Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Isolation of Total RNA
2.3. cDNA Synthesis on RNA Template (Reverse Transcription)
2.4. Determination of mRNA Level
2.5. Determination of 3-HB and PHB in Plant Tissues
2.6. Determination of Phenolic Compound Content by UPLC Method
2.7. Determination of Cellulose Content
2.8. Determination of Lignin Content
2.9. Fractionation of Cell Wall
2.10. Determination of Uronic Acids
2.11. Determination of Monosaccharides by UPLC Method
2.12. Determination of Total Pectin and Hemicellulose
2.13. In Vitro Retting Experiment
2.14. Analysis of Fatty Acids by GC-FID Method
2.15. Analysis of Terpenoid Compounds by UPLC Method
2.16. Analysis of Sterols by GC-FID Method
2.17. IR and X-ray Analysis
2.18. Determination of the Antioxidant Potential of Flax Stem Extract by Spectrophotometric Method Using DPPH
2.19. Statistical Analysis
3. Results
3.1. Productivity of Transgenic Flax
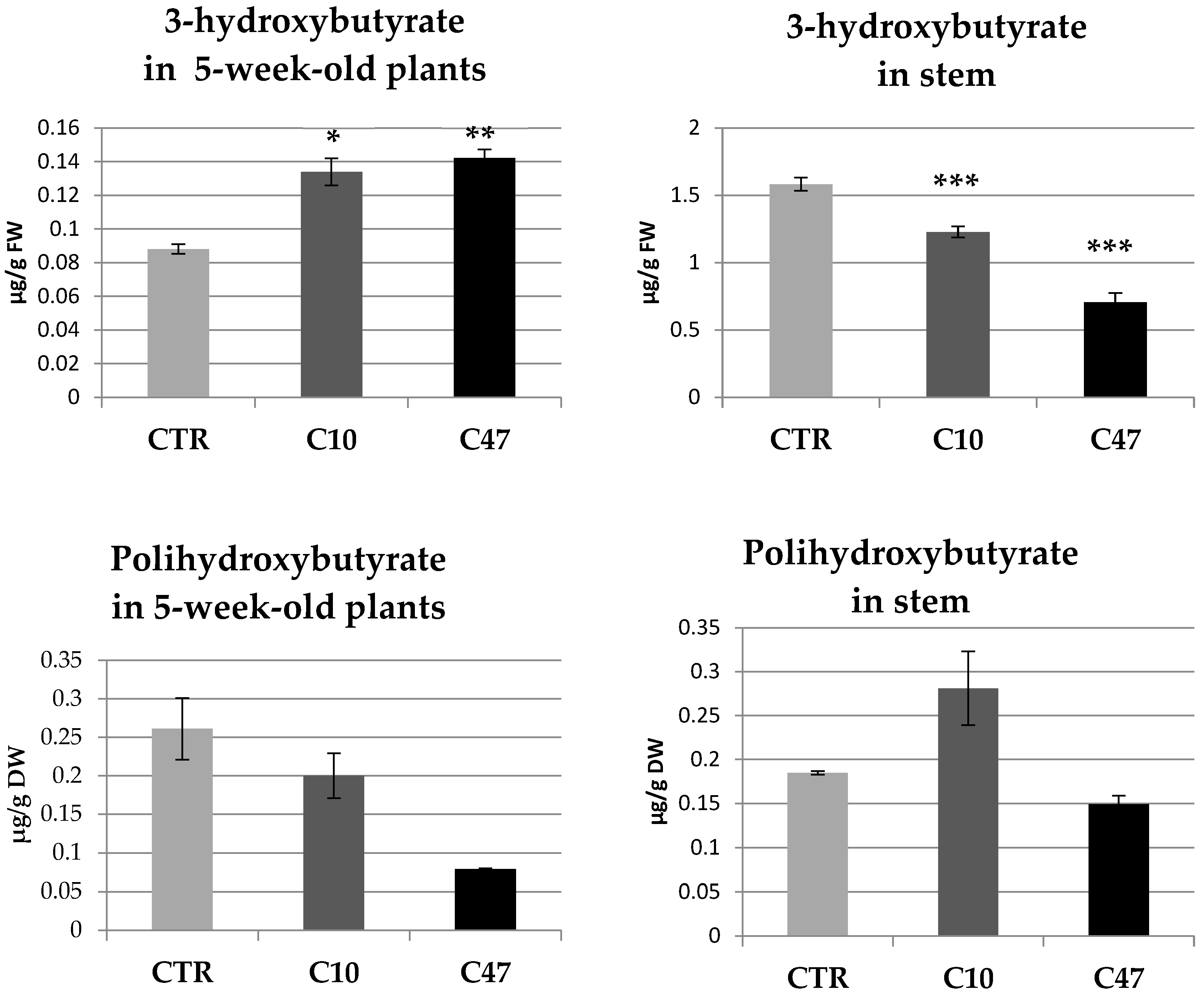
3.2. Determination of 3-Hydroxybutyrate and Polyhydroxybutyrate Content
3.3. Content of Phenolic Compounds in Flax Stems
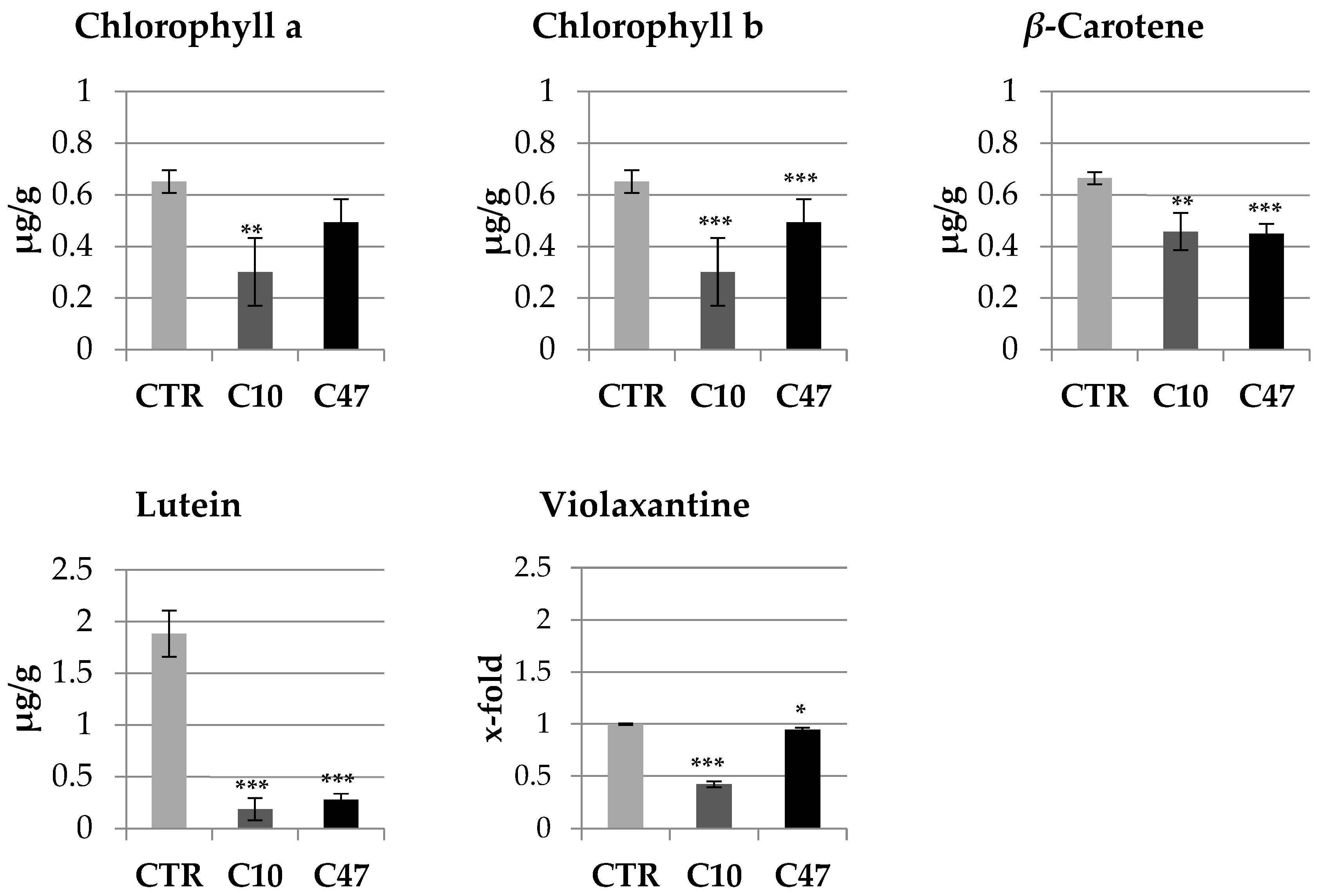
3.4. Content of Carotenoids and Chlorophylls in Flax Stems
3.5. Content of Phytosterols in Flax Stems and Seeds
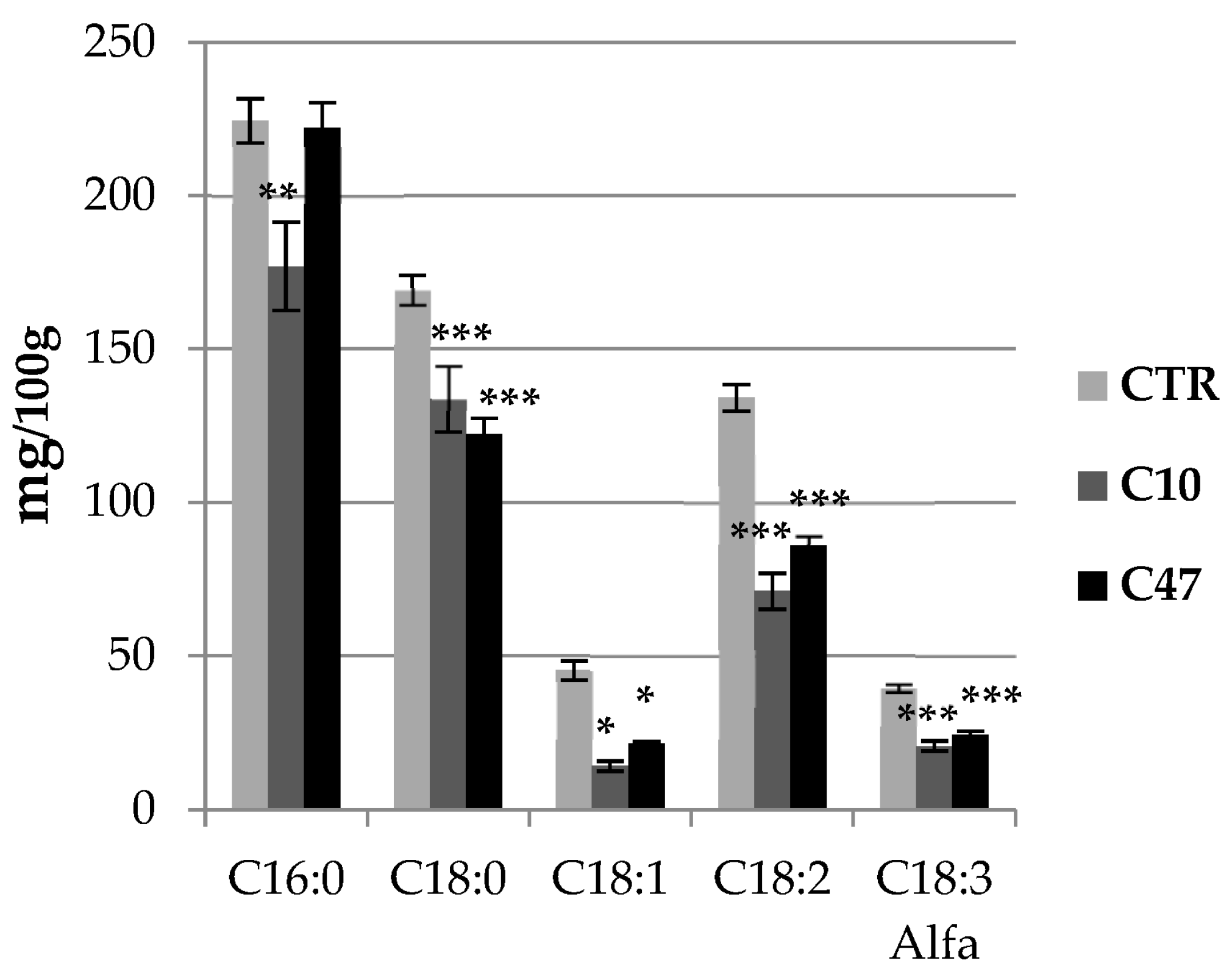
3.6. Content of Fatty Acids in Flax Stems and Seeds
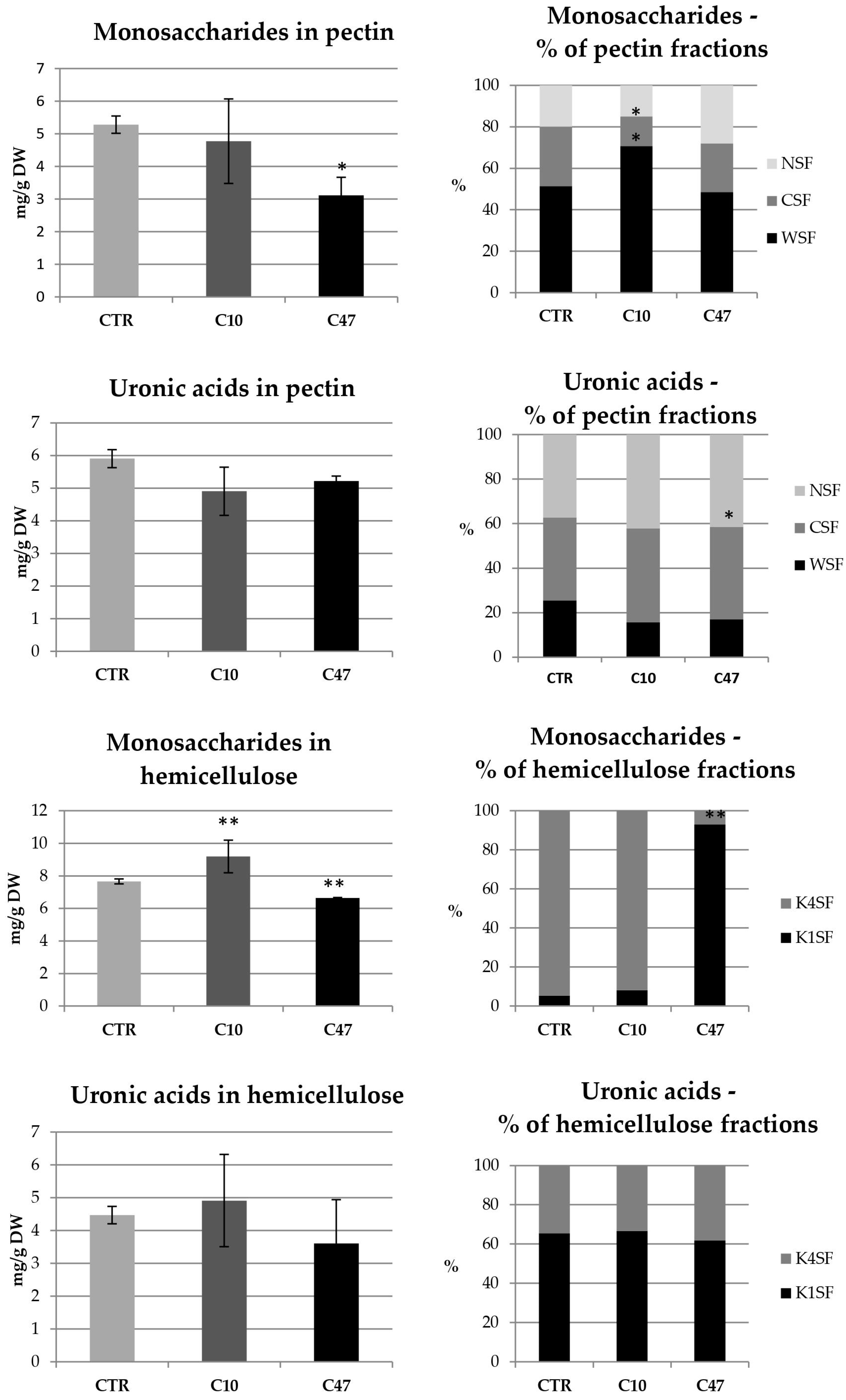
3.7. Content of Cell Wall Components in Flax Stem
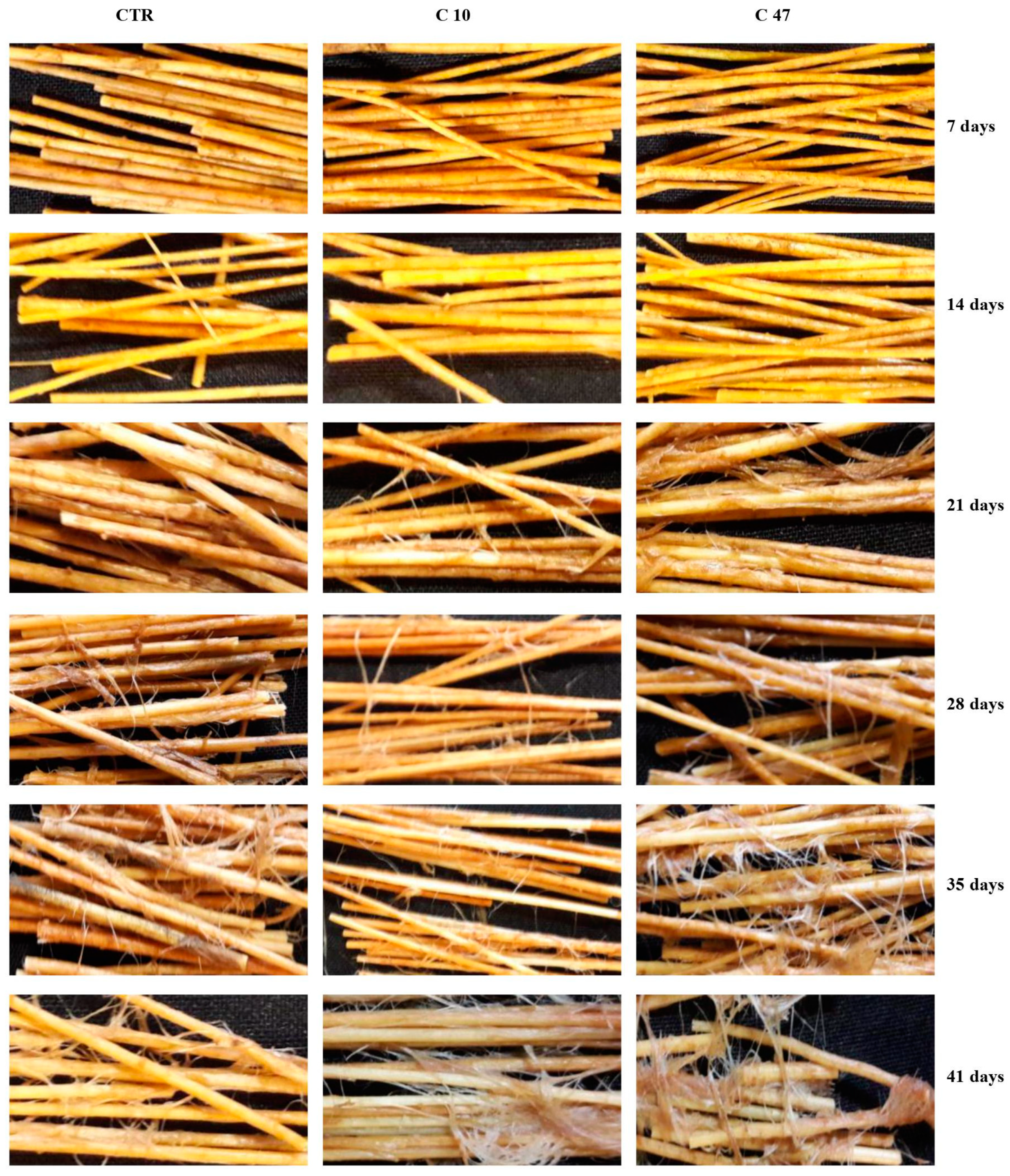
3.8. Retting of Flax Stem
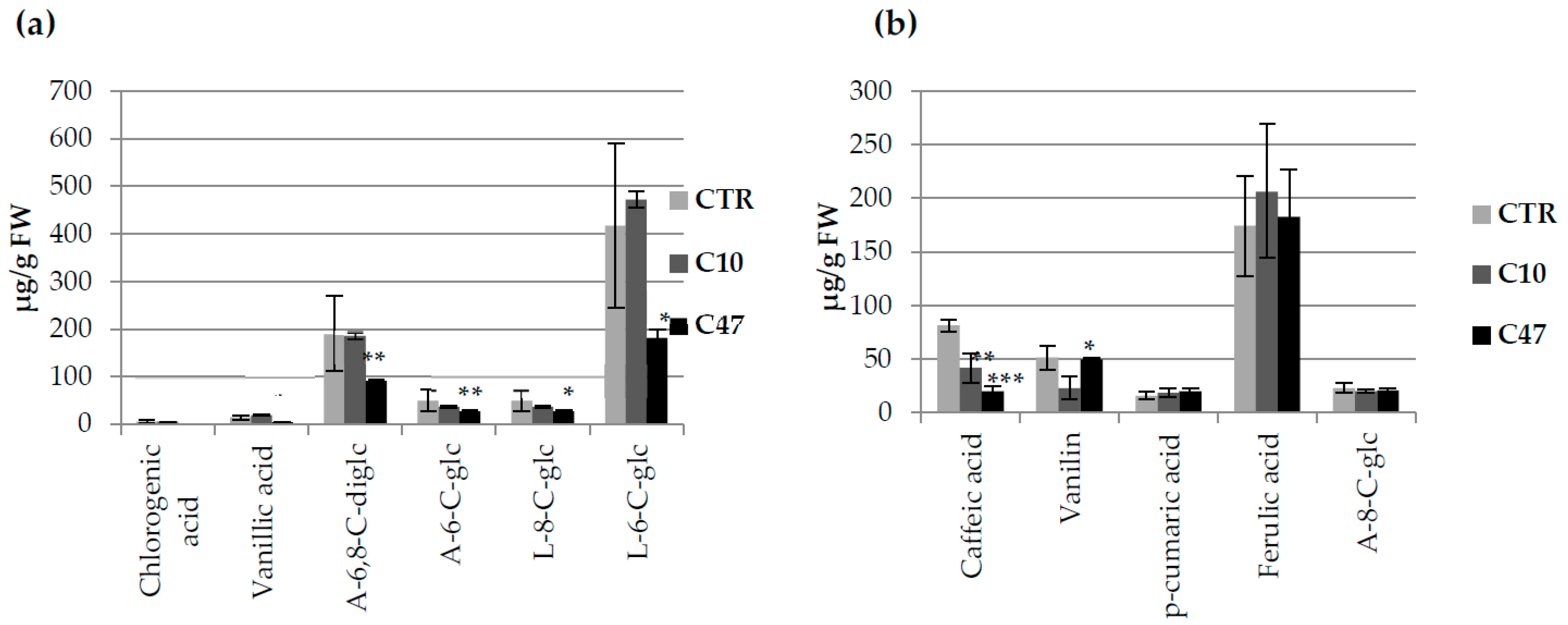
3.9. Content of Phenolic Compounds in Flax Fibres
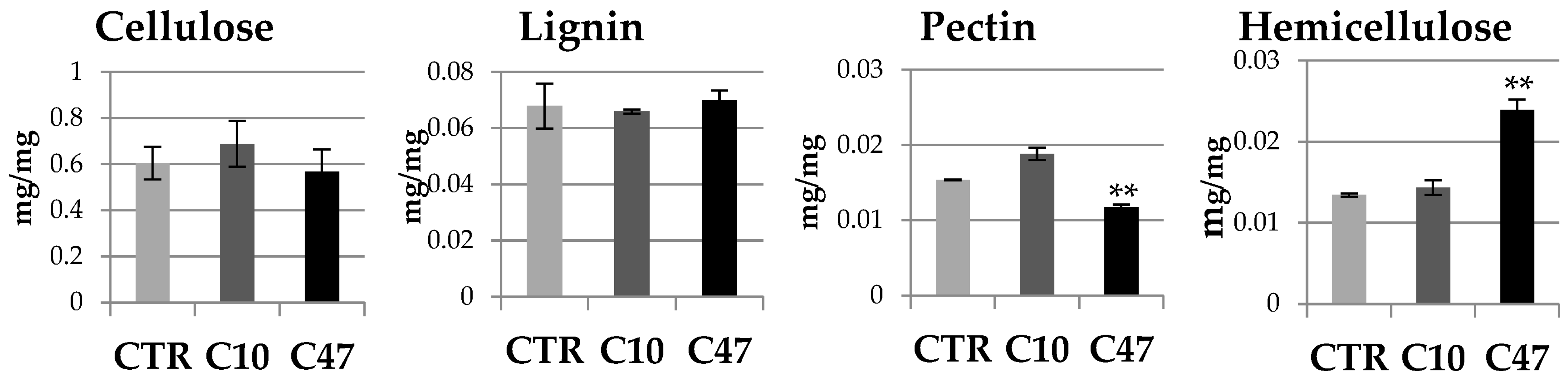
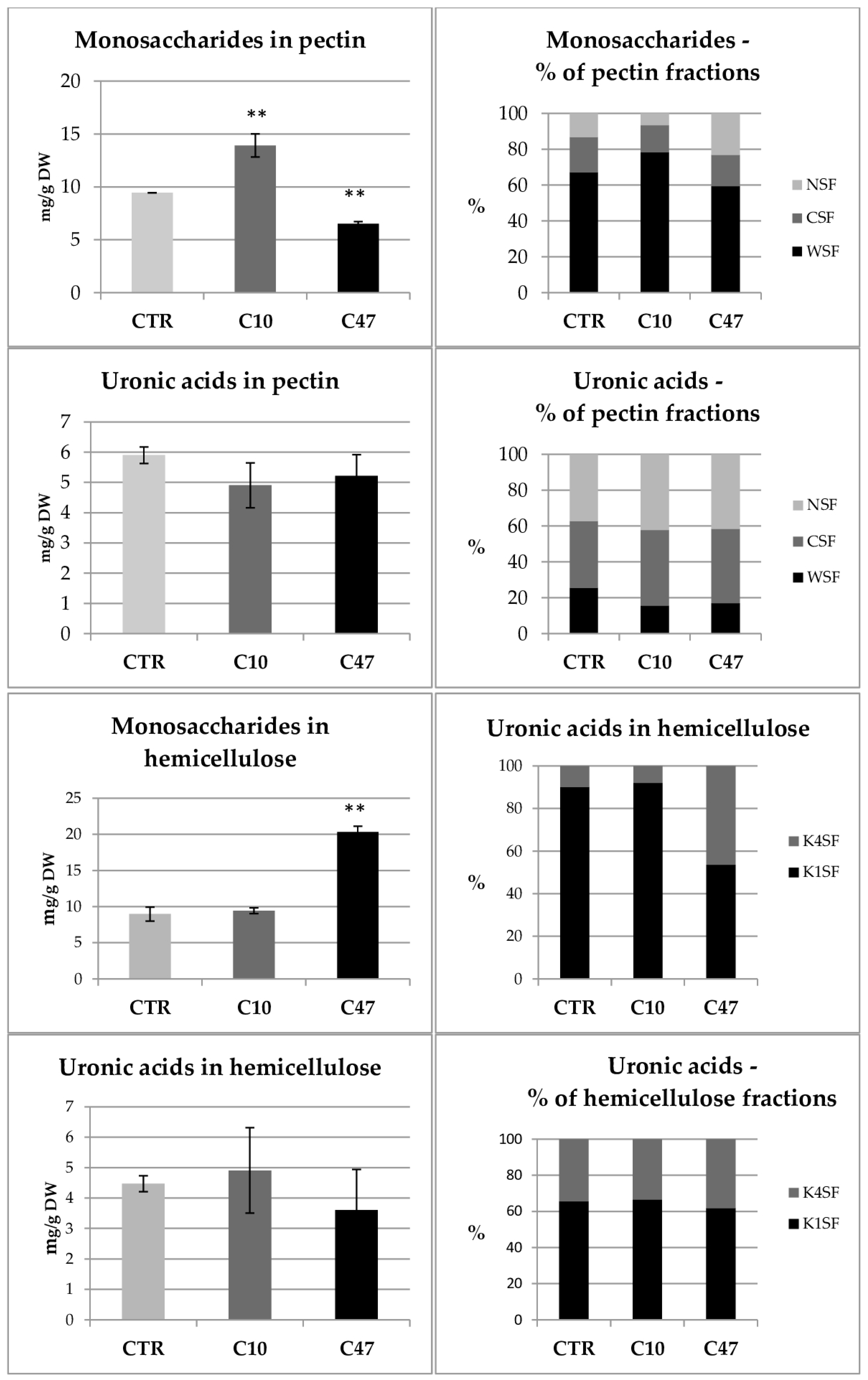
3.10. Content of Cell Wall Components in Flax Fibres
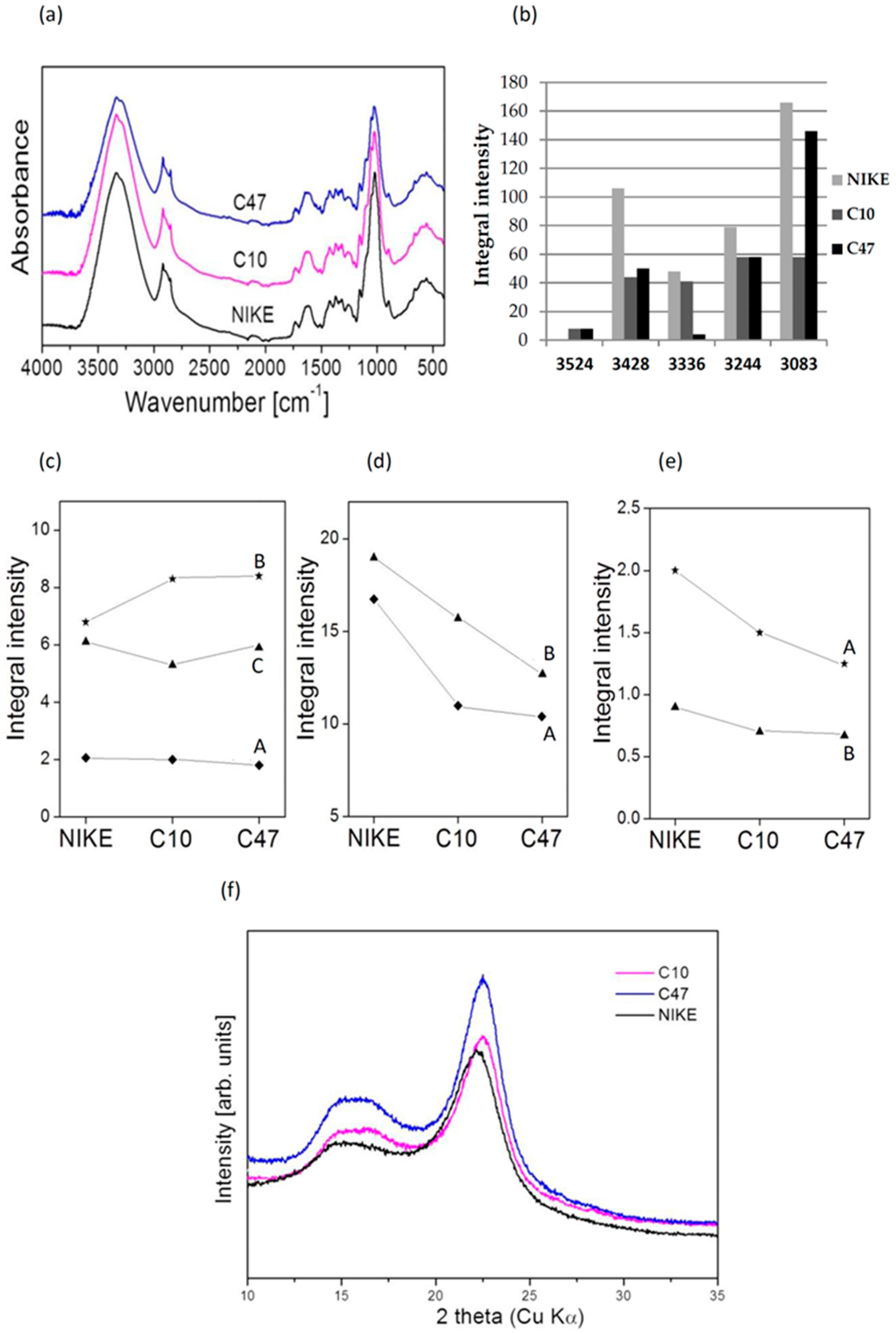
