Evaluation of GC/MS-Based 13C-Positional Approaches for TMS Derivatives of Organic and Amino Acids and Application to Plant 13C-Labeled Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Positional Identification of Carbon Backbones from Mass Fragments of TMS Derivatives
2.2. Analysis of Carbon Isotopologue Distributions (CID) from 13C-PT Standards and Plant 13C-Labeled Experiments
2.3. Calculations of 13C-Positional Enrichments
M13C(Serine)C3 = (2 × M13C(Serine)C2C3) − M13C(Serine)C2
M13C(Serine)C1C2C3 = M13C(Serine)C1 + M13C(Serine)C2 + M13C(Serine)C3
M13C(Serine)C3 = (3 × M13C(Serine)C1C2C3) − (2 × M13C(Serine)C2C3)
M13C(Serine)C1 = (3 × M13C(Serine)C1C2C3) − (M13C(Serine)C1 + M13C(Serine)C1C2)
M13C(Malate)C4 = (3 × M13C(Malate)C2C3C4) − (2 × M13C(Malate)C2C3)
M13C(Malate)C3 = (3 × M13C(Malate)C2C3C4) − (M13C(Malate)C2 + M13C(Malate)C4)
M13C(Malate)C3 = (4 × M13C(Malate)C1C2C3C4) − (M13C(Malate)C1 + M13C(Malate)C2 + M13C(Malate)C4)
M13C(Malate)C3 = (2 × M13C(Malate)C3C4) − (M13C(Malate)C4)
2.4. Data Management, Visualization and Statistical Analysis
3. Results
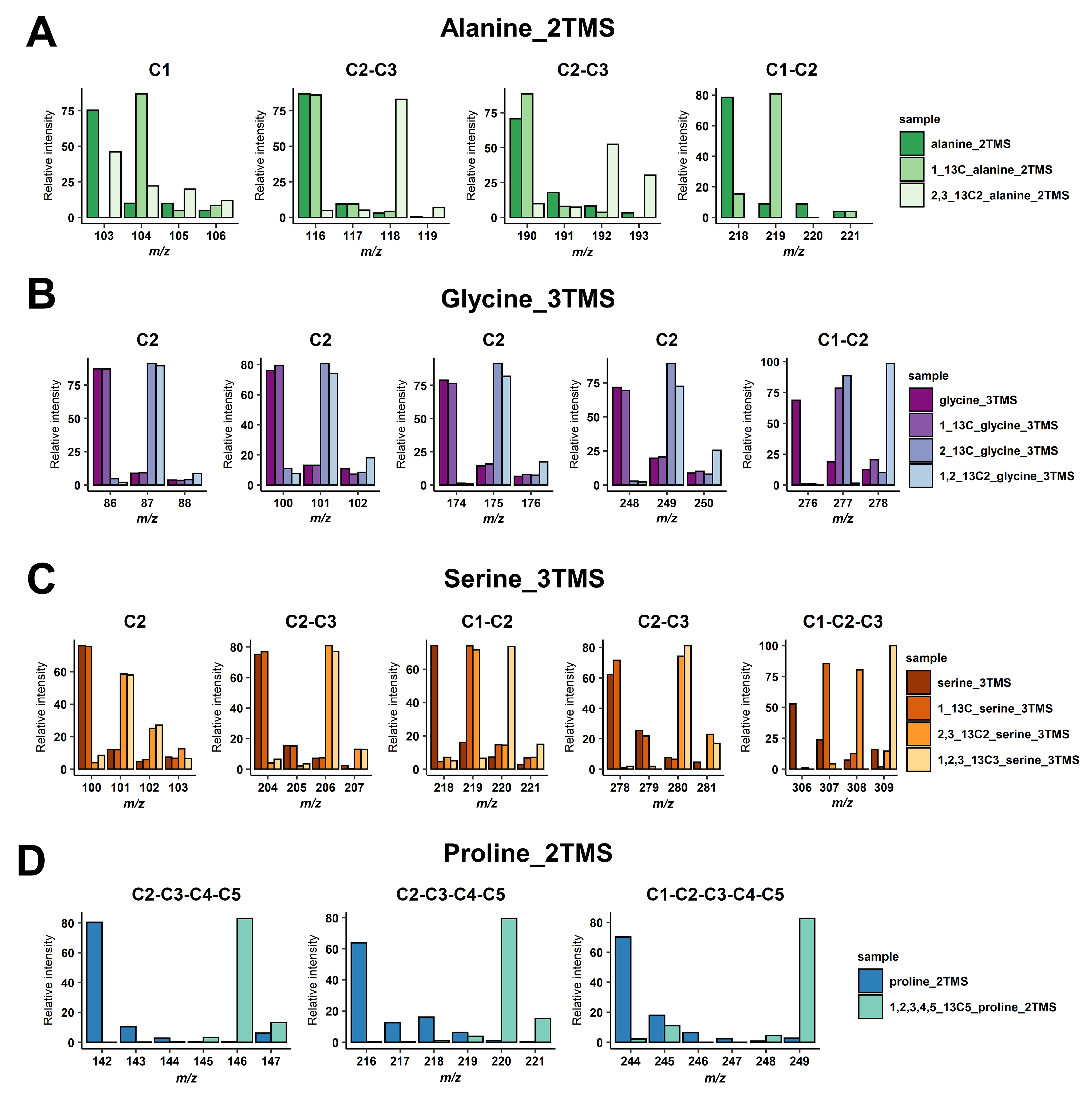
3.1. Selection of Mass Fragments
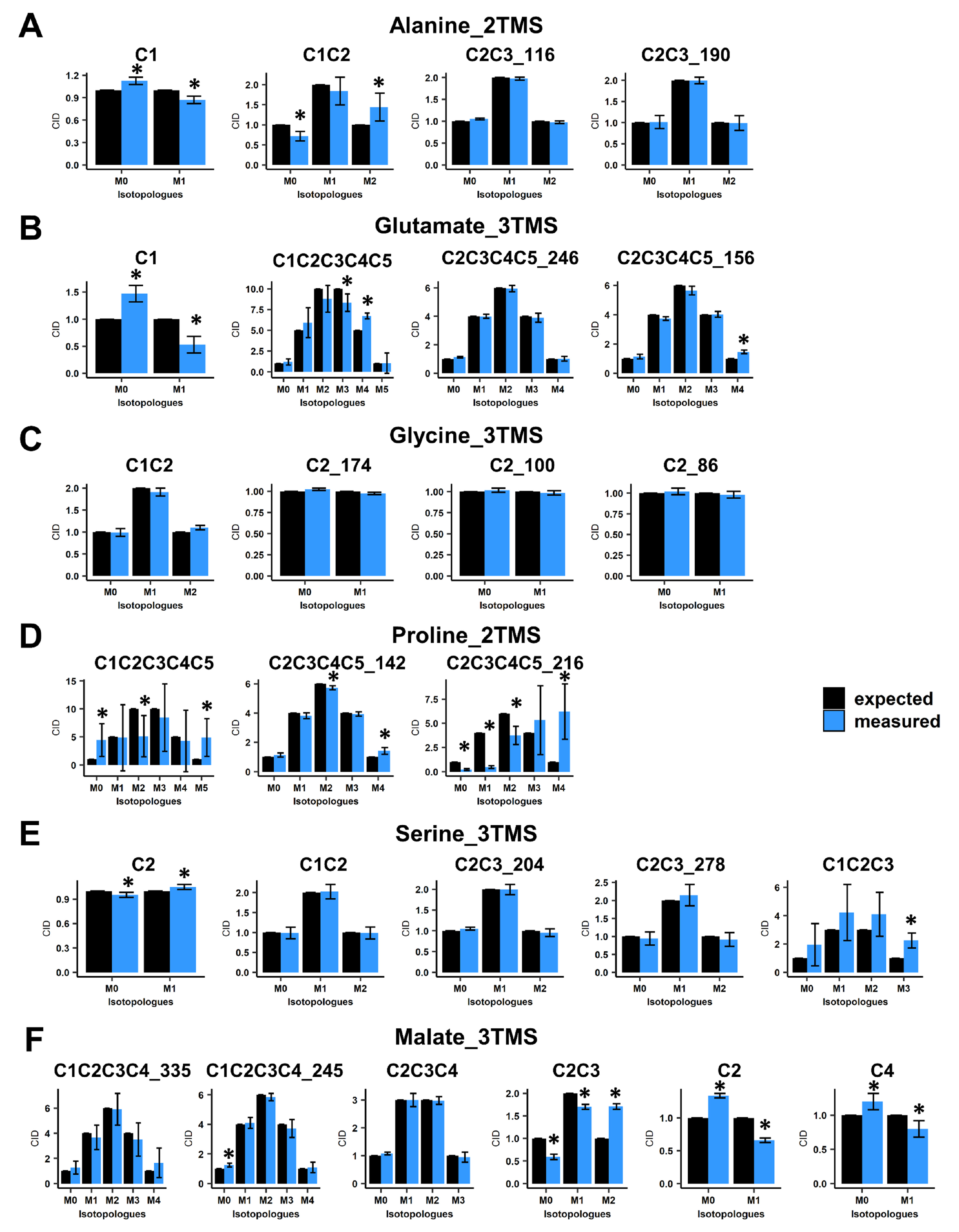
3.2. Validation of GC/MS Based 13C-Positional Approaches
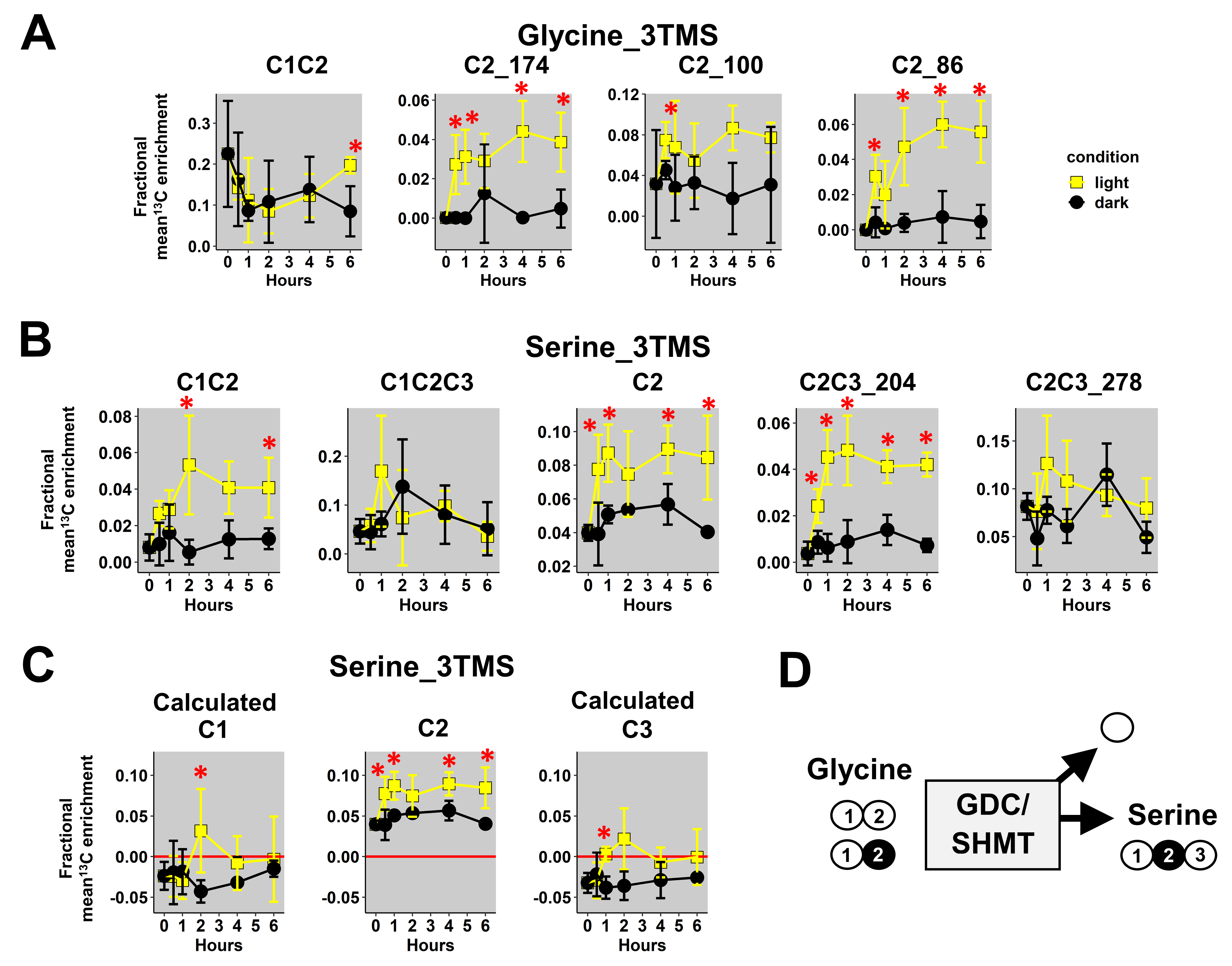
3.3. Application to Plant 13C-Labeled Experiments
4. Discussion
4.1. Identification of Mass Fragment-Specific Analytical Biases and Consequences for GC/MS-Based 13C-Positional Approaches
4.2. A GC/MS-Based 13C-Positional Approach Suitable for Investigating the Metabolic Fluxes Associated to Photorespiration, TCA Cycle and PEPc
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef]
- Dellero, Y. Manipulating Amino Acid Metabolism to Improve Crop Nitrogen Use Efficiency for a Sustainable Agriculture. Front. Plant Sci. 2020, 11, 602548. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Chen, Q.; Havé, M. Regulation of nutrient recycling via autophagy. Curr. Opin. Plant Biol. 2017, 39, 8–17. [Google Scholar] [CrossRef]
