Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
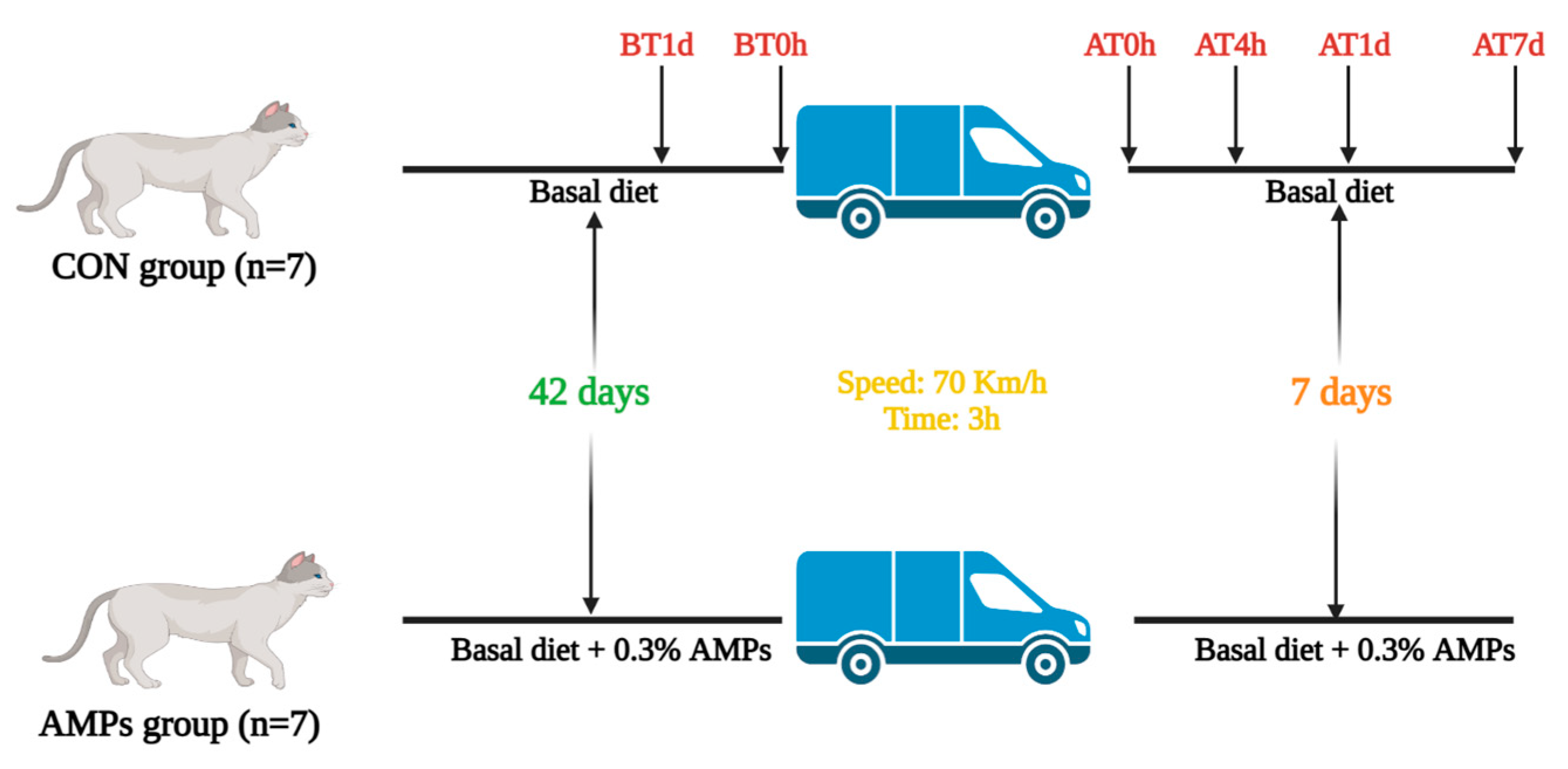
2.2. Experimental Design
2.3. Blood Sample Collection and Analysis
2.4. Fresh Fecal Sample Collection and Analysis
2.4.1. Fecal SCFAs and BCFAs Analysis
2.4.2. 16S rRNA High-Throughput Sequencing
2.5. Serum Untargeted Metabolomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of AMPs on Diarrhea Rate and Serum Hormone in Ragdolls
3.2. Effect of AMPs on Serum Inflammatory Factors and Antioxidant Capacity in Ragdolls
3.3. Effect of AMPs on Fecal Microbiota in Ragdolls
3.4. Effect of AMPs on Fecal SCFAs and BCFAs in Ragdolls
3.5. Effect of AMPs on Serum Metabolic Profiles in Ragdolls
4. Discussion
4.1. AMPs Relieve Transportation Stress in Ragdoll Cats
4.2. AMPs Alter Intestinal Microbiota and Metabolite in Ragdoll Cats
4.3. Future Prospects and Drawbacks of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y. Number of Domestic Cats in China from 2018 to 2021 (in Millions). Available online: https://www.statista.com/statistics/992420/china-number-of-cats/ (accessed on 15 February 2022).
