The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment
Abstract
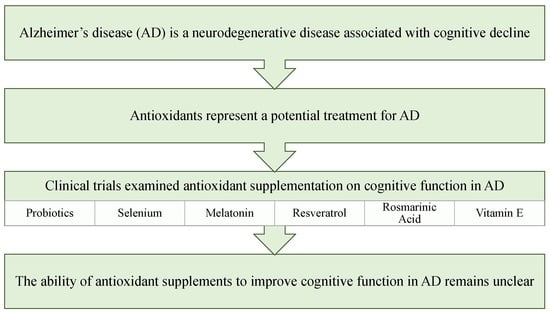
:1. Introduction
2. Probiotics
3. Selenium
4. Melatonin
5. Resveratrol
6. Rosmarinic Acid
7. Carotenoids
8. Curcumin
9. Vitamin E
10. Coenzyme Q
11. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cao, Q.; Tan, C.C.; Xu, W.; Hu, H.; Cao, X.P.; Dong, Q.; Tan, L.; Yu, J.T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimer Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Bacigalupo, I.; Mayer, F.; Lacorte, E.; Di Pucchio, A.; Marzolini, F.; Canevelli, M.; Di Fiandra, T.; Vanacore, N. A Systematic Review and Meta-Analysis on the Prevalence of Dementia in Europe: Estimates from the Highest-Quality Studies Adopting the DSM IV Diagnostic Criteria. J. Alzheimer Dis. 2018, 66, 1471–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, X.; He, R.; Liang, R.; Zhou, L. Measuring the Caregiver Burden of Caring for Community-Residing People with Alzheimer’s Disease. PLoS ONE 2015, 10, e0132168. [Google Scholar] [CrossRef] [Green Version]
- Vu, M.; Mangal, R.; Stead, T.; Lopez-Ortiz, C.; Ganti, L. Impact of Alzheimer’s Disease on Caregivers in the United States. Health Psychol. Res. 2022, 10, 37454. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimer Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxid. Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, F.; Adam, R.H.I.; Bansal, R.; Broersen, K. A Review of Oxidative Stress Products and Related Genes in Early Alzheimer’s Disease. J. Alzheimer Dis. 2021, 83, 977–1001. [Google Scholar] [CrossRef] [PubMed]
- Gustaw-Rothenberg, K.; Kowalczuk, K.; Stryjecka-Zimmer, M. Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 2010, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, H.W.; Wang, W.; Lang, M. Copper Toxicity Links to Pathogenesis of Alzheimer’s Disease and Therapeutics Approaches. Int. J. Mol. Sci. 2020, 21, 7660. [Google Scholar] [CrossRef]
- Ganguly, U.; Kaur, U.; Chakrabarti, S.S.; Sharma, P.; Agrawal, B.K.; Saso, L.; Chakrabarti, S. Oxidative Stress, Neuroinflammation, and NADPH Oxidase: Implications in the Pathogenesis and Treatment of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 7086512. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I. The essential elements of Alzheimer’s disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox. Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Dou, K.X.; Tan, M.S.; Tan, C.C.; Cao, X.P.; Hou, X.H.; Guo, Q.H.; Tan, L.; Mok, V.; Yu, J.T. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimer Res. Ther. 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Grossberg, G.T.; Tong, G.; Burke, A.D.; Tariot, P.N. Present Algorithms and Future Treatments for Alzheimer’s Disease. J. Alzheimer Dis. 2019, 67, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.; Lima, J.; Marchiori, M.F.; Carvalho, I.; Sakamoto-Hojo, E.T. Neuroprotective Effects of Cholinesterase Inhibitors: Current Scenario in Therapies for Alzheimer’s Disease and Future Perspectives. J. Alzheimer Dis. Rep. 2022, 6, 177–193. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia—An Explorative Intervention Study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra, E.O.T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [Green Version]
- Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; Brodtmann, A.; Collins, S.; et al. A Phase IIa Randomized Control Trial of VEL015 (Sodium Selenate) in Mild-Moderate Alzheimer’s Disease. J. Alzheimer Dis. 2016, 54, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; et al. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer Dement. 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Zhao, J.; Wang, L. Effect of Resveratrol Combined with Donepezil Hydrochloride on Inflammatory Factor Level and Cognitive Function Level of Patients with Alzheimer’s Disease. J. Healthc. Eng. 2022, 2022, 9148650. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Samuraki-Yokohama, M.; Iwasa, K.; Yokoyama, K.; Nakamura, H.; et al. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Loskutova, E.; Howard, A.; Mulcahy, R.; Moran, R.; Stack, J.; Bolger, M.; Coen, R.F.; Dennison, J.; Akuffo, K.O.; et al. The impact of supplemental macular carotenoids in Alzheimer’s disease: A randomized clinical trial. J. Alzheimer Dis. 2015, 44, 1157–1169. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimer Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation With Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Arlt, S.; Müller-Thomsen, T.; Beisiegel, U.; Kontush, A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochem. Res. 2012, 37, 2706–2714. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A Phase II Randomized Clinical Trial of a Nutritional Formulation for Cognition and Mood in Alzheimer’s Disease. J. Alzheimer Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations, World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation: Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001, [and] Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April–1 May 2002; Food and Agriculture Organization of the United Nations, World Health Organization: Rome, Italy, 2006.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Douglas, L.C.; Sanders, M.E. Probiotics and Prebiotics in Dietetics Practice. J. Am. Diet. Assoc. 2008, 108, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chang, C.C.; Huang, C.W.; Nouchi, R.; Cheng, C.H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Lekchand Dasriya, V.; Samtiya, M.; Dhewa, T.; Puniya, M.; Kumar, S.; Ranveer, S.; Chaudhary, V.; Vij, S.; Behare, P.; Singh, N.; et al. Etiology and management of Alzheimer’s disease: Potential role of gut microbiota modulation with probiotics supplementation. J. Food Biochem. 2022, 46, e14043. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Mehrabadi, S.; Sadr, S.S. Assessment of Probiotics Mixture on Memory Function, Inflammation Markers, and Oxidative Stress in an Alzheimer’s Disease Model of Rats. Iran Biomed. J. 2020, 24, 220–228. [Google Scholar] [CrossRef]
- OMNi-BiOTiC® STRESS Repair—Stressed? Do Something about It! Available online: https://www.omni-biotic.com/en/products/omni-biotic-stress-repair/ (accessed on 3 March 2023).
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Song, G.L. Roles of Selenoproteins in Brain Function and the Potential Mechanism of Selenium in Alzheimer’s Disease. Front Neurosci. 2021, 15, 646518. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Bukke, S.; Dutt, N.; Rana, P.; Pandey, A.K. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer’s disease: A metal meta-analysis (AMMA study-I). J. Trace Elem. Med. Biol. 2017, 42, 68–75. [Google Scholar] [CrossRef]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef]
- Rita Cardoso, B.; Apolinário, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bansal, M.P.; Sandhir, R. Altered dietary selenium influences brain iron content and behavioural outcomes. Behav. Brain. Res. 2019, 372, 112011. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Damulina, A.; Pirpamer, L.; Soellradl, M.; Sackl, M.; Tinauer, C.; Hofer, E.; Enzinger, C.; Gesierich, B.; Duering, M.; Ropele, S.; et al. Cross-sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease Using 3-T MRI. Radiology 2020, 296, 619–626. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nous, A.; Engelborghs, S.; Smolders, I. Melatonin levels in the Alzheimer’s disease continuum: A systematic review. Alzheimer Res. Ther. 2021, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Homolak, J.; Mudrovčić, M.; Vukić, B.; Toljan, K. Circadian Rhythm and Alzheimer’s Disease. Med. Sci. 2018, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Aldhous, M.; Franey, C.; Wright, J.; Arendt, J. Plasma concentrations of melatonin in man following oral absorption of different preparations. Br. J. Clin. Pharmacol. 1985, 19, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. Melatonin and Sleep. Open Neuroendocrinol. J. 2010, 3, 85–95. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Mozzoni, M.; Antoniello, N.; Cazzola, R.; Savarè, R.; Cerutti, R.; Grossi, E.; Cestaro, B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012, 15, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Aguilar, M.A.; Ramírez-Salado, I.; Cruz-Ulloa, C.; Benítez-King, G.A. Melatonin effects on macro sleep architecture in Alzheimer ‘ s disease patients. Salud Ment. 2013, 36, 243–449. [Google Scholar]