3.11. The IR Analysis of Flax Fibres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preisner, M.; Wojtasik, W.; Kulma, A.; Żuk, M.; Szopa, J. Flax Fiber. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Dyminska, L.; Szatkowski, M.; Wrobel-Kwiatkowska, M.; Zuk, M.; Kurzawa, A.; Syska, W.; Gagor, A.; Zawadzki, M.; Ptak, M.; Maczka, M.; et al. Improved Properties of Micronized Genetically Modified Flax Fibers. J. Biotechnol. 2012, 164, 292–299. [Google Scholar] [CrossRef]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-Glucosides Derived from Flax Straw Extracts Reduce Human Breast Cancer Cell Growth In Vitro and Induce Apoptosis. Front. Pharmacol. 2016, 7, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulma, A.; Skórkowska-Telichowska, K.; Kostyn, K.; Szatkowski, M.; Skała, J.; Drulis-Kawa, Z.; Preisner, M.; Żuk, M.; Szperlik, J.; Wang, Y.F.; et al. New Flax Producing Bioplastic Fibers for Medical Purposes. Ind. Crops Prod. 2015, 68, 80–89. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The Local Treatment and Available Dressings Designed for Chronic Wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K. Plant Cell Walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [Green Version]
- McMurry, J. Organic Chemistry; Brooks/Cole Cengage Learning: Boston, MA, USA, 2011; ISBN 978-0-8400-5453-1. [Google Scholar]
- Germain, V.; Rylott, E.L.; Larson, T.R.; Sherson, S.M.; Bechtold, N.; Carde, J.P.; Bryce, J.H.; Graham, I.A.; Smith, S.M. Requirement for 3-Ketoacyl-CoA Thiolase-2 in Peroxisome Development, Fatty Acid Beta-Oxidation and Breakdown of Triacylglycerol in Lipid Bodies of Arabidopsis Seedlings. Plant J. 2001, 28, 1–12. [Google Scholar] [CrossRef]