- Bedford, E. Number of Pet Cats in Europe from 2010 to 2021 (in 1000s). Available online: https://www.statista.com/statistics/516041/cat-population-europe-europe/ (accessed on 27 September 2022).
- Zhang, L.; Bian, Z.; Liu, Q.; Deng, B. Dealing With Stress in Cats: What Is New About the Olfactory Strategy? Front. Vet. Sci. 2022, 9, 928943. [Google Scholar] [CrossRef]
- Amat, M.; Camps, T.; Manteca, X. Stress in owned cats: Behavioural changes and welfare implications. J. Feline Med. Surg. 2016, 18, 577–586. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The biology of animal stress: Basic principles and implications for animal welfare. Vet. J. 2002, 164, 77. [Google Scholar]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P.; et al. Gallic Acid Alleviates Gut Dysfunction and Boosts Immune and Antioxidant Activities in Puppies Under Environmental Stress Based on Microbiome–Metabolomics Analysis. Front. Immunol. 2022, 12, 813890. [Google Scholar] [CrossRef]
- Bailey, M.T. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 2014, 817, 255–276. [Google Scholar]
- Fazio, F.; Casella, S.; Giannetto, C.; Giudice, E.; Piccione, G. Characterization of acute phase proteins and oxidative stress response to road transportation in the dog. Exp. Anim. 2015, 64, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Wernicki, A.S.A.S.; Urban-Chmiel, R.S.A.S.; Kankofer, M.S.B.S.; Mikucki, P.S.S.; Puchalski, A.S.A.S.; Tokarzewski, S.S.A.S. Evaluation of plasma cortisol and TBARS levels in calves after short-term transportation. Rev. Med. Vet.-Toulouse 2006, 157, 30–34. [Google Scholar]
- Paltrinieri, S. The feline acute phase reaction. Vet. J. 2008, 177, 26–35. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Jacobs, L.; Delezie, E.; Duchateau, L.; Goethals, K.; Ampe, B.; Buyse, J.; Tuyttens, F.A.M. Impact of transportation duration on stress responses in day-old chicks from young and old breeders. Res. Vet. Sci. 2017, 112, 172–176. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, X.; Gao, W.; Ji, C.; Wang, Y.; Feng, P.; Feng, Y.; Zhang, Z.; Li, L.; Zhao, F. Transport stress affects the fecal microbiota in healthy donkeys. J. Vet. Intern. Med. 2021, 35, 2449–2457. [Google Scholar] [CrossRef]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of Microbial Dysbiosis and Metabolomic Changes in Dogs with Acute Diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Su, C.; Verbrugghe, A.; Van de Wiele, T.; Martos Martinez-Caja, A.; Hesta, M. Past, Present, and Future of Gastrointestinal Microbiota Research in Cats. Front. Microbiol. 2020, 11, 1661. [Google Scholar] [CrossRef]
- Schwiertz, A.; Lehmann, U.; Jacobasch, G.; Blaut, M. Influence of resistant starch on the SCFA production and cell counts of butyrate-producing Eubacterium spp. in the human intestine. J. Appl. Microbiol. 2002, 93, 157–162. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Glahn, R.P.; Bae, S.; Brenna, J.T. Branched-chain fatty acids in the neonatal gut and estimated dietary intake in infancy and adulthood. Importance Immunonutrition 2013, 77, 133–143. [Google Scholar]
- Zhang, Q.; Yan, Z.; Meng, Y.; Hong, X.; Shao, G.; Ma, J.; Cheng, X.; Liu, J.; Kang, J.; Fu, C. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, P231–P258. [Google Scholar] [CrossRef]
- Valdez-Miramontes, C.E.; De Haro-Acosta, J.; Aréchiga-Flores, C.F.; Verdiguel-Fernández, L.; Rivas-Santiago, B. Antimicrobial peptides in domestic animals and their applications in veterinary medicine. Peptides 2021, 142, 170576. [Google Scholar] [CrossRef]