- Cruz-Aguilar, M.A.; Ramírez-Salado, I.; Guevara, M.A.; Hernández-González, M.; Benitez-King, G. Melatonin Effects on EEG Activity During Sleep Onset in Mild-to-Moderate Alzheimer’s Disease: A Pilot Study. J. Alzheimer Dis. Rep. 2018, 2, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Singer, C.; Tractenberg, R.E.; Kaye, J.; Schafer, K.; Gamst, A.; Grundman, M.; Thomas, R.; Thal, L.J. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep 2003, 26, 893–901. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef]
- Solís-Chagoyán, H.; Domínguez-Alonso, A.; Valdés-Tovar, M.; Argueta, J.; Sánchez-Florentino, Z.A.; Calixto, E.; Benítez-King, G. Melatonin Rescues the Dendrite Collapse Induced by the Pro-Oxidant Toxin Okadaic Acid in Organotypic Cultures of Rat Hilar Hippocampus. Molecules 2020, 25, 5508. [Google Scholar] [CrossRef]
- Yao, K.; Zhao, Y.F.; Zu, H.B. Melatonin receptor stimulation by agomelatine prevents Aβ-induced tau phosphorylation and oxidative damage in PC12 cells. Drug Des. Devel. Ther. 2019, 13, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Effect of melatonin on neuroinflammation and acetylcholinesterase activity induced by LPS in rat brain. Eur. J. Pharmacol. 2010, 640, 206–210. [Google Scholar] [CrossRef]
- Roy, J.; Tsui, K.C.; Ng, J.; Fung, M.L.; Lim, L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 6841. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol derivatives as potential treatments for Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Grinan-Ferre, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drygalski, K.; Fereniec, E.; Koryciński, K.; Chomentowski, A.; Kiełczewska, A.; Odrzygóźdź, C.; Modzelewska, B. Resveratrol and Alzheimer’s disease. Mol. Pathophysiol. Clin. Trials Exp. Gerontol. 2018, 113, 36–47. [Google Scholar] [CrossRef]
- Ma, T.; Tan, M.-S.; Yu, J.-T.; Tan, L. Resveratrol as a Therapeutic Agent for Alzheimer’s Disease. BioMed Res. Int. 2014, 2014, 350516. [Google Scholar] [CrossRef] [Green Version]
- Tosatti, J.A.G.; Fontes, A.; Caramelli, P.; Gomes, K.B. Effects of Resveratrol Supplementation on the Cognitive Function of Patients with Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. Drugs Aging 2022, 39, 285–295. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem. Int. 2018, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effects of rosmarinic acid on nervous system disorders: An updated review. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Hassanzadeh-Taheri, M.; Ahmadi-Zohan, A.; Mohammadifard, M.; Hosseini, M. Rosmarinic acid attenuates lipopolysaccharide-induced neuroinflammation and cognitive impairment in rats. J. Chem. Neuroanat. 2021, 117, 102008. [Google Scholar] [CrossRef]
- Kola, A.; Hecel, A.; Lamponi, S.; Valensin, D. Novel Perspective on Alzheimer’s Disease Treatment: Rosmarinic Acid Molecular Interplay with Copper(II) and Amyloid β. Life 2020, 10, 118. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Mirza, F.J.; Amber, S.; Sumera; Hassan, D.; Ahmed, T.; Zahid, S. Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ(1-42)-induced mouse model of Alzheimer’s disease. Phytomedicine 2021, 83, 153490. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Rosmarinic acid attenuates obesity and obesity-related inflammation in human adipocytes. Food Chem. Toxicol. 2021, 149, 112002. [Google Scholar] [CrossRef] [PubMed]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Ibarra, A.; Feuillère, N.; Roller, M.; Sukkar, S.G. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Mediterr. J. Nutr. Metab. 2011, 4, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, M.; Firoozabadi, A.; Salehi, A.; Ghorbanifar, Z.; Zarshenas, M.M.; Sadeghniiat-Haghighi, K.; Rezaeizadeh, H. Effects of Herbal combination (Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse) on insomnia severity, anxiety and depression in insomniacs: Randomized placebo controlled trial. Integr. Med. Res. 2018, 7, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, K.A.; Nieman, K.M.; Sanoshy, K.D.; Fonseca, B.A.; Lasrado, J.A.; Schild, A.L.; Maki, K.C.; Wesnes, K.A.; Ceddia, M.A. Spearmint Extract Improves Working Memory in Men and Women with Age-Associated Memory Impairment. J. Altern. Complement. Med. 2018, 24, 37–47. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal el, S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-Kott, A.F.; et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef]