- Footitt, S.; Cornah, J.E.; Pracharoenwattana, I.; Bryce, J.H.; Smith, S.M. The Arabidopsis 3-Ketoacyl-CoA Thiolase-2 (Kat2-1) Mutant Exhibits Increased Flowering but Reduced Reproductive Success. J. Exp. Bot. 2007, 58, 2959–2968. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Song, Z.; Nikolau, B.J. Reverse Genetic Characterization of Two Paralogous Acetoacetyl CoA Thiolase Genes in Arabidopsis Reveals Their Importance in Plant Growth and Development. Plant J. 2012, 70, 1015–1032. [Google Scholar] [CrossRef]
- Wiszniewski, A.A.G.; Bussell, J.D.; Long, R.L.; Smith, S.M. Knockout of the Two Evolutionarily Conserved Peroxisomal 3-Ketoacyl-CoA Thiolases in Arabidopsis Recapitulates the Abnormal Inflorescence Meristem 1 Phenotype. J. Exp. Bot. 2014, 65, 6723–6733. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.C.; León, J. Expression of the Beta-Oxidation Gene 3-Ketoacyl-CoA Thiolase 2 (KAT2) Is Required for the Timely Onset of Natural and Dark-Induced Leaf Senescence in Arabidopsis. J. Exp. Bot. 2008, 59, 2171–2179. [Google Scholar] [CrossRef] [Green Version]
- Graham, I.A. Seed Storage Oil Mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Graham, I.A.; Holdsworth, M.; Smith, S.M.; Theodoulou, F.L. Chewing the Fat: Beta-Oxidation in Signalling and Development. Trends Plant Sci. 2006, 11, 124–132. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Koo, A.J.K.; Howe, G.A. Functional Diversification of Acyl-Coenzyme A Oxidases in Jasmonic Acid Biosynthesis and Action. Plant Physiol. 2007, 143, 812–824. Available online: https://pubmed.ncbi.nlm.nih.gov/17172287/ (accessed on 17 February 2023). [CrossRef] [Green Version]