- Clark, T.J.; Guo, L.; Morgan, J.; Schwender, J. Modeling Plant Metabolism: From Network Reconstruction to Mechanistic Models. Annu. Rev. Plant Biol. 2020, 71, 303–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Have, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar]
- Nelson, C.; Alexova, R.; Jacoby, R.P.; Millar, A.H. Proteins with High Turnover Rate in Barley Leaves Estimated by Proteome Analysis Combined with in Planta Isotope Labeling. Plant Physiol. 2014, 166, 91–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadie, C.; Bathellier, C.; Tcherkez, G. Carbon allocation to major metabolites in illuminated leaves is not just proportional to photosynthesis when gaseous conditions (CO2and O2) vary. New Phytol. 2018, 218, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Dellero, Y.; Jossier, M.; Glab, N.; Oury, C.; Tcherkez, G.; Hodges, M. Decreased glycolate oxidase activity leads to altered carbon allocation and leaf senescence after a transfer from high CO2 to ambient air in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 3149–3163. [Google Scholar] [CrossRef] [Green Version]
- Daloso, D.M.; Müller, K.; Obata, T.; Florian, A.; Tohge, T.; Bottcher, A.; Riondet, C.; Bariat, L.; Carrari, F.; Nunes-Nesi, A.; et al. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, E1392–E1400. [Google Scholar] [CrossRef] [Green Version]
- Dellero, Y.; Heuillet, M.; Marnet, N.; Bellvert, F.; Millard, P.; Bouchereau, A. Sink/Source Balance of Leaves Influences Amino Acid Pools and Their Associated Metabolic Fluxes in Winter Oilseed Rape (Brassica napus L.). Metabolites 2020, 10, 150. [Google Scholar] [CrossRef]
- Chu, K.L.; Koley, S.; Jenkins, L.M.; Bailey, S.R.; Kambhampati, S.; Foley, K.; Arp, J.J.; Morley, S.A.; Czymmek, K.J.; Bates, P.D.; et al. Metabolic flux analysis of the non-transitory starch tradeoff for lipid production in mature tobacco leaves. Metab. Eng. 2021, 69, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Daloso, D.M.; Antunes, W.C.; Pinheiro, D.P.; Waquim, J.P.; Araújo, W.L.; Loureiro, M.E.; Fernie, A.R.; Williams, T.C.R. Tobacco guard cells fix CO2by both Rubisco and PEPcase while sucrose acts as a substrate during light-induced stomatal opening. Plant Cell Environ. 