0.36 ± 0.02 | 0.58 ± 0.30 |
| 1254 | 1254 | Geraniol | 0.40 ± 0.02 | 1.10 ± 0.18 |
| 1260 | 1260 | (E)-2-Decenal | 0.71 ± 0.04 | 0.55 ± 0.09 |
| 1285 | 1285 | Dihydroedulan II | 0.31 ± 0.02 | 0.61 ± 0.12 |
| 1295 | 1298 | Theaspirane A | 0.28 ± 0.02 | 0.54 ± 0.10 |
| 1313 | 1315 | Theaspirane B | 0.35 ± 0.02 | 0.66 ± 0.10 |
| 1314 | 1316 | (E,E)-2,4-Decadienal | 0.71 ± 0.06 | 0.53 ± 0.05 |
| 1349 | - | Methyl 5-vinylnicotinate | 0.43 ± 0.06 | 1.49 ± 0.60 |
| 1355 | 1356 | Eugenol * | 0.60 ± 0.04 | 0.32 ± 0.02 |
| 1400 | 1400 | Tetradecane | 0.85 ± 0.07 | 0.88 ± 0.14 |
| 1407 | 1405 | 1,3,5-Trimethoxybenzene * | 12.60 ± 0.97 | 2.12 ± 0.30 |
| 1463 | 1465 | 2,6,10-Trimethyltridecane | 0.54 ± 0.07 | 0.81 ± 0.07 |
| 1512 | 1512 | Butylated hydroxytoluene | 0.49 ± 0.12 | 0.53 ± 0.40 |
| 1600 | 1600 | Hexadecane | 0.59 ± 0.02 | 0.65 ± 0.07 |
| 1651 | 1656 | Neointermedeol | n.d. | 0.55 ± 0.39 |
| 1670 | 1669 | (E,E)-6,8-Heptadecadiene | 0.88 ± 0.10 | 0.55 ± 0.17 |
| 1677 | 1692 | (Z)-3-Heptadecene | 16.25 ± 1.60 | 8.13 ± 2.15 |
| 1700 | 1700 | Heptadecane | 2.57 ± 0.21 | 1.37 ± 0.35 |
| 1845 | 1845 | Hexahydrofarnesyl acetone | 1.02 ± 0.14 | 2.54 ± 0.66 |
| 1880 | 1904 | Homosalate | 0.17 ± 0.01 | 0.53 ± 0.1 |
| 1900 | 1900 | Nonadecane | 1.50 ± 0.06 | 1.46 ± 0.44 |
| 1965 | 1965 | Hexadecanoic acid | 1.06 ± 0.15 | 2.53 ± 0.55 |
| 2085 | 2087 | 1-Henicosene | 2.85 ± 0.07 | 1.68 ± 0.55 |
| 2100 | 2100 | Heneicosane | 7.92 ± 0.16 | 6.02 ± 1.39 |
| 2114 | 2114 | Phytol | 0.12 ± 0.02 | 1.69 ± 0.35 |
| 2200 | 2200 | Docosane | 0.83 ± 0.03 | 0.68 ± 0.13 |
| 2274 | 2274 | (Z)-9-Tricosene | 2.53 ± 0.32 | 2.39 ± 0.61 |
| 2300 | 2300 | Tricosane | 7.08 ± 0.55 | 4.02 ± 0.67 |
| 2500 | 2500 | Pentacosane | 2.04 ± 0.18 | 1.51 ± 0.27 |
| 2700 | 2700 | Heptacosane | 2.05 ± 0.25 | 4.10 ± 0.29 |
| 2800 | 2800 | Octacosane | 0.31 ± 0.03 | 0.76 ± 0.06 |
| 2821 | 2833 | Squalene | 0.54 ± 0.08 | 0.28 ± 0.03 |
| 2888 | 2900 | Nonacosane | 3.81 ± 0.44 | 7.93 ± 0.5 |
| 3100 | 3100 | Hentriacontane | 1.28 ± 0.14 | 2.10 ± 0.16 |
| Phenolic Compound | Lastovka | Oblica |
|---|---|---|
| 3-Hydroxytyrosol | 43.12 ± 9.94 | 39.07 ± 1.71 |
| Tyrosol | 3.79 ± 0.75 | 3.95 ± 0.26 |
| Caffeic acid | 0.12 ± 0.05 | 0.20 ± 0.02 |
| Vanilin | 0.03 ± 0.01 | 0.59 ± 0.53 |
| trans-p-Coumaric acid | 0.13 ± 0.02 | 0.24 ± 0.03 |
| Rutin | 16.32 ± 2.11 | 20.65 ± 2.90 |
| Verbascoside | 18.38 ± 2.43 | 29.98 ± 2.41 |
| Luteolin-7-glucoside | 4.98 ± 0.11 | 8.11 ± 0.74 |
| trans-o-Coumaric acid | 1.90 ± 0.35 | 3.08 ± 0.38 |
