A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites
Abstract
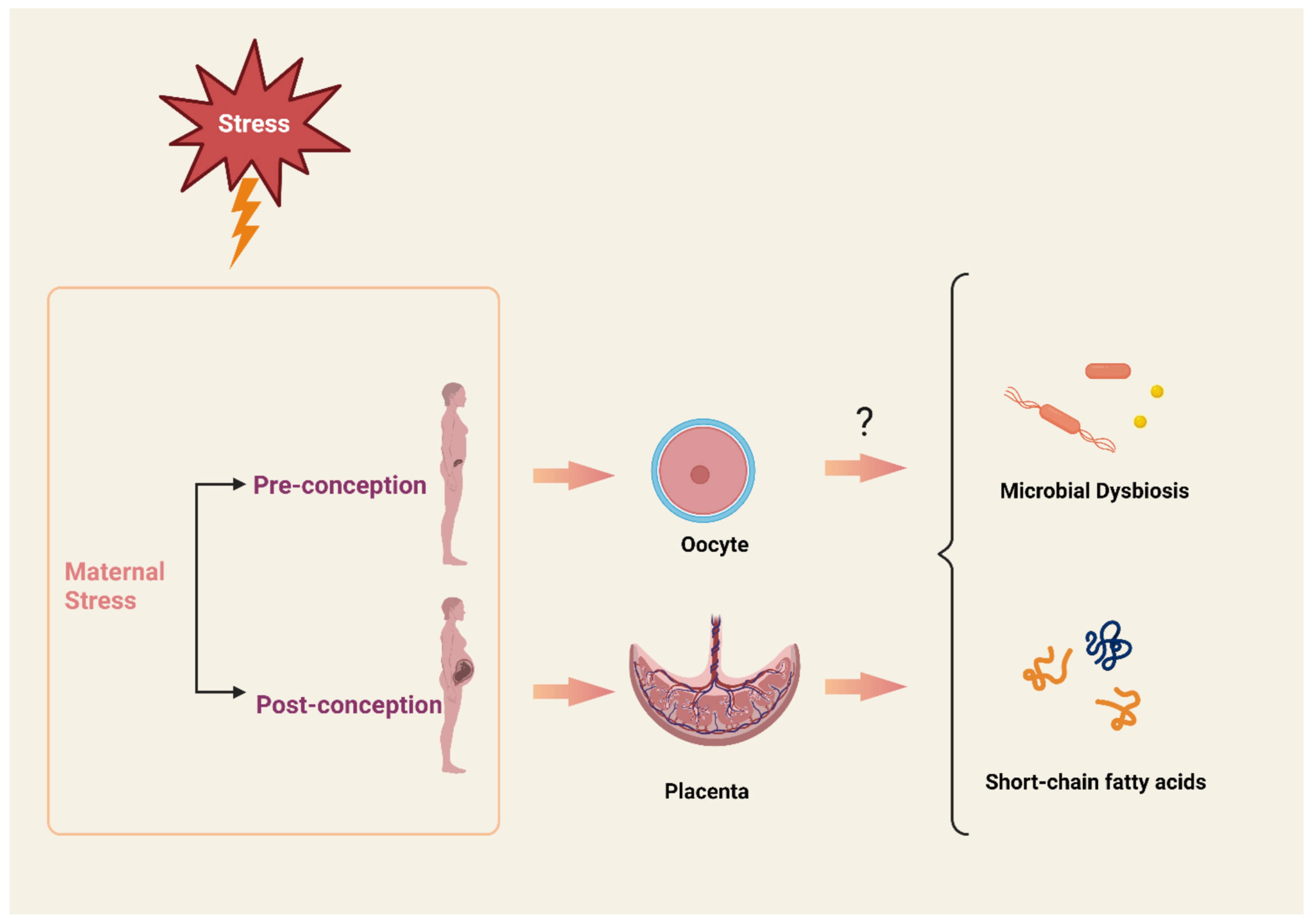
1. Increasing Prevalence of Anxiety and Stress
2. Effect of Stress on Microbiota Composition
3. The Gut-Brain Axis and Microbial Metabolites
4. The Effect of Probiotics Supplementation on SCFA Production
5. The Effect of Probiotic Supplementation on the Stress Response
6. Father’s Role in Offspring Microbiome
7. Mechanisms of Cross-Generational Transmission of Prenatal Stress
8. Non-Traditional Models to Investigate Stress–Microbiome–Behavior Relationships
9. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Francis, L.; DePriest, K.; Wilson, M.; Gross, D. Child Poverty, Toxic Stress, and Social Determinants of Health: Screening and Care Coordination. Online J. Issues Nurs. 2018, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.W.H.; Curran, E.; Ames, D.; Lautenschlager, N.T.; Castle, D.J. Mental health of older adults during the COVID-19 pandemic: Lessons from history to guide our future. Int. Psychogeriatry 2020, 32, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Kandola, A.; Vancampfort, D.; Herring, M.; Rebar, A.; Hallgren, M.; Firth, J.; Stubbs, B. Moving to Beat Anxiety: Epidemiology and Therapeutic Issues with Physical Activity for Anxiety. Curr. Psychiatry Rep. 2018, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Weinberger, A.H.; Kim, J.H.; Wu, M.; Galea, S. Trends in anxiety among adults in the United States, 2008–2018: Rapid increases among young adults. J. Psychiatr. Res. 2020, 130, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Robinson, O.J.; Cornwell, B.; Ernst, M. Modeling anxiety in healthy humans: A key intermediate bridge between basic and clinical sciences. Neuropsychopharmacology 2019, 44, 1999–2010. [Google Scholar] [CrossRef]
- Comer, J.S.; Blanco, C.; Hasin, D.S.; Liu, S.M.; Grant, B.F.; Turner, J.B.; Olfson, M. Health-related quality of life across the anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions (NESARC). J. Clin. Psychiatry 2011, 72, 43–50. [Google Scholar] [CrossRef]
- Chandola, T.; Brunner, E.; Marmot, M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ 2006, 332, 521–525. [Google Scholar] [CrossRef]
- Jasarevic, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Gur, T.L.; Palkar, A.V.; Rajasekera, T.; Allen, J.; Niraula, A.; Godbout, J.; Bailey, M.T. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav. Brain Res. 2019, 359, 886–894. [Google Scholar] [CrossRef]
- Brawner, K.M.; Yeramilli, V.A.; Kennedy, B.A.; Patel, R.K.; Martin, C.A. Prenatal stress increases IgA coating of offspring microbiota and exacerbates necrotizing enterocolitis-like injury in a sex-dependent manner. Brain Behav. Immun. 2020, 89, 291–299. [Google Scholar] [CrossRef]
- Jasarevic, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, K.E.; Lee, H.J.; Kim, D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-kappaB activation through gut microbiota disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef]