- Chia, T.Y.P.; Pike, M.J.; Rawsthorne, S. Storage Oil Breakdown during Embryo Development of Brassica napus (L.). J. Exp. Bot. 2005, 56, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Cruz Castillo, M.; Martínez, C.; Buchala, A.; Métraux, J.-P.; León, J. Gene-Specific Involvement of Beta-Oxidation in Wound-Activated Responses in Arabidopsis. Plant Physiol. 2004, 135, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Afitlhile, M.M.; Fukushige, H.; Nishimura, M.; Hildebrand, D.F. A Defect in Glyoxysomal Fatty Acid Beta-Oxidation Reduces Jasmonic Acid Accumulation in Arabidopsis. Plant Physiol. Biochem. 2005, 43, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, X.-F.; Wang, X.-F.; Zhang, D.-P. Arabidopsis 3-Ketoacyl-CoA Thiolase-2 (KAT2), an Enzyme of Fatty Acid β-Oxidation, Is Involved in ABA Signal Transduction. Plant Cell Physiol. 2011, 52, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Bussell, J.D.; Reichelt, M.; Wiszniewski, A.A.G.; Gershenzon, J.; Smith, S.M. Peroxisomal ATP-Binding Cassette Transporter COMATOSE and the Multifunctional Protein Abnormal INFLORESCENCE MERISTEM Are Required for the Production of Benzoylated Metabolites in Arabidopsis Seeds. Plant Physiol. 2014, 164, 48–54. [Google Scholar] [CrossRef]
- Van Moerkercke, A.; Schauvinhold, I.; Pichersky, E.; Haring, M.A.; Schuurink, R.C. A Plant Thiolase Involved in Benzoic Acid Biosynthesis and Volatile Benzenoid Production. Plant J. 2009, 60, 292–302. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Han, J.; Ahn, C.; Park, E.; Choi, Y. Exploring Genes Involved in Benzoic Acid Biosynthesis in the Populus Davidiana Transcriptome and Their Transcriptional Activity upon Methyl Jasmonate Treatment. J. Chem. Ecol. 2017, 43, 1097–1108. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kostyn, A.; Wojtasik, W.; Dziadas, M.; Preisner, M.; Szopa, J.; Kulma, A. Methyl Salicylate Level Increase in Flax after Fusarium Oxysporum Infection Is Associated with Phenylpropanoid Pathway Activation. Front. Plant Sci. 2016, 