| Apigenin-7-glucoside | 6.84 ± 0.84 | 10.82 ± 1.00 |
| Oleacein | 120.37 ± 21.18 | 85.11 ± 20.84 |
| Oleuropein | 59.27 ± 8.86 | 79.39 ± 12.67 |
| Oleuroside | 16.83 ± 2.96 | 15.68 ± 1.96 |
| Ligstroside | 2.58 ± 0.33 | 4.10 ± 0.41 |
| Pinoresinol | 3.51 ± 0.39 | 2.54 ± 1.05 |
| Quercetin | 8.26 ± 1.66 | 10.43 ± 0.22 |
| Luteolin | 1.80 ± 0.33 | 7.10 ± 0.92 |
| Apigenin | 2.02 ± 0.29 | 2.26 ± 0.04 |
| Diosmetin | 0.62 ± 0.12 | 0.71 ± 0.12 |
| Essential Oil | Extracts | |||
|---|---|---|---|---|
| Antioxidant Assay | Lastovka | Oblica | Lastovka | Oblica |
| ORAC (µM TE) | 139.95 ± 18.06 | 43.11 ± 2.53 | 1835.42 ± 38.31 | 1297.8 ± 73.82 |
| DPPH (IC50, mg/mL) | 30.51 ± 4.9 | 55.36 ± 9.6 | 0.274 ± 0.03 | 0.358 ± 0.01 |
| Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. cereus | L. monocytogenes | E. faecalis | E. coli | S. enteridis | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Essential oils | Lastovka | >4 | / | >4 | / | >4 | / | >4 | / | >4 | / | >4 | / |
| Oblica | >4 | / | >4 | / | >4 | / | >4 | / | >4 | / | >4 | / | |
| Extracts | Lastovka | 4 | >4 | 4 | >4 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Oblica | 4 | 4 | 4 | >4 | 2 | >4 | 4 | 4 | 4 | 4 | 4 | >4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, M.; Burčul, F.; Veršić Bratinčević, M.; Režić Mužinić, N.; Skroza, D.; Frleta Matas, R.; Nazlić, M.; Ninčević Runjić, T.; Jukić Špika, M.; Bego, A.; et al. In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties. Metabolites 2023, 13, 237. https://doi.org/10.3390/metabo13020237
Popović M, Burčul F, Veršić Bratinčević M, Režić Mužinić N, Skroza D, Frleta Matas R, Nazlić M, Ninčević Runjić T, Jukić Špika M, Bego A, et al. In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties. Metabolites. 2023; 13(2):237. https://doi.org/10.3390/metabo13020237
Chicago/Turabian StylePopović, Marijana, Franko Burčul, Maja Veršić Bratinčević, Nikolina Režić Mužinić, Danijela Skroza, Roberta Frleta Matas, Marija Nazlić, Tonka Ninčević Runjić, Maja Jukić Špika, Ana Bego, and et al. 2023. "In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties" Metabolites 13, no. 2: 237. https://doi.org/10.3390/metabo13020237
APA StylePopović, M., Burčul, F., Veršić Bratinčević, M., Režić Mužinić, N., Skroza, D., Frleta Matas, R., Nazlić, M., Ninčević Runjić, T., Jukić Špika, M., Bego, A., Dunkić, V., & Vitanović, E. (2023). In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties. Metabolites, 13(2), 237. https://doi.org/10.3390/metabo13020237