- Bailey, M.T. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 2014, 817, 255–276. [Google Scholar] [CrossRef]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Sakuma, K.; Funabashi, H.; Matsuoka, H.; Saito, M. Potential use of Lactobacillus cell density in feces as a non-invasive bio-indicator for evaluating environmental stress during mouse breeding. Biocontrol. Sci. 2013, 18, 101–104. [Google Scholar] [CrossRef]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef]
- Lyte, M.; Bailey, M.T. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J. Surg. Res. 1997, 70, 195–201. [Google Scholar] [CrossRef]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Moreira, C.G.; Russell, R.; Mishra, A.A.; Narayanan, S.; Ritchie, J.M.; Waldor, M.K.; Curtis, M.M.; Winter, S.E.; Weinshenker, D.; Sperandio, V. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuan, P.Q.; Wang, L.; Tache, Y. Activation of the parapyramidal region in the ventral medulla stimulates gastric acid secretion through vagal pathways in rats. Neuroscience 2000, 95, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Messmer, B.; Zimmerman, F.G.; Lenz, H.J. Regulation of exocrine pancreatic secretion by cerebral TRH and CGRP: Role of VIP, muscarinic, and adrenergic pathways. Am. J. Physiol. 1993, 264 Pt 1, G237–G242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yin, J.; Wang, L.; Chen, J.D. Diffused and sustained inhibitory effects of intestinal electrical stimulation on intestinal motility mediated via sympathetic pathway. Neuromodulation 2014, 17, 373–379, discussion 380. [Google Scholar] [CrossRef]
- Jarillo-Luna, A.; Rivera-Aguilar, V.; Garfias, H.R.; Lara-Padilla, E.; Kormanovsky, A.; Campos-Rodriguez, R. Effect of repeated restraint stress on the levels of intestinal IgA in mice. Psychoneuroendocrinology 2007, 32, 681–692. [Google Scholar] [CrossRef]
- Reyna-Garfias, H.; Miliar, A.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Pacheco-Yepez, J.; Baeza, I.; Campos-Rodriguez, R. Repeated restraint stress increases IgA concentration in rat small intestine. Brain Behav. Immun. 2010, 24, 110–118. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.t.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharm. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef]
- Zaboski, B.A.; Storch, E.A. Comorbid autism spectrum disorder and anxiety disorders: A brief review. Future Neurol. 2018, 13, 31–37. [Google Scholar] [CrossRef]
- Jasarevic, E.; Rodgers, A.B.; Bale, T.L. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol. Stress 2015, 1, 81–88. [Google Scholar] [CrossRef]
- Russell, S.L.; Gold, M.J.; Willing, B.P.; Thorson, L.; McNagny, K.M.; Finlay, B.B. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 2013, 4, 158–164. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fulling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Fung, C.; Cools, B.; Malagola, S.; Martens, T.; Tack, J.; Kazwiny, Y.; Vanden Berghe, P. Luminal short-chain fatty acids and 5-HT acutely activate myenteric neurons in the mouse proximal colon. Neurogastroenterol. Motil. 2021, 33, e14186. [Google Scholar] [CrossRef]
