Elimination of Cefquinome Sulfate Residue in Cow’s Milk after Intrauterine Infusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Sample Collection
2.3. Establishment of a UPLC-MS/MS Assay
2.3.1. UPLC Conditions
2.3.2. Mass Spectrometry Conditions
2.4. Sample Pretreatment
Extraction and Purification
2.5. Method Validation
3. Results and Discussion
3.1. Optimization of UPLC-MS/MS Analysis Conditions
3.2. Method Validation
3.3. Application to Real Samples
3.4. Withdrawal Period
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, L.; McMahon, K.J.; Gier, H.T.; Marion, G.B. Uterus of the cow after parturition: Bacterial content. Am. J. Vet. Res. 1968, 29, 77–81. [Google Scholar] [PubMed]
- Griffin, J.F.; Hartigan, P.J.; Nunn, W.R. Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology 1974, 1, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Borsberry, S.; Dobson, H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet. Rec. 1989, 124, 217–219. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef]
- Runciman, D.J.; Anderson, G.A.; Malmo, J. Comparison of two methods of detecting purulent vaginal discharge in postpartum dairy cows and effect of intrauterine cephapirin on reproductive performance. Aust. Vet. J. 2009, 87, 369–378. [Google Scholar] [CrossRef]
- Moore, K. Cluster headache: The challenge of clinical trials. Curr. Pain Headache Rep. 2002, 6, 52–56. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Rycroft, A.N.; Zhou, C. Association between postpartum pyrexia and uterine bacterial infection in dairy cattle. Vet. Rec. 2004, 154, 289–293. [Google Scholar] [CrossRef]
- Nehru, D.A.; Dhaliwal, G.S.; Jan, M.H.; Cheema, R.S.; Kumar, S. Clinical efficacy of intrauterine cephapirin benzathine administration on clearance of uterine bacteria and subclinical endometritis in postpartum buffaloes. Reprod. Domest. Anim. 2019, 54, 317–324. [Google Scholar] [CrossRef]
- Uney, K.; Altan, F.; Altan, S.; Erol, H.; Arican, M.; Elmas, M. Plasma and synovial fluid pharmacokinetics of cefquinome following the administration of multiple doses in horses. J. Vet. Pharmacol. Ther. 2017, 40, 239–247. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, X.; Yan, K.; Jiang, L.; Li, R.; Liu, Y.; Wang, M.; Wang, Z. PK/PD integration and pharmacodynamic cutoff of cefquinome against cow mastitis due to Escherichia coli. J. Vet. Pharmacol. Ther. 2022, 45, 83–91. [Google Scholar] [CrossRef]
- Limbert, M.; Isert, D.; Klesel, N.; Markus, A.; Seeger, K.; Seibert, G.; Schrinner, E. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 1991, 35, 14–19. [Google Scholar] [CrossRef]
- Qi, R.; Wang, J.; Ye, J.; Hao, H.; Bao, Y. The solubility of cefquinome sulfate in pure and mixed solvents. Front. Chem. Sci. Eng. 2016, 10, 245–254. [Google Scholar] [CrossRef]
- Yehia, A.M.; Arafa, R.M.; Abbas, S.S.; Amer, S.M. Ratio manipulating spectrophotometry versus chemometry as stability indicating methods for cefquinome sulfate determination. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2016, 153, 231–240. [Google Scholar] [CrossRef]
- Elghobashy, M.R.; Bebawy, L.I.; Abbas, S.S.; Shokry, R.F. Stability-Indicating HPLC and RP-TLC Determination of Cefpirome Sulfate with Kinetic Study. Chromatographia 2013, 76, 1141–1151. [Google Scholar] [CrossRef]