- Sierra, J.M.; Vinas, M. Future prospects for Antimicrobial peptide development: Peptidomimetics and antimicrobial combinations. Expert Opin. Drug Discov. 2021, 16, 601–604. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Xie, Y.; Chen, Y.; Yi, H.; He, D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int. Immunopharmacol. 2020, 85, 106658. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, F.; Cao, R.; Ni, X.; Xin, Z.; Deng, J.; Wu, G.; Ren, W.; Yin, Y.; Deng, B. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice With DSS-Induced IBD. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Choi, S.C.; Ingale, S.L.; Kim, J.S.; Park, Y.K.; Kwon, I.K.; Chae, B.J. Antimicrobial peptide-A3: Effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Brit. Poultry Sci. 2013, 54, 738–746. [Google Scholar] [CrossRef]
- Baillon, M.A.; Marshall-Jones, Z.V.; Butterwick, R.F. Effects of probiotic Lactobacillus acidophilus strain DSM13241 in healthy adult dogs. Am. J. Vet. Res. 2004, 65, 338–343. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, S.; Ren, D.; Liu, W.; Han, B.; Zhang, Y.; Xiao, J.; Yi, L.; Deng, B. UPLC-Orbitrap-MS/MS combined with chemometrics establishes variations in chemical components in green tea from Yunnan and Hunan origins. Food Chem. 2018, 266, 534–544. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- De, A.K.; Sawhney, S.; Ponraj, P.; Sunder, J.; Banik, S.; Bhattacharya, D. Physiological and immune responses to long road transportation in Andaman local pigs. Trop. Anim. Health Pro. 2021, 53, 247. [Google Scholar] [CrossRef]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Shooshtarizadeh, P.; Zhang, D.; Chich, J.; Gasnier, C.; Schneider, F.; Haïkel, Y.; Aunis, D.; Metz-Boutigue, M. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul. Pept. 2010, 165, 102–110. [Google Scholar] [CrossRef]
- Khan, S.R.; van der Burgh, A.C.; Peeters, R.P.; van Hagen, P.M.; Dalm, V.A.S.H.; Chaker, L. Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 664526. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Sapolsky, R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007, 21, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Ni, X.; Jin, C.; Ren, W.; Li, J.; Deng, J.; Deng, B.; Yin, Y. Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway. Int. J. Mol. Sci. 2018, 19, 1941. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xie, S.; Zhou, A.; Zhang, C.; Wen, L.; Xu, G.; Zou, J. Effects of mixed antimicrobial peptide on the growth performance, antioxidant and immune responses and disease resistance of Pengze crucian carp (Carassius auratus var. Pengze). Fish Shellfish Immun. 2021, 114, 112–118. [Google Scholar] [CrossRef]
- Todorova, I.; Simeonova, G.; Kyuchukova, D.; Dinev, D.; Gadjeva, V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp. Clin. Pathol. 2005, 13, 190–194. [Google Scholar] [CrossRef]
- Cecerska-Heryc, E.; Surowska, O.; Heryc, R.; Serwin, N.; Napiontek-Balinska, S.; Dolegowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Liu, X.; Wen, L.; Zou, J. Effects of dietary antimicrobial peptides on intestinal morphology, antioxidant status, immune responses, microbiota and pathogen disease resistance in grass carp Ctenopharyngodon idellus. Microb. Pathog. 2022, 165, 105386. [Google Scholar] [CrossRef]