7, 1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, G.; Stritzler, M.; Lisi, C.; Alleva, K.; Pagano, M.; Ardila, F.; Mozzicafreddo, M.; Cuccioloni, M.; Angeletti, M.; Ayub, N. Acetoacetyl-CoA Thiolase Regulates the Mevalonate Pathway during Abiotic Stress Adaptation. J. Exp. Bot. 2011, 62, 5699–5711. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones Inhibit Mitochondrial Production of Reactive Oxygen Species Production Following Glutamate Excitotoxicity by Increasing NADH Oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kulma, A.; Dziadas, M.; Kostyn, K.; Dymińska, L.; Hanuza, J.; Żuk, M.; Szopa, J. 3-Hydroxybutyrate Is Active Compound in Flax That Upregulates Genes Involved in DNA Methylation. Int. J. Mol. Sci. 2020, 21, 2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snell, K.D.; Peoples, O.P. PHA Bioplastic: A Value-Added Coproduct for Biomass Biorefineries. Biofuels Bioprod. Biorefining 2009, 3, 456–467. [Google Scholar] [CrossRef]
- Wróbel, M.; Zebrowski, J.; Szopa, J. Polyhydroxybutyrate Synthesis in Transgenic Flax. J. Biotechnol. 2004, 107, 41–54. [Google Scholar] [CrossRef]
- Wrobel-Kwiatkowska, M.; Zebrowski, J.; Starzycki, M.; Oszmianski, J.; Szopa, J. Engineering of PHB Synthesis Causes Improved Elastic Properties of Flax Fibers. Biotechnol. Prog. 2007, 23, 269–277. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Żuk, M.; Szopa, J.; Dymińska, L.; Mączka, M.; Hanuza, J. Poly-3-Hydroxy Butyric Acid Interaction with the Transgenic Flax Fibers: FT-IR and Raman Spectra of the Composite Extracted from a GM Flax. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 286–294. [Google Scholar] [CrossRef]