- Thomas, E.; Thomas, V.; Wilhelm, C. Antibacterial activity of cefquinome against equine bacterial pathogens. Vet. Microbiol. 2006, 115, 140–147. [Google Scholar] [CrossRef]
- Murphy, S.P.; Erwin, M.E.; Jones, R.N. Cefquinome (HR 111V). In vitro evaluation of a broad-spectrum cephalosporin indicated for infections in animals. Diagn. Microbiol. Infect. Dis. 1994, 20, 49–55. [Google Scholar] [CrossRef]
- Shpigel, N.Y.; Levin, D.; Winkler, M.; Saran, A.; Ziv, G.; Bottner, A. Efficacy of cefquinome for treatment of cows with mastitis experimentally induced using Escherichia coli. J. Dairy Sci. 1997, 80, 318–323. [Google Scholar] [CrossRef]
- Pengov, A. Treatment of clinical and subclinical mastitis cases with COBACTAN. Vet. Novice 2001, 27, 145–148. [Google Scholar]
- Zhang, B.-X.; Lu, X.-X.; Gu, X.-Y.; Li, X.-H.; Gu, M.-X.; Zhang, N.; Shen, X.-G.; Ding, H.-Z. Pharmacokinetics and ex vivo pharmacodynamics of cefquinome in porcine serum and tissue cage fluids. Vet. J. 2014, 199, 399–405. [Google Scholar] [CrossRef]
- Rohdich, N.; Zschiesche, E.; Heckeroth, A.; Wilhelm, C.; Leendertse, I.; Thomas, E. Treatment of septicaemia and severe bacterial infections in foals with a new cefquinome formulation: A field study. Dtsch. Tierarztl. Wochenschr. 2009, 116, 316–320. [Google Scholar] [PubMed]
- Widmer, A.; Kummer, M.; Eser, M.W.; Fuerst, A. Comparison of the clinical efficacy of cefquinome with the combination of penicillin G and gentamicin in equine patients. Eqyine Vet. Educ. 2009, 21, 430–435. [Google Scholar] [CrossRef]
- Guerin-Faublee, V.; Carret, G.; Houffschmitt, P. In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Vet. Rec. 2003, 152, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Amiridis, G.S.; Fthenakis, G.C.; Dafopoulos, J.; Papanikolaou, T.; Mavrogianni, V.S. Use of cefquinome for prevention and treatment of bovine endometritis. J. Vet. Pharmacol. Ther. 2003, 26, 387–390. [Google Scholar] [CrossRef]
- Ehinger, A.M.; Schmidt, H.; Kietzmann, M. Tissue distribution of cefquinome after intramammary and “systemic” administration in the isolated perfused bovine udder. Vet. J. 2006, 172, 147–153. [Google Scholar] [CrossRef]
- Lamar, J.; Petz, M. Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal. Chim. Acta 2007, 586, 296–303. [Google Scholar] [CrossRef]
- Le Breton, M.H.; Savoy-Perroud, M.C.; Diserens, J.M. Validation and comparison of the Copan Milk Test and Delvotest SP-NT for the detection of antimicrobials in milk. Anal. Chim. Acta 2007, 586, 280–283. [Google Scholar] [CrossRef]
- Suhren, G.; Knappstein, K. Detection of cefquinome in milk by liquid chromatography and screening methods. Anal. Chim. Acta 2003, 483, 363–372. [Google Scholar] [CrossRef]
- Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Available online: https://op.europa.eu/en/publication-detail/-/publication/ed928116-a955-4a84-b10a-cf7a82bad858 (accessed on 24 March 2020).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MSMS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta. 2015, 880, 103–121. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Liu, Y.; Du, Z.; Feng, Y.; Jia, L.; Zhang, J. Extraction optimization of sixteen cephalosporins in milk by filtered solid phase extraction and ultra high pressure liquid chromatography coupled to tandem mass spectrometry. Anal. Methods 2017, 9, 1282–1289. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Schmidt, K.; Bertulat, S.; Klein, L.; Finnah, A.; Heuwieser, W.; Richter, A. Quantitative analysis of cefquinome considering different matrix compositions of bovine colostrum and raw milk. Anal. Bioanal. Chem. 2018, 410, 7465–7475. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, D.; Rodriguez-Ramirez, R.; Del, O.M.; Garcia-Campana, A.M. Validation of a new method based on salting-out assisted liquid-liquid extraction and UHPLC-MS/MS for the determination of betalactam antibiotics in infant dairy products. Talanta 2017, 167, 493–498. [Google Scholar] [CrossRef]
- Stockler, R.M.; Morin, D.E.; Lantz, R.K.; Constable, P.D. Effect of milking frequency and dosing interval on the pharmacokinetics of cephapirin after intramammary infusion in lactating dairy cows. J. Dairy. Sci. 2009, 92, 4262–4275. [Google Scholar] [CrossRef]
- Cobb, D.V. Who’s to blame for inappropriate use of drugs? J. Am. Vet. Med. Assoc. 1998, 213, 338–339. [Google Scholar]
- Jank, L.; Martins, M.T.; Arsand, J.B.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M. High-throughput method for the determination of residues of beta-lactam antibiotics in bovine milk by LC-MS/MS. Food Addit. Contam. Part A Chem Anal. Control. Expo. Risk Assess. 2015, 32, 1992–2001. [Google Scholar] [CrossRef]
- Hoff, R.B.; Rubensam, G.; Jank, L.; Barreto, F.; Peralba, M.C.; Pizzolato, T.M.; Silvia, D.M.; Barcelo, D. Analytical quality assurance in veterinary drug residue analysis methods: Matrix effects determination and monitoring for sulfonamides analysis. Talanta 2015, 132, 443–450. [Google Scholar] [CrossRef]
- Sachi, S.; Ferdous, J.; Sikder, M.H.; Azizul, K.H.S. Antibiotic residues in milk: Past, present, and future. J. Adv. Vet. Anim. Res. 2019, 6, 315–332. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Moghadam, M.M.; Amiri, M.; Riabi, H.R.; Riabi, H.R. Evaluation of Antibiotic Residues in Pasteurized and Raw Milk Distributed in the South of Khorasan-e Razavi Province, Iran. J. Clin. Diagn. Res. 2016, 10, C31–C35. [Google Scholar] [CrossRef] [PubMed]
- Nouws, J.F.M.R.; Egmond, H.V.; Loeffen, G.; Keukens, H.; Smulders, I.; Stegeman, H. Suitability of the Charm HVS and a microbiological multiplate system for detection of residues in raw milk at EU maximum residue levels. Vet. Q. 1999, 21, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ibraimi, Z.S.A.H.Z. Detection and Risk Assessment of Beta-Lactam Residues in Kosovo’s Milk using Elisa Method. Int. J. Pharm. Pharm. Sci. 2013, 5, 50–446. Available online: https://www.researchgate.net/publication/286052502 (accessed on 10 August 2021).
- Sulejmani, Z.S.A.H.Z. Abuse of pharmaceutical drugs-Antibiotics in dairy cattle in Kosovo and detection of their residues in Abuse of pharmaceutical drugs-Antibiotics in dairy cattle in Kosovo and detection of their residues in milk. J. Ecosyst. Ecogr. 2012, 2, 112. [Google Scholar] [CrossRef]
- Al-Mazeedi, H.M.; Abbas, A.B.; Alomirah, H.F.; Al-Jouhar, W.Y.; Al-Mufty, S.A.; Ezzelregal, M.M.; Al-Owaish, R.A. Screening for tetracycline residues in food products of animal origin in the State of Kuwait using Charm II radio-immunoassay and LC/MS/MS methods. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 291–301. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Available online: http://data.europa.eu/eli/reg/2010/37(1)/2022-05-09 (accessed on 9 May 2022).