- Liu, N.; Ma, X.; Jiang, X. Effects of Immobilized Antimicrobial Peptides on Growth Performance, Serum Biochemical Index, Inflammatory Factors, Intestinal Morphology, and Microbial Community in Weaning Pigs. Front. Immunol. 2022, 13, 872990. [Google Scholar] [CrossRef]
- Ren, Z.; Yao, R.; Liu, Q.; Deng, Y.; Shen, L.; Deng, H.; Zuo, Z.; Wang, Y.; Deng, J.; Cui, H.; et al. Effects of antibacterial peptides on rumen fermentation function and rumen microorganisms in goats. PLoS ONE 2019, 14, e0221815. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Shang, L.; Wang, F.; Zeng, X.; Yu, H.; Liu, L.; Zhou, J.; Qiao, S. Effects of Antimicrobial Peptide Microcin C7 on Growth Performance, Immune and Intestinal Barrier Functions, and Cecal Microbiota of Broilers. Front. Vet. Sci. 2022, 8, 1542. [Google Scholar] [CrossRef]
- Shang, L.; Yu, H.; Liu, H.; Chen, M.; Zeng, X.; Qiao, S. Recombinant antimicrobial peptide microcin J25 alleviates DSS-induced colitis via regulating intestinal barrier function and modifying gut microbiota. Biomed. Pharmacother. 2021, 139, 111127. [Google Scholar] [CrossRef]
- Schwab, C.; Berry, D.; Rauch, I.; Rennisch, I.; Ramesmayer, J.; Hainzl, E.; Heider, S.; Decker, T.; Kenner, L.; Muller, M.; et al. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 2014, 8, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Garrigues, Q.; Apper, E.; Chastant, S.; Mila, H. Gut microbiota development in the growing dog: A dynamic process influenced by maternal, environmental and host factors. Front. Vet. Sci. 2022, 9, 964649. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Buyukleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef] [Green Version]
- King, M.; Hurley, H.; Davidson, K.R.; Dempsey, E.C.; Barron, M.A.; Chan, E.D.; Frey, A. The Link between Fusobacteria and Colon Cancer: A Fulminant Example and Review of the Evidence. Immune Netw. 2020, 20, e30. [Google Scholar] [CrossRef]
- Fox, B.; Berger, M.; Roncallo, M.; Pinoche, L.; Ibáñez, M.; Gonzalez-Fraga, S.; Fernández-Canigia, L. MALDI-TOF MS in Anaerobiospirillum succiniciproducens bacteremia: A report of 4 cases in different hosts. Anaerobe 2018, 154, 267–270. [Google Scholar] [CrossRef]
- Schaumburg, F.; Dieckmann, R.; Auml, T.S.; Kling; Becker, K.; Idelevich, E.A. First description of an Anaerobiospirillum succiniciproducens prosthetic joint infection. New Microbes New Infect. 2017, 18, 1–2. [Google Scholar] [CrossRef]
- Tang, S.; Zhong, R.; Yin, C.; Su, D.; Xie, J.; Chen, L.; Liu, L.; Zhang, H. Exposure to High Aerial Ammonia Causes Hindgut Dysbiotic Microbiota and Alterations of Microbiota-Derived Metabolites in Growing Pigs. Front. Nutr. 2021, 8, 689818. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, F.; Hu, H.; Li, X.; Dong, F.; Sun, M.; Xiao, Y.; Ge, T.; et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut Microbes 2021, 13, 1865708. [Google Scholar] [CrossRef]