- Linan-Rico, A.; Ochoa-Cortes, F.; Beyder, A.; Soghomonyan, S.; Zuleta-Alarcon, A.; Coppola, V.; Christofi, F.L. Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation. Front. Neurosci. 2016, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Sajdel-Sulkowska, E.M. Neuropsychiatric Ramifications of COVID-19: Short-Chain Fatty Acid Deficiency and Disturbance of Microbiota-Gut-Brain Axis Signaling. Biomed. Res. Int. 2021, 2021, 7880448. [Google Scholar] [CrossRef] [PubMed]
- Ravi M, M.A.; Michopoulos, V. The immunology of stress and the impact of inflammation on the brain and behaviour. BJPsych Adv. 2020, 82, 158–165. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Udina, M.; Castellvi, P.; Moreno-Espana, J.; Navines, R.; Valdes, M.; Forns, X.; Langohr, K.; Sola, R.; Vieta, E.; Martin-Santos, R. Interferon-induced depression in chronic hepatitis C: A systematic review and meta-analysis. J. Clin. Psychiatry 2012, 73, 1128–1138. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Schiopu, C.; Stefanescu, G.; Diaconescu, S.; Balan, G.G.; Gimiga, N.; Rusu, E.; Moldovan, C.A.; Popa, B.; Tataranu, E.; Olteanu, A.V.; et al. Magnesium Orotate and the Microbiome-Gut-Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders. Nutrients 2022, 14, 1567. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jorgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sorensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783 e771. [Google Scholar] [CrossRef]
- Ho, S.T.; Hsieh, Y.T.; Wang, S.Y.; Chen, M.J. Improving effect of a probiotic mixture on memory and learning abilities in d-galactose-treated aging mice. J. Dairy Sci. 2019, 102, 1901–1909. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Pilar Francino, M.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66. [Google Scholar] [CrossRef]
- dos Reis, S.A.; da Conceicao, L.L.; Siqueira, N.P.; Rosa, D.D.; da Silva, L.L.; Peluzio, M.D. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef]
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral administration of butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Hongwittayakorn, P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 2013, 169, 511–525. [Google Scholar] [CrossRef]
- Moens, F.; Van den Abbeele, P.; Basit, A.W.; Dodoo, C.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 2010, 57, 1411–1415. [Google Scholar] [PubMed]
- Moya-Perez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e01269762015. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Vasodavan, K.; Saipudin, N.A.; Yusof, B.N.M.; Kumar, S.; Nordin, S.A. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 2019, 103–111. [Google Scholar] [CrossRef]
- Nagata, S.; Chiba, Y.; Wang, C.; Yamashiro, Y. The effects of the Lactobacillus casei strain on obesity in children: A pilot study. Benef. Microbes 2017, 8, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Cavalcante, R.G.S.; de Albuquerque, T.M.R.; de Luna Freire, M.O.; Ferreira, G.A.H.; Carneiro Dos Santos, L.A.; Magnani, M.; Cruz, J.C.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1408–1417. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Forty-five-year evolution of probiotic therapy. Microb. Cell 2019, 6, 184–196. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Moller, C.M.; Olsa, E.J.A.; Ginty, A.T.; Rapelje, A.L.; Tindall, C.L.; Holesh, L.A.; Petersen, K.L.; Conklin, S.M. Influence of Acute Multispecies and Multistrain Probiotic Supplementation on Cardiovascular Function and Reactivity to Psychological Stress in Young Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Psychosom. Med. 2017, 79, 914–919. [Google Scholar] [CrossRef]