2015, 38, 2353–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dethloff, F.; Orf, I.; Kopka, J. Rapid in situ 13C tracing of sucrose utilization in Arabidopsis sink and source leaves. Plant Methods 2017, 13, 87. [Google Scholar] [CrossRef] [Green Version]
- Dellero, Y.; Clouet, V.; Marnet, N.; Pellizzaro, A.; Dechaumet, S.; Niogret, M.-F.; Bouchereau, A. Leaf status and environmental signals jointly regulate proline metabolism in winter oilseed rape. J. Exp. Bot. 2019, 71, 2098–2111. [Google Scholar] [CrossRef]
- Abadie, C.; Lothier, J.; Boex-Fontvieille, E.; Carroll, A.; Tcherkez, G. Direct assessment of the metabolic origin of carbon atoms in glutamate from illuminated leaves using 13 C- NMR. New Phytol. 2017, 216, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Buescher, J.M.; Antoniewicz, M.R.; Boros, L.G.; Burgess, S.C.; Brunengraber, H.; Clish, C.B.; DeBerardinis, R.J.; Feron, O.; Frezza, C.; Ghesquiere, B.; et al. A roadmap for interpreting 13 C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 2015, 34, 189–201. [Google Scholar] [CrossRef]
- Schulze, D.; Kohlstedt, M.; Becker, J.; Cahoreau, E.; Peyriga, L.; Makowka, A.; Hildebrandt, S.; Gutekunst, K.; Portais, J.C.; Wittmann, C. Gc/ms-based (13)c metabolic flux analysis resolves the parallel and cyclic photomixotrophic metabolism of synechocystis sp. Pcc 6803 and selected deletion mutants including the entner-doudoroff and phosphoketolase pathways. Microb. Cell Fact. 2022, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Masakapalli, S.K.; Le Lay, P.; Huddleston, J.E.; Pollock, N.L.; Kruger, N.J.; Ratcliffe, R.G. Subcellular Flux Analysis of Central Metabolism in a Heterotrophic Arabidopsis Cell Suspension Using Steady-State Stable Isotope Labeling. Plant Physiol. 2009, 152, 602–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadie, C.; Tcherkez, G. In vivo phosphoenolpyruvate carboxylase activity is controlled by CO2 and O2 mole fractions and represents a major flux at high photorespiration rates. New Phytol. 2019, 221, 1843–1852. [Google Scholar] [CrossRef]