- Dovrolis, N.; Michalopoulos, G.; Theodoropoulos, G.E.; Arvanitidis, K.; Kolios, G.; Sechi, L.A.; Eliopoulos, A.G.; Gazouli, M. The Interplay between Mucosal Microbiota Composition and Host Gene-Expression is Linked with Infliximab Response in Inflammatory Bowel Diseases. Microorganisms 2020, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on the immune response, antioxidant capacity, and intestinal microbiota composition of broilers. Poultry Sci. 2022, 101, 101996. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, Y.; Zhang, F.; Wang, C.; Yang, X.; Bai, S.; Xue, Y.; Shen, Q. Heat-Treated Adzuki Bean Protein Hydrolysates Reduce Obesity in Mice Fed a High-Fat Diet via Remodeling Gut Microbiota and Improving Metabolic Function. Mol. Nutr. Food Res. 2022, 66, 2100907. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Tr. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Palbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Z.; Niu, X.; Wang, W.; Zhao, J. The role of short-chain fatty acids in Clostridioides difficile infection: A review. Anaerobe 2022, 75, 102585. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef]
- Yang, K.; Jian, S.; Wen, C.; Guo, D.; Liao, P.; Wen, J.; Kuang, T.; Han, S.; Liu, Q.; Deng, B. Gallnut Tannic Acid Exerts Anti-stress Effects on Stress-Induced Inflammatory Response, Dysbiotic Gut Microbiota, and Alterations of Serum Metabolic Profile in Beagle Dogs. Front. Nutr. 2022, 9, 847966. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhang, T.; Huang, L.; Yan, W.; Lu, L.; Jia, J.; Tao, Y.; Cai, W.; Wang, Y. Alterations of gut microbiota and serum bile acids are associated with parenteral nutrition-associated liver disease. J. Pediatr. Surg. 2021, 56, 738–744. [Google Scholar] [CrossRef]
- Huang, H.; Wu, X.; Yi, S.; Zhou, Z.; Zhu, J.; Fang, Z.; Yue, J.; Bao, S. Rifamycin S and its geometric isomer produced by a newly found actinomycete, Micromonospora rifamycinica. Antonie Van Leeuwenhoek 2009, 95, 143–148. [Google Scholar] [CrossRef]
- Arora, S.K. Correlation of structure and activity in ansamycins: Structure, conformation, and interactions of antibiotic rifamycin S. J. Med. Chem. 1985, 28, 1099–1102. [Google Scholar] [CrossRef]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [Green Version]





| Ingredients | Basis % | Analytical Composition | DM Basis % |
|---|---|---|---|
| Fish | 23.00 | DM | 93.38 |
| Chicken meat meal | 24.00 | CP | 40.17 |
| Duck meat meal | 12.00 | CF | 22.26 |
| Fish meal | 8.00 | Ash | 8.13 |
| Chicken oil | 6.00 | OM | 91.87 |
| Tapioca | 5.00 | ||
| Tapioca flour | 4.00 | ||
| Alfalfa grass grain | 4.00 | ||
| Chicken liver | 2.70 | ||
| Chicken heart | 2.00 | ||
| Flavoring paste | 1.60 | ||
| Salmon oil | 1.50 | ||
| Beer yeast power | 1.20 | ||
| Egg power | 1.00 | ||
| Kelp power | 1.00 | ||
| Yucca | 1.00 | ||
| Semen plantaginis | 1.00 | ||
| Madder | 1.00 | ||
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Yang, K.; Wen, J.; Kuang, T.; Cao, Z.; Zhang, L.; Han, S.; Jian, S.; Chen, X.; Zhang, L.; et al. Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota. Metabolites 2023, 13, 326. https://doi.org/10.3390/metabo13030326
He S, Yang K, Wen J, Kuang T, Cao Z, Zhang L, Han S, Jian S, Chen X, Zhang L, et al. Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota. Metabolites. 2023; 13(3):326. https://doi.org/10.3390/metabo13030326
Chicago/Turabian StyleHe, Shansong, Kang Yang, Jiawei Wen, Tao Kuang, Zhihao Cao, Lingna Zhang, Sufang Han, Shiyan Jian, Xin Chen, Limeng Zhang, and et al. 2023. "Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota" Metabolites 13, no. 3: 326. https://doi.org/10.3390/metabo13030326
APA StyleHe, S., Yang, K., Wen, J., Kuang, T., Cao, Z., Zhang, L., Han, S., Jian, S., Chen, X., Zhang, L., Deng, J., & Deng, B. (2023). Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota. Metabolites, 13(3), 326. https://doi.org/10.3390/metabo13030326