- Bhatti, A.B.; Usman, M.; Ali, F.; Satti, S.A. Vitamin Supplementation as an Adjuvant Treatment for Alzheimer’s Disease. J. Clin. Diagn. Res. 2016, 10, OE07–OE11. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, F.; Tanimukai, S.; Sasaki, M.; Ikejima, C.; Yamashita, F.; Kodama, C.; Mizukami, K.; Asada, T. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimer Dis. 2012, 32, 895–903. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 631–638. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimer Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef] [Green Version]
- Polidori, M.C.; Nelles, G. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease—Challenges and perspectives. Curr. Pharm. Des. 2014, 20, 3083–3092. [Google Scholar] [CrossRef]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.P.; Nyunt, M.S.Z.; Gao, Q.; Gwee, X.; Chua, D.Q.L.; Yap, K.B. Curcumin-Rich Curry Consumption and Neurocognitive Function from 4.5-Year Follow-Up of Community-Dwelling Older Adults (Singapore Longitudinal Ageing Study). Nutrients 2022, 14, 1189. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Chen, M.; Du, Z.Y.; Zheng, X.; Li, D.L.; Zhou, R.P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; van Amelsvoort, T.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free. Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaaboul, F.; Liu, Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 964–998. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimer Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Page, R.; Morrell, C.; Shea, T.B. Maintenance of Cognitive Performance and Mood for Individuals with Alzheimer’s Disease Following Consumption of a Nutraceutical Formulation: A One-Year, Open-Label Study. J. Alzheimer Dis. 2016, 51, 991–995. [Google Scholar] [CrossRef]
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimer Dement. Transl. Res. Clin. Interv. 2016, 2, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Viña, J. Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, N.F.; Yanagisawa, D.; Durani, L.W.; Hamezah, H.S.; Damanhuri, H.A.; Wan Ngah, W.Z.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. Tocotrienol-Rich Fraction Modulates Amyloid Pathology and Improves Cognitive Function in AβPP/PS1 Mice. J. Alzheimer Dis. 2017, 55, 597–612. [Google Scholar] [CrossRef] [Green Version]
- Desrumaux, C.; Pisoni, A.; Meunier, J.; Deckert, V.; Athias, A.; Perrier, V.; Villard, V.; Lagrost, L.; Verdier, J.M.; Maurice, T. Increased amyloid-β peptide-induced memory deficits in phospholipid transfer protein (PLTP) gene knockout mice. Neuropsychopharmacology 2013, 38, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, A.; Samadi, M.; Shirvani, H.; Sepandi, M.; Tahmasebi, W. Coenzyme Q10 Supplementation and Oxidative Stress Parameters: An Updated Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Asian J. Sports Med. 2022, 13, e131308. [Google Scholar] [CrossRef]
- Akbari, A.; Mobini, G.R.; Agah, S.; Morvaridzadeh, M.; Omidi, A.; Potter, E.; Fazelian, S.; Ardehali, S.H.; Daneshzad, E.; Dehghani, S. Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 2020, 76, 1483–1499. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Flori, L.; Cicero, A.F.G.; Colletti, A. Coenzyme Q(10): Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10, Ageing and the Nervous System: An Overview. Antioxidants 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Coenzyme Q10 and Dementia: A Systematic Review. Antioxidants 2023, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- University Hospital, Geneva. Dynamic Response to Probiotics in Context of Alzheimer Disease: A Proof-of-Concept Study. Available online: https://ClinicalTrials.gov/show/NCT05521477 (accessed on 10 January 2023).
- Preventive Medicine Research Institute; University of California, San Francisco; Harvard Medical School; University of California, San Diago; The Cleveland Clinic; Renown Health. Can Lifestyle Changes Reverse Early-Stage Alzheimer’s Disease. Available online: https://ClinicalTrials.gov/show/NCT04606420 (accessed on 10 January 2023).
- Hsieh-Hsun, H. Effect of Probiotics in Alzheimer’s Disease. Available online: https://ClinicalTrials.gov/show/NCT05145881 (accessed on 10 January 2023).
- Natalie, D. Disease Modifying Potential of 5mg of Melatonin on Cognition and Brain Health in Aging. Available online: https://ClinicalTrials.gov/show/NCT03954899 (accessed on 10 January 2023).