- Teichman, E.M.; O’Riordan, K.J.; Gahan, C.G.M.; Dinan, T.G.; Cryan, J.F. When Rhythms Meet the Blues: Circadian Interactions with the Microbiota-Gut-Brain Axis. Cell. Metab. 2020, 31, 448–471. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Li, M.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.; Huang, J.; Tian, D.; et al. Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Moughnyeh, M.M.; Brawner, K.M.; Kennedy, B.A.; Yeramilli, V.A.; Udayakumar, N.; Graham, J.A.; Martin, C.A. Stress and the Gut-Brain Axis: Implications for Cancer, Inflammation and Sepsis. J. Surg. Res. 2021, 266, 336–344. [Google Scholar] [CrossRef]
- Coelho, G.D.P.; Ayres, L.F.A.; Barreto, D.S.; Henriques, B.D.; Prado, M.; Passos, C.M.D. Acquisition of microbiota according to the type of birth: An integrative review. Rev. Lat. Am. Enferm. 2021, 29, e3446. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Kaan, A.M.; Zaura, E. Oral Microbiome Transmission and Infant Feeding Habits. mBio 2022, 13, e00325222022. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, F. Microbial transmission, colonisation and succession: From pregnancy to infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations with the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma. Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Linner, A.; Westrup, B.; Lode-Kolz, K.; Klemming, S.; Lillieskold, S.; Markhus Pike, H.; Morgan, B.; Bergman, N.J.; Rettedal, S.; Jonas, W. Immediate parent-infant skin-to-skin study (IPISTOSS): Study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open 2020, 10, e038938. [Google Scholar] [CrossRef]
- Dill-McFarland, K.A.; Tang, Z.Z.; Kemis, J.H.; Kerby, R.L.; Chen, G.; Palloni, A.; Sorenson, T.; Rey, F.E.; Herd, P. Close social relationships correlate with human gut microbiota composition. Sci. Rep. 2019, 9, 703. [Google Scholar] [CrossRef]
- Kulkarni, S.; Heeb, P. Social and sexual behaviours aid transmission of bacteria in birds. Behav. Process. 2007, 74, 88–92. [Google Scholar] [CrossRef]
- Gurevich, Y.; Lewin-Epstein, O.; Hadany, L. The evolution of paternal care: A role for microbes? Philos. Trans R. Soc. Lond. B Biol. Sci. 2020, 375, 20190599. [Google Scholar] [CrossRef]
- Korgan, A.C.; Foxx, C.L.; Hashmi, H.; Sago, S.A.; Stamper, C.E.; Heinze, J.D.; O’Leary, E.; King, J.L.; Perrot, T.S.; Lowry, C.A.; et al. Effects of paternal high-fat diet and maternal rearing environment on the gut microbiota and behavior. Sci. Rep. 2022, 12, 10179. [Google Scholar] [CrossRef]
- Yehuda, R.; Lehrner, A. Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Hemmings, S.M.J.; Qulu, L. Prenatal maternal stress and offspring aggressive behavior: Intergenerational and transgenerational inheritance. Front. Behav. Neurosci. 2022, 16, 1–19. [Google Scholar] [CrossRef]