- Gredes, T.; Kunert-Keil, C.; Dominiak, M.; Gedrange, T.; Wróbel-Kwiatkowska, M.; Szopa, J. The Influence of Biocomposites Containing Genetically Modified Flax Fibers on Gene Expression in Rat Skeletal Muscle. Biomed. Tech. 2010, 55, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gredes, T.; Wróbel-Kwiatkowska, M.; Dominiak, M.; Gedrange, T.; Kunert-Keil, C. Osteogenic Capacity of Transgenic Flax Scaffolds. Biomed. Tech. 2012, 57, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gebarowski, T.; Gebczak, K.; Wiatrak, B.; Kulma, A.; Pelc, K.; Czuj, T.; Szopa, J.; Gasiorowski, K. Flax oil from transgenic linum usitatissimum selectively inhibits in vitro proliferation of human cancer cell lines. Acta Pol. Pharm. 2017, 74, 653–659. [Google Scholar] [PubMed]
- Skórkowska-Telichowska, K.; Kulma, A.; Gębarowski, T.; Wojtasik, W.; Kostyn, K.; Moreira, H.; Szyjka, A.; Boba, A.; Preisner, M.; Mierziak, J.; et al. V79 Fibroblasts Are Protected Against Reactive Oxygen Species by Flax Fabric. Appl. Biochem. Biotechnol. 2018, 184, 366–385. [Google Scholar] [CrossRef]
- Tsuda, H. Generation of Poly-β-Hydroxybutyrate from Externally Provided Acetate in Rice Root. Plant Physiol. Biochem. PPB 2012, 50, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bohmert, K.; Balbo, I.; Kopka, J.; Mittendorf, V.; Nawrath, C.; Poirier, Y.; Tischendorf, G.; Trethewey, R.N.; Willmitzer, L. Transgenic Arabidopsis Plants Can Accumulate Polyhydroxybutyrate to up to 4% of Their Fresh Weight. Planta 2000, 211, 841–845. [Google Scholar] [CrossRef]
- Mierziak, J.; Wojtasik, W.; Kostyn, K.; Czuj, T.; Szopa, J.; Kulma, A. Crossbreeding of Transgenic Flax Plants Overproducing Flavonoids and Glucosyltransferase Results in Progeny with Improved Antifungal and Antioxidative Properties. Mol. Breed. 