- India Globalization Capital Inc. IGC-AD1 Trial on Agitation in Dementia Due to Alzheimer’s. Available online: https://ClinicalTrials.gov/show/NCT05543681 (accessed on 10 January 2023).
- Venturelli, M.; University of Perugia; INCLIVA; Molde University College; University of Liverpool; Molecular Horizon S.r.l.; Nestlé Italiana, S.p.A. Chocolate and Physical Exercise to Reduce Malnutrition in Pre-dementia Aged People. Available online: https://ClinicalTrials.gov/show/NCT05343611 (accessed on 10 January 2023).

| Authors | Year Published | Location | Participants | Intervention | Duration of Treatment Period | Main Results | Trial Numbers |
|---|---|---|---|---|---|---|---|
| Akarbi et al. [40] | 2016 | Iran | AD | 200 mL/day of milk either with (n = 30) or without (n = 30) probiotic supplementation (2 × 109 CFU/g of Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum, and Bifidobacterium bifidum). | 12 weeks | Compared to the control group, the probiotic group experienced a significant improvement in MMSE scores, a decline in hs-CRP, MDA, HOMA-IR, HOMA-B, and increased QUICKI. | IRCT201511305623N60 c |
| Agahi et al. [41] | 2018 | Iran | AD | Placebo (n = 30) or a probiotic (n = 30) consisting of two capsules (3 × 109 each of either Lactobacillus fermentum, Lactobacillus plantarum, and Bifidobacterium lactis or Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum) every other day. | 12 weeks | Either no or negligible significant differences were reported for indicators of cognitive function (TYM), oxidative stress (TAC, MDA, NO, total glutathione, and 8-hydroxy-2′-deoxyguanosine), and inflammation (TNF-α, IL-6, and IL-10). | IRCT2017061534549N1 c |
| Leblhuber et al. [42] | 2018 | Austria | AD | 3 g of a probiotic supplement containing Lactobacillus casei, Lactococcus lactis, Lactobacillus acidophilus, Bifidobacterium lactis, Lactobacillus paracasei, Lactobacillus plantarum, Bifidobacterium lactis, Bifidobacterium bifidum, and Lactobacillus salivarius (n = 20) | 4 weeks | No significant changes in cognitive function (MMSE, clock drawing test). Fecal Faecalibacterium prausnitzii RNA increased, with no change in Clostridium cluster I or Akkermansia muciniphila RNA. Fecal zonulin significantly declined, and serum kynurenine increased. | |
| Ton et al. [43] | 2020 | Brazil | Probable AD | 2 mL/kg/day kefir-fermented milk containing Acetobacter aceti, Acetobacter sp., Candida famata, Candida krusei, Enterococcus faecium, Lactobacillus delbrueckii delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Lactobacillus kefiranofaciens, and Leuconostoc spp. (n = 16). | 90 days | Participants experienced a significant improvement in MMSE scores, inflammation (TNF-α, IL-8, and IL12p70), and oxidative stress (ROS, advanced oxidative protein products, and NO). | |
| Malpas et al. [44] | 2016 | Australia | Mild-to-moderate AD | Supranutrional (10 mg sodium selenate; n = 20), nutritional (0.32 mg sodium selenate; n = 10), or a placebo (n = 10) three times a day. | 24 weeks | Supplementation with supranutritional selenate was tolerable and safe. No significant changes in AD-related CSF biomarkers (phosphorylated tau, t-tau, and Aβ1–42) or cognitive function (MMSE, ADAS-Cog, COWAT, CFT, OCL, IDN, and DET). | ACTRN12611001200976 d |
| Cardoso et al. [45] a | 2019 | Australia | Mild-to-moderate AD | Supranutrional (10 mg sodium selenate; n = 20), nutritional (0.32 mg sodium selenate; n = 10), or a placebo (n = 10) three times a day. | 24 weeks | There was no difference in the number of drug-related adverse events between the responsive and non-responsive groups. Compared to non-responsive individuals, participants in the responsive group did not experience a significant decline in MMSE. No other measures of cognitive function (ADAS-Cog, COWAT, CFT, OCL, IDN, and DET) were significant. | ACTRN12611001200976 d |