- Cao-Lei, L.; de Rooij, S.R.; King, S.; Matthews, S.G.; Metz, G.A.S.; Roseboom, T.J.; Szyf, M. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 2020, 117, 198–210. [Google Scholar] [CrossRef]
- Walsh, K.; McCormack, C.A.; Webster, R.; Pinto, A.; Lee, S.; Feng, T.; Krakovsky, H.S.; O’Grady, S.M.; Tycko, B.; Champagne, F.A.; et al. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc. Natl. Acad. Sci. USA 2019, 116, 23996–24005. [Google Scholar] [CrossRef]
- Yeramilli, V.A.; Brawner, K.M.; Crossman, D.K.; Barnum, S.R.; Martin, C.A. RNASeq analysis reveals upregulation of complement C3 in the offspring gut following prenatal stress in mice. Immunobiology 2020, 225, 151983. [Google Scholar] [CrossRef]
- Bowers, M.E.; Yehuda, R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology 2016, 41, 232–244. [Google Scholar] [CrossRef]
- Yehuda, R.; Halligan, S.L.; Bierer, L.M. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J. Psychiatr. Res. 2001, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Mesiano, S.; Chan, E.C.; Brown, S.; Jaffe, R.B. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J. Clin. Endocrinol. Metab. 1998, 83, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Sandman, C.A.; Chicz-DeMet, A.; Porto, M. Placental CRH modulates maternal pituitary adrenal function in human pregnancy. Ann. N. Y. Acad. Sci. 1997, 814, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef]
- Challis, J.R.; Sloboda, D.; Matthews, S.G.; Holloway, A.; Alfaidy, N.; Patel, F.A.; Whittle, W.; Fraser, M.; Moss, T.J.; Newnham, J. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol. Cell Endocrinol. 2001, 185, 135–144. [Google Scholar] [CrossRef]
- Duthie, L.; Reynolds, R.M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 2013, 98, 106–115. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2020, 14, 601939. [Google Scholar] [CrossRef]
- Thomas, J.C.; Letourneau, N.; Campbell, T.S.; Giesbrecht, G.F. Social buffering of the maternal and infant HPA axes: Mediation and moderation in the intergenerational transmission of adverse childhood experiences. Dev. Psychopathol. 2018, 30, 921–939. [Google Scholar] [CrossRef]
- Bronson, S.L.; Bale, T.L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 2014, 155, 2635–2646. [Google Scholar] [CrossRef]
- Webster, J.I.; Tonelli, L.; Sternberg, E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002, 20, 125–163. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Furcron, A.E.; Levenson, D.; Galaz, J.; Zou, C.; Hassan, S.S.; Hsu, C.D.; Olson, D.; Metz, G.A.S.; et al. Prenatal Maternal Stress Causes Preterm Birth and Affects Neonatal Adaptive Immunity in Mice. Front. Immunol. 2020, 11, 254. [Google Scholar] [CrossRef]
- Herrera, J.A.; Alvarado, J.P.; Martinez, J.E. The psychosocial environment and the cellular immunity in the pregnant patient. Stress Med. 1988, 4, 49–56. [Google Scholar] [CrossRef]
- Benediktsson, R.; Calder, A.A.; Edwards, C.R.W.; Seckl, J.R. Placental 11β-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. 1997, 46, 161–166. [Google Scholar] [CrossRef]