- Smith, G.W.; Gehring, R.; Riviere, J.E.; Yeatts, J.L.; Baynes, R.E. Elimination kinetics of ceftiofur hydrochloride after intramammary administration in lactating dairy cows. J. Am. Vet. Med. Assoc. 2004, 224, 1827–1830. [Google Scholar] [CrossRef]
- Sato, K.; Ohashi, E.; Tsukano, K.; Ikeda, K.; Ajito, T.; Suzuki, K. Effects of temporary cessation of milking following intramammary cefazolin sodium infusion on residual antibiotic in lactating dairy cows. J. Vet. Med. Sci. 2021, 83, 814–818. [Google Scholar] [CrossRef]
- Zeng, S.S.; Escobar, E.N.; BrownCrowder, I. Evaluation of screening tests for detection of antibiotic residues in goat milk. Small Rumiant. Res. 1996, 21, 155–160. [Google Scholar] [CrossRef]
- The, E. Reducing the use of antimicrobial agents in animals and man. J. Med. Microbiol. 2000, 49, 111–113. [Google Scholar] [CrossRef]
- Rollin, B.E. An ethicist’s commentary on extra-label drug use. Can. Vet. J. 2002, 43, 749–750. [Google Scholar]
- Pengov, A.; Kirbis, A. Risks of antibiotic residues in milk following intramammary and intramuscular treatments in dairy sheep. Anal. Chim. Acta. 2009, 637, 13–17. [Google Scholar] [CrossRef]
- Poole, K. Resistance to beta-lactam antibiotics. Cell Mol Life Sci. 2004, 61, 2200–2223. [Google Scholar] [CrossRef]
- Khotaei, G.T.; Fattahi, F.; Pourpak, Z.; Moinfar, Z.; Aghaee, F.M.; Gholami, K.; Moin, M. Adverse reactions to antibiotics in hospitalized Iranian children. J. Microbiol. Immunol. Infect. 2008, 41, 160–164. [Google Scholar]