- Tcherkez, G.; Mahé, A.; Gauthier, P.; Mauve, C.; Gout, E.; Bligny, R.; Cornic, G.; Hodges, M. In Folio Respiratory Fluxomics Revealed by 13C Isotopic Labeling and H/D Isotope Effects Highlight the Noncyclic Nature of the Tricarboxylic Acid “Cycle” in Illuminated Leaves. Plant Physiol. 2009, 151, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalvin, C.; Drevensek, S.; Gilard, F.; Mauve, C.; Chollet, C.; Morin, H.; Nicol, E.; Héripré, E.; Kriegshauser, L.; Gakière, B.; et al. Sclareol and linalyl acetate are produced by glandular trichomes through the MEP pathway. Hortic. Res. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Ratcliffe, R.G.; Shachar-Hill, Y. Measuring multiple fluxes through plant metabolic networks. Plant J. 2006, 45, 490–511. [Google Scholar] [CrossRef] [PubMed]
- Rosiers, C.D.; Lloyd, S.; Comte, B.; Chatham, J. A critical perspective of the use of 13C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab. Eng. 2003, 6, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.B.; Souza, L.P.; Antunes, W.C.; Araújo, W.L.; Daloso, D.M.; Fernie, A.R. Sucrose breakdown within guard cells provides substrates for glycolysis and glutamine biosynthesis during light-induced stomatal opening. Plant J. 2018, 94, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, V.F.; de Souza, L.P.; Williams, T.C.R.; Fernie, A.R.; Daloso, D.M. Gas Chromatography–Mass Spectrometry-Based 13C-Labeling Studies in Plant Metabolomics. Plant Metab. Methods Protoc. 2018, 1778, 47–58. [Google Scholar] [CrossRef]
- Dellero, Y.; Berardocco, S.; Berges, C.; Filangi, O.; Bouchereau, A. Validation of carbon isotopologue distribution measurements by GC-MS and application to 13C-metabolic flux analysis of the tricarboxylic acid cycle in Brassica napus leaves. Front. Plant Sci. 2023, 13, 885051. [Google Scholar] [CrossRef]