| Tamtaji et al. [46] | 2019 | Iran | Institutionalized individuals with AD | 200 μg selenium with (n = 30) and without (n = 30) a probiotic (2 × 109 CFU of Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum) supplement or a placebo (n = 30) daily. | 12 weeks | The selenium-only group experienced a significant improvement in markers of inflammation (hs-CRP), oxidative stress (glutathione), and insulin resistance (insulin, HOMA-IR, and QUICKI) compared to the placebo group. Compared to the placebo and selenium-only groups, the combination group experienced a significant improvement in cognitive function (MMSE), inflammation (hs-CRP), oxidative stress (TAC and glutathione), and insulin resistance (insulin, HOMA-IR, and QUICKI). | IRCT20170612034497N5 c |
| Wade et al. [47] | 2014 | United Kingdom, United States | Mild-to-moderate AD | 2 mg prolonged-release melatonin supplement (n = 39) or a placebo (n = 34) daily. | 24 weeks | Supplementation with prolonged-release melatonin was tolerable and safe. The melatonin group experienced an improvement in overall sleep quality (PSQI), less decline in cognitive function (MMSE), and an improvement in IADL. No changes were observed for ADAS-Cog. | |
| Turner et al. [48] | 2015 | United States | Mild-to-moderate AD | Resveratrol (n = 62) or placebo (n = 55) daily, starting at a 500 mg dose and increasing by 500 mg every 13 weeks. | 52 weeks | Supplementation with resveratrol was safe and tolerable. Compared to the placebo group, the resveratrol group experienced less decline in CSF, plasma Aβ40, and ADCS-ADL after 52 weeks. No changes were observed for AD-related biomarkers (CSF Aβ42, plasma Aβ42, CSF tau, and CSF p-tau), cognitive function (ADAS-Cog, CDR-sum of boxes, MMSE, and NPI), or plasma glucose and insulin metabolism. | NCT01504854 e |
| Moussa et al. [49] b | 2017 | United States | Mild-to-moderate AD | Resveratrol (n = 62) or placebo (n = 55) daily, starting at a 500 mg dose and increasing by 500 mg every 13 weeks. | 52 weeks | ADCS-ADL and MMSE scores significantly declined in the placebo group. CSF Aβ40 declined significantly in the resveratrol group. Compared to the placebo group, the resveratrol group experienced significantly less decline in CSF Aβ42 and greater increase in MMP9. | NCT01504854 e |
| Zhu et al. [50] | 2018 | United States | Mild-to-moderate AD | A supplement containing 5 mg resveratrol, 5 g dextrose, and 5 g malate (n = 17) or a placebo (n = 15). | 1 year | No significant differences between groups for ADAS-Cog, ADCS-ADL, ADCS-CGIC, MMSE, or NPI were observed. | NCT00678431 e |
| Fang et al. [51] | 2022 | China | Hospitalized individuals with AD | Donepezil hydrochloride with (n = 45) or without (n = 45) 1 g or 2 g of resveratrol daily. | 2 months | The resveratrol group had significantly improved cognitive function (MMSE and ADAS-Cog), living ability (FIM), and biomarkers of inflammation (IL-6 and TNF-α). | |
| Noguchi-Shinohara et al. [52] | 2020 | Japan | Mild AD | Melissa officinalis extract containing 500 mg of rosmarinic acid (n = 12) or a placebo (n = 11). | 24 weeks | Supplementation with Melissa officinalis was safe and tolerable. Compared to the placebo group, the Melissa officinalis group experienced a significant improvement in NPI. No significant changes were observed for cognitive function (MMSE, ADAS-Cog, and CDR), functional ability (DAD), or CSF AD-related biomarkers (Aβ1–42, tau, and p-tau). | UMIN000007734 f |
| Nolan et al. [53] | 2015 | Ireland | Mild-to-moderate AD Healthy controls | Four groups: AD and carotenoid (n = 16; 10 mg lutein, 2 mg zeaxanthin, 10 mg meso-zeaxanthin); AD and placebo (n = 15); healthy control and carotenoid (n = 15); healthy control and placebo (n = 16). | 6 months | Both groups receiving the carotenoid supplement experienced an increase in serum lutein, zeaxanthin, and meso-zeaxanthin levels. No significant changes were observed for cognitive function (MMSE). | |
| Nolan et al. [54] | 2018 | Ireland | AD | Carotenoid supplement (n = 12; 10 mg lutein, 2 mg zeaxanthin, 10 mg meso-zeaxanthin) or the carotenoid supplement in combination with a fish oil supplement (n = 13; 430 mg DHA, 90 mg EPA) | 18 months | Compared to the carotenoid-only group, the combined intervention group experienced less functional decline based on medical observations and a greater increase in serum lutein and meso-zeaxanthin levels. | |