2014, 34, 1917–1932. [Google Scholar] [CrossRef] [Green Version]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber Dimensions, Lignin and Cellulose Content of Various Plant Materials and Their Suitability for Paper Production. Ind. Crop Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Iiyama, K.; Wallis, A.F.A. Determination of Lignin in Herbaceous Plants by an Improved Acetyl Bromide Procedure. J. Sci. Food Agric. 1990, 51, 145–161. [Google Scholar] [CrossRef]
- Wojtasik, W.; Kulma, A.; Dymińska, L.; Hanuza, J.; Żebrowski, J.; Szopa, J. Fibres from Flax Overproducing β-1,3-Glucanase Show Increased Accumulation of Pectin and Phenolics and Thus Higher Antioxidant Capacity. BMC Biotechnol. 2013, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Baley, C. Analysis of the Flax Fibres Tensile Behaviour and Analysis of the Tensile Stiffness Increase. Compos. Part A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kozak, B.; Wojtasik, W.; Preisner, M.; Prescha, A.; Gola, E.M.; Lysh, D.; Dudek, B.; Szopa, J.; et al. Fusarium Oxysporum Infection Activates the Plastidial Branch of the Terpenoid Biosynthesis Pathway in Flax, Leading to Increased ABA Synthesis. Planta 2020, 251, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, C.-M.; Vasile, C.; Popescu, M.-C.; Singurel, G.; Popa, V.; Munteanu, B. Analytical Methods for Lignin Characterization. II. Spectroscopic Studies. Cellul. Chem. Technol. 2006, 40, 597–621. [Google Scholar]
- Dymińska, L.; Gągor, A.; Hanuza, J.; Kulma, A.; Preisner, M.; Żuk, M.; Szatkowski, M.; Szopa, J. Spectroscopic Characterization of Genetically Modified Flax Fibers. J. Mol. Struct. 2014, 1074, 321–329. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; Available online: https://www.wiley.com/en-us/Infrared+and+Raman+Characteristic+Group+Frequencies%3A+Tables+and+Charts%2C+3rd+Edition-p-9780470093078 (accessed on 17 February 2023).