- Allen, D.K.; Young, J.D. Carbon and Nitrogen Provisions Alter the Metabolic Flux in Developing Soybean Embryos. Plant Physiol. 2013, 161, 1458–1475. [Google Scholar] [CrossRef] [Green Version]
- Schwender, J.; Shachar-Hill, Y.; Ohlrogge, J.B. Mitochondrial Metabolism in Developing Embryos of Brassica napus. J. Biol. Chem. 2006, 281, 34040–34047. [Google Scholar] [CrossRef] [Green Version]
- Lima, V.F.; Erban, A.; Daubermann, A.G.; Freire, F.B.S.; Porto, N.P.; Candido-Sobrinho, S.A.; Medeiros, D.B.; Schwarzlander, M.; Fernie, A.R.; Dos Anjos, L.; et al. Establishment of a gc-ms-based (13) c-positional isotopomer approach suitable for investigating metabolic fluxes in plant primary metabolism. Plant J. 2021, 108, 21. [Google Scholar] [CrossRef]
- Choi, J.; Grossbach, M.T.; Antoniewicz, M.R. Measuring Complete Isotopomer Distribution of Aspartate Using Gas Chromatography/Tandem Mass Spectrometry. Anal. Chem. 2012, 84, 4628–4632. [Google Scholar] [CrossRef]
- Okahashi, N.; Kawana, S.; Iida, J.; Shimizu, H.; Matsuda, F. Fragmentation of Dicarboxylic and Tricarboxylic Acids in the Krebs Cycle Using GC-EI-MS and GC-EI-MS/MS. Mass Spectrom. 2019, 8, A0073. [Google Scholar] [CrossRef] [Green Version]
- Harvey, D.J.; Vouros, P. Mass Spectrometric Fragmentation of Trimethylsilyl and Related Alkylsilyl Derivatives. Mass Spectrom. Rev. 2020, 39, 105–211. [Google Scholar] [CrossRef]
- Lai, Z.; Fiehn, O. Mass spectral fragmentation of trimethylsilylated small molecules. Mass Spectrom. Rev. 2016, 37, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Porto, N.P.; Bret, R.S.; Souza, P.V.; Cândido-Sobrinho, S.A.; Medeiros, D.B.; Fernie, A.R.; Daloso, D.M. Thioredoxins regulate the metabolic fluxes throughout the tricarboxylic acid cycle and associated pathways in a light-independent manner. Plant Physiol. Biochem. 