- Toutain, P.L.; Del, C.J.; Bousquet-Melou, A. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef]
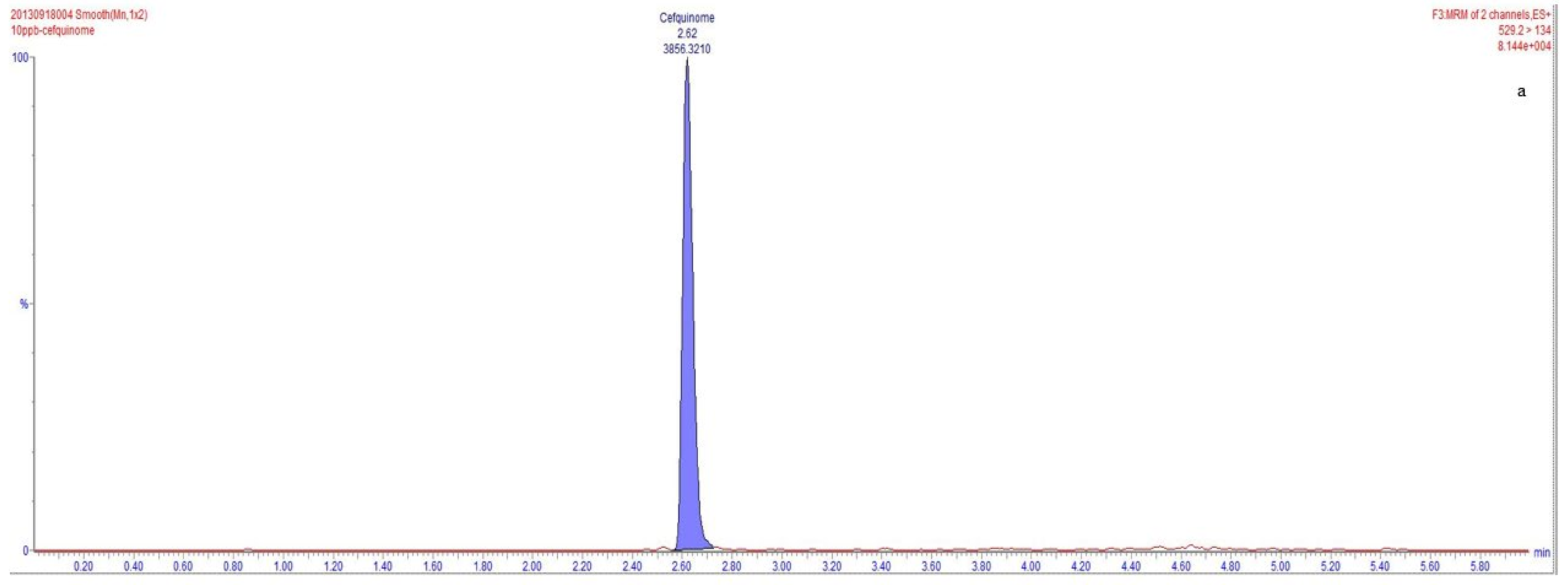

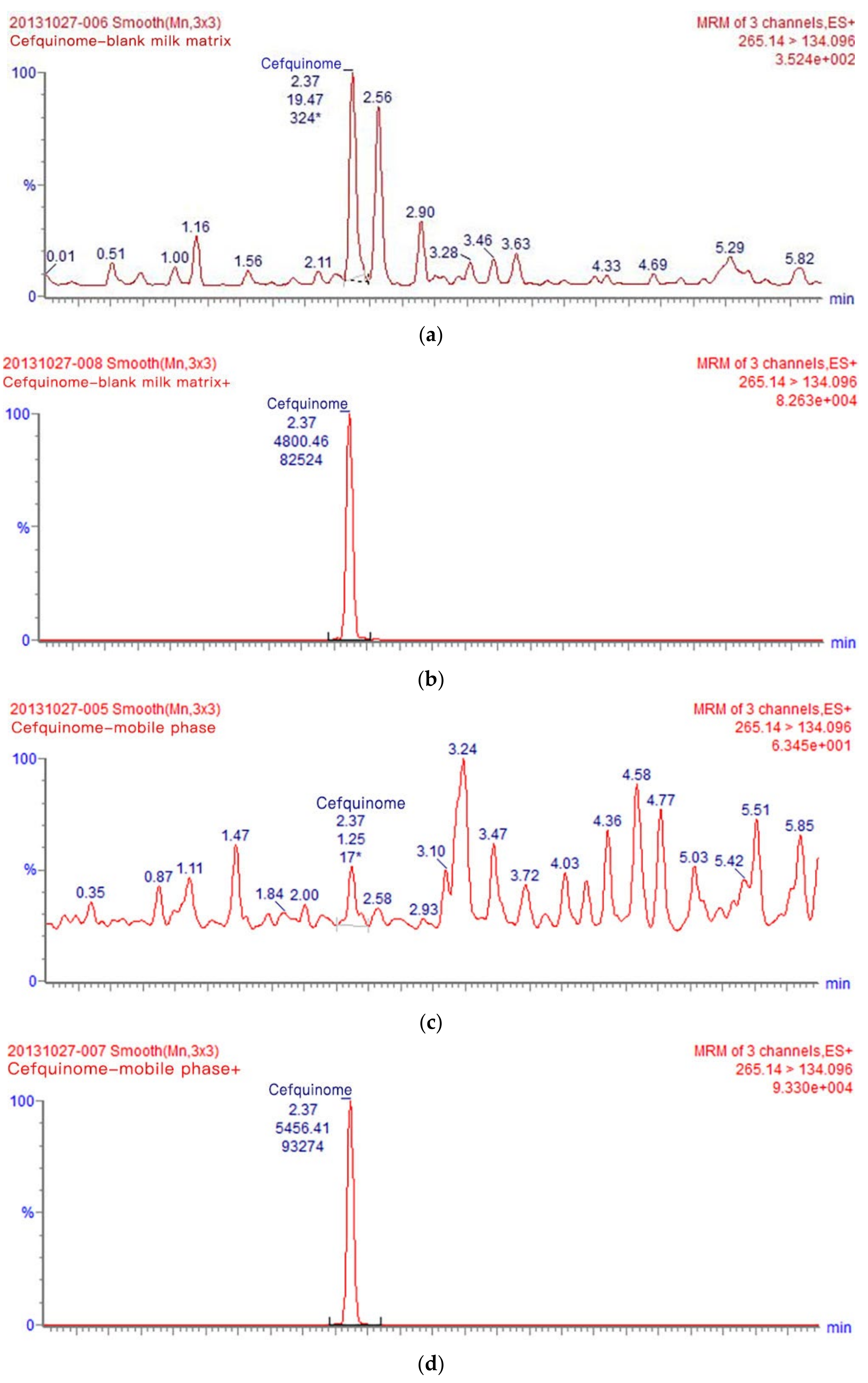
| Precursorion (m/z) | Production (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|
| 265.1 | 134.1 * | 28 | 16 |
| 265.1 | 199.3 | 28 | 14 |
| Spiking Concentration (μg·kg−1) | Recovery Rate (%) | SD (%) | Intra-Day RSD (%) | Inter-Day RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||
| 50 | 97.29 | 1.77 | 1.67 | 1.56 | 1.35 | 1.28 | 1.52 | 1.81 |
| 10 | 100.95 | 2.54 | 2.26 | 2.22 | 1.69 | 1.97 | 3.45 | 2.51 |
| 0.2 | 88.60 | 16.33 | 12.92 | 13.00 | 10.50 | 13.73 | 12.29 | 18.44 |
| Name | Content |
|---|---|
| Calibration Equation | Y = 250.86X − 102.29 |
| A linear range (μg·kg−1) | 0.2–50 |
| Correlation coefficient (R2) | 0.9996 |
| The limits of detection (LOD) (μg·kg−1) | 0.1 |
| The limits of quantitation (LOQ) (μg·kg−1) | 0.2 |
| Time (h) | Concentration of Cefquinome in Milk (μg·kg−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cow #1 | Cow #2 | Cow #3 | Cow #4 | Cow #5 | Cow #6 | Cow #7 | Cow #8 | Cow #9 | Cow #10 | Cow #11 | Cow #12 | Average | SD | |
| 12 | 347.76 | 197.37 | 260.08 | 106.18 | 204.30 | 139.31 | 117.15 | 192.52 | 104.22 | 115.81 | 229.16 | 131.00 | 178.74 | 74.66 |