| Nolan et al. [55] | 2022 | Ireland | Mild-to-moderate AD | Supplement with 10 mg lutein, 10 mg meso-zeaxanthin, 2 mg zeaxanthin, 500 mg DHA, 150 mg EPA, and 15 mg α-tocopherol (n = 50) or a placebo (n = 27) | 12 months | Compared to the placebo group, the intervention group experienced a significant increase in serum nutrient levels (lutein, meso-zeaxanthin, zeaxanthin, DHA, EPA, and vitamin E). No significant changes were observed for cognitive function (MMSE and DSRS). Categorizing participants by dementia severity (DSRS), a significant improvement was observed in the intervention group, while the placebo group experienced a significant decline. | ISRCTN11892249 g |
| Ringman et al. [56] | 2012 | United States | Mild-to-moderate AD | RCT: 2 g curcumin (n = 12), 4 g curcumin (n = 12), or a placebo (n = 12) daily. Open-label: the placebo group was randomized to either 2 g or 4 g of curcumin. | 24 week RCT followed by 24 week open-label period. | No significant between-group differences for measures of cognitive function (MMSE, ADAS-Cog, ADCS-ADL, and NPI), AD biomarkers (plasma Aβ40 and Aβ42; CSF Aβ42, t-tau, and p-tau), or isoprostanes. | NCT00099710 e |
| Arlt et al. [57] | 2012 | Germany | Mild-to-moderate AD | Standard care (n = 11) or supplementation (n = 12) with 400 IU vitamin E and 1000 mg vitamin C daily. | 1 year | No significant difference between groups for cognitive function (MMSE, immediate and delayed word recall, word fluency, trail-making test). The intervention group experienced a significant increase in CSF α-tocopherol and ascorbate and a significant decrease in the rate of CSF oxidation. | |
| Galasko et al. [58] | 2012 | United States | Mild-to-moderate AD | A supplement containing 800 IU vitamin E, 500 mg vitamin C, and 900 mg α-lipoic acid (n = 26); 400 mg coenzyme Q (n = 26) three times a day; or a placebo (n = 26). | 16 weeks | Compared to the other groups, the combined antioxidant group experienced a significant decline in cognitive function (MMSE) and oxidative stress (F2-isoprostane). No significant between-group differences emerged for ADAS-ADL or CSF biomarkers (Aβ42, t-tau, or p-tau). | NCT00117403 e |
| Dysken et al. [59] | 2014 | United States | Mild-to-moderate AD | 2000 IU vitamin E (n = 152), 20 mg memantine (n = 155), vitamin E and memantine (n = 154), or placebo (n = 152) daily. | 6 months to 4 years | Supplementation with vitamin E was safe and tolerable. Compared to the placebo group, participants receiving vitamin E alone experienced significantly less decline in ADCS-ADL scores. No significant differences were detected for MMSE, ADAS-Cog, NPI, or Dependence Scale. | NCT00235716 e |
| Remington et al. [60] | 2015 | United States | Individuals with AD | A supplement containing 400 μg folic acid, 30 IU α-tocopherol, 6 μg vitamin B12, 400 mg S-adenosyl methionine, 600 mg N-acetyl cysteine, and 500 mg acetyl-L-carnitine (n = 86) or placebo (n = 57) twice daily. | 3 to 6 months | After three months, the intervention group had a significant improvement in cognitive function (Dementia Rating Scale), but no significant changes in NPI or ADCS-ADL scores. | NCT01320527 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knight, E.; Geetha, T.; Broderick, T.L.; Babu, J.R. The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment. Metabolites 2023, 13, 438. https://doi.org/10.3390/metabo13030438
Knight E, Geetha T, Broderick TL, Babu JR. The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment. Metabolites. 2023; 13(3):438. https://doi.org/10.3390/metabo13030438
Chicago/Turabian StyleKnight, Emily, Thangiah Geetha, Tom L. Broderick, and Jeganathan Ramesh Babu. 2023. "The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment" Metabolites 13, no. 3: 438. https://doi.org/10.3390/metabo13030438
APA StyleKnight, E., Geetha, T., Broderick, T. L., & Babu, J. R. (2023). The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment. Metabolites, 13(3), 438. https://doi.org/10.3390/metabo13030438