2022, 193, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Heuillet, M.; Bellvert, F.; Cahoreau, E.; Letisse, F.; Millard, P.; Portais, J.-C. Methodology for the Validation of Isotopic Analyses by Mass Spectrometry in Stable-Isotope Labeling Experiments. Anal. Chem. 2017, 90, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Massou, S.; Portais, J.-C.; Létisse, F. Isotopic Studies of Metabolic Systems by Mass Spectrometry: Using Pascal’s Triangle To Produce Biological Standards with Fully Controlled Labeling Patterns. Anal. Chem. 2014, 86, 10288–10295. [Google Scholar] [CrossRef] [PubMed]
- Méret, M.; Kopetzki, D.; Degenkolbe, T.; Kleessen, S.; Nikoloski, Z.; Tellstroem, V.; Barsch, A.; Kopka, J.; Antonietti, M.; Willmitzer, L. From systems biology to systems chemistry: Metabolomic procedures enable insight into complex chemical reaction networks in water. RSC Adv. 2014, 4, 16777–16781. [Google Scholar] [CrossRef]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Dellero, Y.; Filangi, O. Corrective Method Dedicated to Isocor for Calculations of Positional 13c-Enrichments from GCMS Runs, V1 ed.; Recherche Data Gouv: Paris, France, 2023. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. Available online: https://dplyr.tidyverse.org/ (accessed on 20 February 2023).
- Wickham, H.; Girlich, M. Tidyr: Tidy Messy Data. Available online: https://github.com/tidyverse/tidyr (accessed on 20 February 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- RStudio Team. Rstudio: Integrated Development for r; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Tovar-Mendez, A.; Miernyk, J.A.; Randall, D.D. Regulation of pyruvate dehydrogenase complex activity in plant cells. JBIC J. Biol. Inorg. Chem. 2003, 270, 1043–1049. [Google Scholar] [CrossRef]
- Tcherkez, G.; Cornic, G.; Bligny, R.; Gout, E.; Ghashghaie, J. In Vivo Respiratory Metabolism of Illuminated Leaves. Plant Physiol. 2005, 138, 1596–1606. [Google Scholar] [CrossRef] [Green Version]