- Jensen Peña, C.; Monk, C.; Champagne, F.A. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE 2012, 7, e397912012. [Google Scholar] [CrossRef]
- Conradt, E.; Lester, B.M.; Appleton, A.A.; Armstrong, D.A.; Marsit, C.J. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 2013, 8, 1321–1329. [Google Scholar] [CrossRef]
- Appleton, A.A.; Lester, B.M.; Armstrong, D.A.; Lesseur, C.; Marsit, C.J. Examining the joint contribution of placental NR3C1 and HSD11B2 methylation for infant neurobehavior. Psychoneuroendocrinology 2015, 52, 32–42. [Google Scholar] [CrossRef]
- Howerton, C.L.; Morgan, C.P.; Fischer, D.B.; Bale, T.L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl. Acad. Sci. USA 2013, 110, 5169–5174. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Bierer, L.M.; Bader, H.N.; Daskalakis, N.P.; Lehrner, A.; Provencal, N.; Wiechmann, T.; Klengel, T.; Makotkine, I.; Binder, E.B.; Yehuda, R. Intergenerational Effects of Maternal Holocaust Exposure on FKBP5 Methylation. Am. J. Psychiatry. 2020, 177, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, H.; Leshem, M.; Gaisler-Salomon, I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol. Psychiatry 2013, 74, 680–687. [Google Scholar] [CrossRef]
- Moog, N.K.; Buss, C.; Entringer, S.; Shahbaba, B.; Gillen, D.L.; Hobel, C.J.; Wadhwa, P.D. Maternal Exposure to Childhood Trauma Is Associated during Pregnancy with Placental-Fetal Stress Physiology. Biol. Psychiatry 2016, 79, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.H.; Ly, N.H.; Katou, Y.; Yokota, N.; Nakato, R.; Murakami, S.; Hirayama, A.; Fukuda, S.; Kang, S.; Soga, T.; et al. Paternal restraint stress affects offspring metabolism via ATF-2 dependent mechanisms in Drosophila melanogaster germ cells. Commun. Biol. 2020, 3, 208. [Google Scholar] [CrossRef]
- Jia, Y.; Jin, S.; Hu, K.; Geng, L.; Han, C.; Kang, R.; Pang, Y.; Ling, E.; Tan, E.K.; Pan, Y.; et al. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.A.; Lee, W.J. Drosophila as a model system for deciphering the ‘host physiology-nutrition-microbiome’ axis. Curr. Opin. Insect. Sci. 2020, 41, 112–119. [Google Scholar] [CrossRef]
- Ludington, W.B.; Ja, W.W. Drosophila as a model for the gut microbiome. PLoS Pathog. 2020, 16, e10083982020. [Google Scholar] [CrossRef]
- Uren Webster, T.M.; Consuegra, S.; Garcia de Leaniz, C. Early life stress causes persistent impacts on the microbiome of Atlantic salmon. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100888. [Google Scholar] [CrossRef]
- Uren Webster, T.M.; Rodriguez-Barreto, D.; Consuegra, S.; Garcia de Leaniz, C. Cortisol-Related Signatures of Stress in the Fish Microbiome. Front. Microbiol. 2020, 11, 1621. [Google Scholar] [CrossRef]
- Davis, D.J.; Bryda, E.C.; Gillespie, C.H.; Ericsson, A.C. Microbial modulation of behavior and stress responses in zebrafish larvae. Behav. Brain Res. 2016, 311, 219–227. [Google Scholar] [CrossRef]
- Cavalieri, V.; Spinelli, G. Environmental epigenetics in zebrafish. Epigenetics Chromatin 2017, 10, 46. [Google Scholar] [CrossRef]
- Cypser, J.R.; Johnson, T.E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B109–B114. [Google Scholar] [CrossRef]