- Wojtkowiak, B.; Chabanel, M. Spectrochimie Moleculaire; Technique et Documentation; PWN: Warszawa, Poland, 1984; Chapter 4; p. 11. [Google Scholar]
- Bikales, N.M.; Segal, L. Cellulose and Cellulose Derivatives; Willey: New York, NY, USA, 1971; Volume 1. [Google Scholar]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part II. A New Infrared Ratio for Estimation of Crystallinity in Celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Żuk, M.; Kulma, A.; Bugajska-Prusak, A.; Ratajczak, K.; Gąsiorowski, K.; Kostyn, K.; Szopa, J. New Dressing Materials Derived from Transgenic Flax Products to Treat Long-Standing Venous Ulcers—A Pilot Study. Wound Repair Regen. 2010, 18, 168–179. [Google Scholar] [CrossRef]
- Morvan, C.; Andème-Onzighi, C.; Girault, R.; Himmelsbach, D.S.; Driouich, A.; Akin, D.E. Building Flax Fibres: More than One Brick in the Walls. Plant Physiol. Biochem. 2003, 41, 935–944. [Google Scholar] [CrossRef]
- Musialak, M.; Wróbel-Kwiatkowska, M.; Kulma, A.; Starzycka, E.; Szopa, J. Improving Retting of Fibre through Genetic Modification of Flax to Express Pectinases. Transgenic Res. 2008, 17, 133–147. [Google Scholar] [CrossRef]
- Akin, D.E. Linen Most Useful: Perspectives on Structure, Chemistry, and Enzymes for Retting Flax. ISRN Biotechnol. 2013, 2013, 186534. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; Lorenzo, G.D. Oligogalacturonides: Plant Damage-Associated Molecular Patterns and Regulators of Growth and Development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaumie, S.; Goffner, D.; Barbier, O.; Martinant, J.P.; Pichon, M.; Barrière, Y. Expression of Cell Wall Related Genes in Basal and Ear Internodes of Silking Brown-Midrib-3, Caffeic Acid O-Methyltransferase (COMT) down-Regulated, and Normal Maize Plants. BMC Plant Biol. 2008, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, W.; Zhao, Y.; Xiang, Y.; Jiang, H.; Zhu, S.; Cheng, B. Downregulation of Caffeoyl-CoA O-Methyltransferase (CCoAOMT) by RNA Interference Leads to Reduced Lignin Production in Maize Straw. Genet. Mol. Biol. 2013, 36, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wróbel-Kwiatkowska, M.; Skórkowska-Telichowska, K.; Dymińska, L.; Mączka, M.; Hanuza, J.; Szopa, J. Biochemical, Mechanical, and Spectroscopic Analyses of Genetically Engineered Flax Fibers Producing Bioplastic (Poly-β-Hydroxybutyrate). Biotechnol. Prog. 2009, 25, 1489–1498. [Google Scholar] [CrossRef]
- Carrie, C.; Murcha, M.W.; Millar, A.H.; Smith, S.M.; Whelan, J. Nine 3-Ketoacyl-CoA Thiolases (KATs) and Acetoacetyl-CoA Thiolases (ACATs) Encoded by Five Genes in Arabidopsis Thaliana Are Targeted Either to Peroxisomes or Cytosol but Not to Mitochondria. Plant Mol. Biol. 2007, 63, 97–108. [Google Scholar] [CrossRef]
- Stracke, R.; Favory, J.-J.; Gruber, H.; Bartelniewoehner, L.; Bartels, S.; Binkert, M.; Funk, M.; Weisshaar, B.; Ulm, R. The Arabidopsis BZIP Transcription Factor HY5 Regulates Expression of the PFG1/MYB12 Gene in Response to Light and Ultraviolet-B Radiation. Plant Cell Environ. 2010, 33, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential Regulation of Closely Related R2R3-MYB Transcription Factors Controls Flavonol Accumulation in Different Parts of the Arabidopsis Thaliana Seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef] [Green Version]
- Wróbel-Kwiatkowska, M.; Czemplik, M.; Kulma, A.; Zuk, M.; Kaczmar, J.; Dymińska, L.; Hanuza, J.; Ptak, M.; Szopa, J. New Biocomposites Based on Bioplastic Flax Fibers and Biodegradable Polymers. Biotechnol. Prog. 2012, 28, 1336–1346. [Google Scholar] [CrossRef]
- Buitink, J.; Leger, J.J.; Guisle, I.; Vu, B.L.; Wuillème, S.; Lamirault, G.; Le Bars, A.; Le Meur, N.; Becker, A.; Küster, H.; et al. Transcriptome Profiling Uncovers Metabolic and Regulatory Processes Occurring during the Transition from Desiccation-Sensitive to Desiccation-Tolerant Stages in Medicago Truncatula Seeds. Plant J. 2006, 47, 735–750. [Google Scholar] [CrossRef]
- Borecký, J.; Vercesi, A.E. Plant Uncoupling Mitochondrial Protein and Alternative Oxidase: Energy Metabolism and Stress. Biosci. Rep. 2005, 25, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Koskimäki, J.J.; Kajula, M.; Hokkanen, J.; Ihantola, E.L.; Kim, J.H.; Hautajärvi, H.; Hankala, E.; Suokas, M.; Pohjanen, J.; Podolich, O.; et al. Methyl-Esterified 3-Hydroxybutyrate Oligomers Protect Bacteria from Hydroxyl Radicals. Nat. Chem. Biol. 2016, 12, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Sacomboio, E.N.M.; Kim, E.Y.S.; Correa, H.L.R.; Bonato, P.; Pedrosa, F.O.; de Souza, E.M.; Chubatsu, L.S.; Müller-Santos, M. The Transcriptional Regulator NtrC Controls Glucose-6-Phosphate Dehydrogenase Expression and Polyhydroxybutyrate Synthesis through NADPH Availability in Herbaspirillum Seropedicae. Sci. Rep. 2017, 7, 13546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What Is Stress? Concepts, Definitions and Applications in Seed Science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-Hydroxybutyrate as a Metabolite and a Signal Molecule Regulating Processes of Living Organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierziak, J.; Wojtasik, W.; Kulma, A.; Żuk, M.; Grajzer, M.; Boba, A.; Dymińska, L.; Hanuza, J.; Szperlik, J.; Szopa, J. Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties. Metabolites 2023, 13, 437. https://doi.org/10.3390/metabo13030437
Mierziak J, Wojtasik W, Kulma A, Żuk M, Grajzer M, Boba A, Dymińska L, Hanuza J, Szperlik J, Szopa J. Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties. Metabolites. 2023; 13(3):437. https://doi.org/10.3390/metabo13030437
Chicago/Turabian StyleMierziak, Justyna, Wioleta Wojtasik, Anna Kulma, Magdalena Żuk, Magdalena Grajzer, Aleksandra Boba, Lucyna Dymińska, Jerzy Hanuza, Jakub Szperlik, and Jan Szopa. 2023. "Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties" Metabolites 13, no. 3: 437. https://doi.org/10.3390/metabo13030437
APA StyleMierziak, J., Wojtasik, W., Kulma, A., Żuk, M., Grajzer, M., Boba, A., Dymińska, L., Hanuza, J., Szperlik, J., & Szopa, J. (2023). Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties. Metabolites, 13(3), 437. https://doi.org/10.3390/metabo13030437