- Antoniewicz, M.R.; Kelleher, J.K.; Stephanopoulos, G. Accurate Assessment of Amino Acid Mass Isotopomer Distributions for Metabolic Flux Analysis. Anal. Chem. 2007, 79, 7554–7559. [Google Scholar] [CrossRef] [PubMed]
- Abadie, C.; Lalande, J.; Tcherkez, G. Exact mass GC-MS analysis: Protocol, database, advantages and application to plant metabolic profiling. Plant Cell Environ. 2022, 45, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Cukier, C.; Lea, P.J.; Canas, R.; Marmagne, A.; Limami, A.M.; Hirel, B. Labeling maize (zea mays l.) leaves with (15) nh4(+) and monitoring nitrogen incorporation into amino acids by gc/ms analysis. Curr. Protoc. Plant Biol. 2018, 3, e20073. [Google Scholar] [CrossRef] [PubMed]
- Szecowka, M.; Heise, R.; Tohge, T.; Nunes-Nesi, A.; Vosloh, D.; Huege, J.; Feil, R.; Lunn, J.; Nikoloski, Z.; Stitt, M.; et al. Metabolic Fluxes in an Illuminated Arabidopsis Rosette. Plant Cell 2013, 25, 694–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Gregory, L.M.; Weise, S.E.; Walker, B.J. Integrated flux and pool size analysis in plant central metabolism reveals unique roles of glycine and serine during photorespiration. Nat. Plants 2022, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Hagemann, M. Photorespiration—How is it regulated and regulates overall plant metabolism? J. Exp. Bot. 2020, 71, 3955–3965. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [Green Version]
- Abadie, C.; Tcherkez, G. 13C Isotope Labelling to Follow the Flux of Photorespiratory Intermediates. Plants 2021, 10, 427. [Google Scholar] [CrossRef]


| TMS Derivative | Loss | Fragment | |||
|---|---|---|---|---|---|
| Formula | Mass | Formula | m/z | Carbon Backbone | |
| Alanine(2TMS) | CH3 | 15 | C8H20NO2Si2 | 218 | 1-2 |
| CH3, CO | 43 | C7H20NOSi2 | 190 | 2-3 | |
| TMS-CO2 | 117 | C5H14NSi | 116 | 2-3 | |
| unknown | 130 | C4H11OSi | 103 | 1 | |
| Glutamate(3TMS) | CH3 | 15 | C13H30NO4Si3 | 348 | 1-2-3-4-5 |
| TMS-CO2 | 117 | C10H24NO2Si2 | 245 | 2-3-4-5 | |
| TMS-CO2, TMS-OH | 207 | C7H14NOSi | 156 | 2-3-4-5 | |
| TMS-NH-C3H5-CO2-TMS | 246 | C4H9O2Si | 117 | 1 | |