- Fabrizio, P.; Garvis, S.; Palladino, F. Histone Methylation and Memory of Environmental Stress. Cells 2019, 8, 339. [Google Scholar] [CrossRef]
- Lithgow, G.J.; White, T.M.; Melov, S.; Johnson, T.E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA 1995, 92, 7540–7544. [Google Scholar] [CrossRef]
- Antoni, M.H.; Moreno, P.I.; Penedo, F.J. Stress Management Interventions to Facilitate Psychological and Physiological Adaptation and Optimal Health Outcomes in Cancer Patients and Survivors. Annu. Rev. Psychol. 2023, 74, 423–455. [Google Scholar] [CrossRef]
- Spar, D. The egg trade—Making sense of the market for human oocytes. N. Engl. J. Med. 2007, 356, 1289–1291. [Google Scholar] [CrossRef]
- Zakarin Safier, L.; Gumer, A.; Kline, M.; Egli, D.; Sauer, M.V. Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. J. Assist. Reprod. Genet. 2018, 35, 1219–1225. [Google Scholar] [CrossRef]
| Probiotic | Species | Effects (↑ = Increase ↓ = Decrease) | Reference |
|---|---|---|---|
| Lactobacilus plantarum P-8 | Human | ↑ Bifidobacterium ↓ Desulfovibrio ↑ Acetate & Propionate | [54] |
| Probiotic mixture containing Lactobacillus paracasei ssp. paracasei BCRC 12188, Lactobacillus plantarum BCRC 12251, and Streptococcus thermophilus BCRC 13869 | Mice | ↑ SCFAs ↑ Improved memory and learning ability | [55] |
| Fermented salami + Lactobacillus rhamnous + citrus fiber | Human | ↑ Butyrate | [56] |
| SymproveTM containing Lactobacillus plantarum NCIM 30173, Lactobacillus rhamnosus NCIMB 30174, and Enterococcus faecium NCIMB 30176 | Human | ↑ Proximal and distal colonic lactate ↑ Butyrate | [60] |
| Lactobacillus gasseri OLL27A | Human | ↑ Lactobacillus spp. ↓ Clostridium perfringens ↑ Fecal isobutyric acid | [61] |
| Lactobacillus casei | Human | ↑ Fecal butyric, propionic, and acetic acid | [63,64] |
| Probiotic | Species | Effects (↑ = Increase ↓ = Decrease) | Reference |
|---|---|---|---|
| Bifidobacterial infants | Mice | ↓ Corticosterone and restore HPA axis homeostasis | [71] |
| Lactobacillus helveticus R0052 + Bifidobacterium longum RO175 | Rats | ↓ Anxiety | [73] |
| L. Rhamnos | Rats | ↓ Anxiety | [74,75] |
| Lactobacillus plantarum DR7 or P8 | Human | ↓ Stress Symptoms | [76,80] |
| Lactobacilli, Bifidobacteria, Streptocci | Human | No change in stress symptoms | [77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeramilli, V.; Cheddadi, R.; Shah, J.; Brawner, K.; Martin, C. A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites. Metabolites 2023, 13, 535. https://doi.org/10.3390/metabo13040535
Yeramilli V, Cheddadi R, Shah J, Brawner K, Martin C. A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites. Metabolites. 2023; 13(4):535. https://doi.org/10.3390/metabo13040535
Chicago/Turabian StyleYeramilli, Venkata, Riadh Cheddadi, Juhi Shah, Kyle Brawner, and Colin Martin. 2023. "A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites" Metabolites 13, no. 4: 535. https://doi.org/10.3390/metabo13040535
APA StyleYeramilli, V., Cheddadi, R., Shah, J., Brawner, K., & Martin, C. (2023). A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites. Metabolites, 13(4), 535. https://doi.org/10.3390/metabo13040535