| 18 | 115.53 | 73.29 | 82.20 | 66.93 | 85.46 | 70.01 | 74.99 | 100.85 | 81.37 | 63.39 | 78.02 | 89.06 | 101.26 | 14.77 |
| 24 | 45.31 | 35.48 | 47.22 | 35.65 | 43.75 | 21.67 | 14.26 | 33.53 | 34.21 | 10.43 | 52.84 | 11.96 | 32.19 | 14.47 |
| 36 | 9.70 | 9.46 | 9.25 | 6.52 | 4.90 | 9.25 | 3.83 | 3.11 | 7.01 | 2.96 | 9.64 | 9.99 | 7.14 | 2.78 |
| 42 | 7.74 | 2.33 | 4.28 | 1.20 | 2.37 | 4.46 | 2.29 | 0.95 | 3.35 | 1.04 | 5.01 | 1.03 | 3.00 | 2.07 |
| 48 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Han, M.; Wang, H.; Chen, X.; Tang, Y.; Zhang, D.; Li, X.; Liu, Y. Elimination of Cefquinome Sulfate Residue in Cow’s Milk after Intrauterine Infusion. Metabolites 2023, 13, 492. https://doi.org/10.3390/metabo13040492
Liu C, Han M, Wang H, Chen X, Tang Y, Zhang D, Li X, Liu Y. Elimination of Cefquinome Sulfate Residue in Cow’s Milk after Intrauterine Infusion. Metabolites. 2023; 13(4):492. https://doi.org/10.3390/metabo13040492
Chicago/Turabian StyleLiu, Chunshuang, Mingyue Han, Honglei Wang, Xiaojie Chen, Yaoxin Tang, Daokang Zhang, Xiubo Li, and Yiming Liu. 2023. "Elimination of Cefquinome Sulfate Residue in Cow’s Milk after Intrauterine Infusion" Metabolites 13, no. 4: 492. https://doi.org/10.3390/metabo13040492
APA StyleLiu, C., Han, M., Wang, H., Chen, X., Tang, Y., Zhang, D., Li, X., & Liu, Y. (2023). Elimination of Cefquinome Sulfate Residue in Cow’s Milk after Intrauterine Infusion. Metabolites, 13(4), 492. https://doi.org/10.3390/metabo13040492