| Glycine(3TMS) | CH3 | 15 | C10H26NO2Si3 | 276 | 1-2 |
| CH3, CO | 43 | C9H26NOSi3 | 248 | 2 | |
| TMS-CO2, TMS-H | 191 | C4H10NSi | 100 | 2 | |
| TMS-CO2, TMS-CH3 | 205 | C3H8NSi | 86 | 2 | |
| Malate(3TMS) | CH3 | 15 | C12H27O5Si3 | 335 | 1-2-3-4 |
| C3HO3 | 85 | C10H29O2Si3 | 265 | 2 | |
| TMS-OH, CH3 | 90 | C9H17O4Si2 | 245 | 1-2-3-4 | |
| TMS-CO2 | 117 | C9H21O3Si2 | 233 | 2-3-4 | |
| TMS-CO2, CH3, COH | 161 | C7H17O2Si2 | 189 | 2-3 | |
| C9H21O3Si2 | 233 | TMS-CO2 | 117 | 4 | |
| Proline(2TMS) | CH3 | 15 | C10H22NO2Si | 244 | 1-2-3-4-5 |
| CH3, CO | 43 | C9H22NOSi | 216 | 2-3-4-5 | |
| TMS-CO2 | 117 | C7H16N | 142 | 2-3-4-5 | |
| Serine(3TMS) | CH3 | 15 | C11H28NO3Si3 | 306 | 1-2-3 |
| CH3, CO | 43 | C10H28NO2Si3 | 278 | 2-3 | |
| TMS-CH2O | 103 | C8H20NO2Si2 | 218 | 1-2 | |
| TMS-CO2 | 117 | C8H22NOSi2 | 204 | 2-3 | |
| unknown | 221 | C4H10NSi | 100 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellero, Y.; Filangi, O.; Bouchereau, A. Evaluation of GC/MS-Based 13C-Positional Approaches for TMS Derivatives of Organic and Amino Acids and Application to Plant 13C-Labeled Experiments. Metabolites 2023, 13, 466. https://doi.org/10.3390/metabo13040466
Dellero Y, Filangi O, Bouchereau A. Evaluation of GC/MS-Based 13C-Positional Approaches for TMS Derivatives of Organic and Amino Acids and Application to Plant 13C-Labeled Experiments. Metabolites. 2023; 13(4):466. https://doi.org/10.3390/metabo13040466
Chicago/Turabian StyleDellero, Younès, Olivier Filangi, and Alain Bouchereau. 2023. "Evaluation of GC/MS-Based 13C-Positional Approaches for TMS Derivatives of Organic and Amino Acids and Application to Plant 13C-Labeled Experiments" Metabolites 13, no. 4: 466. https://doi.org/10.3390/metabo13040466
APA StyleDellero, Y., Filangi, O., & Bouchereau, A. (2023). Evaluation of GC/MS-Based 13C-Positional Approaches for TMS Derivatives of Organic and Amino Acids and Application to Plant 13C-Labeled Experiments. Metabolites, 13(4), 466. https://doi.org/10.3390/metabo13040466








