Abstract
The presence of mycotoxins in cereals can pose a significant health risk to animals and humans. China is one of the countries that is facing cereal contamination by mycotoxins. Treating mycotoxin-contaminated cereals with established physical and chemical methods can lead to negative effects, such as the loss of nutrients, chemical residues, and high energy consumption. Therefore, microbial detoxification techniques are being considered for reducing and treating mycotoxins in cereals. This paper reviews the contamination of aflatoxins, zearalenone, deoxynivalenol, fumonisins, and ochratoxin A in major cereals (rice, wheat, and maize). Our discussion is based on 8700 samples from 30 provincial areas in China between 2005 and 2021. Previous research suggests that the temperature and humidity in the highly contaminated Chinese cereal-growing regions match the growth conditions of potential antagonists. Therefore, this review takes biological detoxification as the starting point and summarizes the methods of microbial detoxification, microbial active substance detoxification, and other microbial inhibition methods for treating contaminated cereals. Furthermore, their respective mechanisms are systematically analyzed, and a series of strategies for combining the above methods with the treatment of contaminated cereals in China are proposed. It is hoped that this review will provide a reference for subsequent solutions to cereal contamination problems and for the development of safer and more efficient methods of biological detoxification.
1. Introduction
Cereal farming has always been a major part of human agricultural production, as cereals are essential human foods and animal feed resources. Based on global survey data and thresholds, Marin et al. [1] confirmed the Food and Agriculture Organization’s (FAO) claim that about 25% of global cereals are contaminated with mycotoxins. Mycotoxins are secondary metabolites produced by certain fungi in oilseeds, cereals, legumes, nuts, and processed products during the pre-harvest and post-harvest stages [2]. The common mycotoxins include aflatoxins (AFs), ochratoxin A (OTA), zearalenone (ZEN), deoxynivalenol (DON), T-2 toxin (T-2), and fumonisin (FB), all of which exhibit a high melting point, poor solubility, a long half-life, and are carcinogenic, mutagenic, and teratogenic [3]. The International Agency for Research on Cancer (IARC) has classified many natural mycotoxins as being linked to carcinogenicity [4] and recognized aflatoxin as the most serious carcinogen as it has the strongest biological toxicity. Specifically, AF exposure in combination with hepatitis B virus infection increases the risk of liver carcinogens (hepatocellular carcinoma) in some areas of South Africa and China [5,6]. Histological analysis has confirmed that cattle mortality due to liver damage is strongly associated with the consumption of AF-contaminated peanuts [7]. In addition, continuous exposure to high doses of aflatoxin can cause growth retardation in children [8]. Additionally, ochratoxin, which is as harmful as aflatoxin, is often detected in pork for human consumption [9]. According to Petkova-Bocharova et al. [10], as a nephrotoxin, ochratoxin may be related to the Balkan endemic nephropathy and urinary tract tumors. Table 1 lists the mycotoxins commonly found in cereals and their effects.

Table 1.
Common mycotoxins in cereals and their toxicology.
The growing public attention paid to food quality and safety has strengthened the scientific research into mycotoxins and toxin-producing molds in cereals [30,31,32]. Developing countries generally face a greater threat than developed countries because of their inadequate storage and transportation facilities. Moreover, the U.S. Department of Agriculture reports that China’s total grain production reached 548.5 million tons in 2019–2020, accounting for about 20% of the world’s total production, among which more than 10 million tons of cereals were contaminated above national standards [33]. Therefore, the decontamination of mycotoxins in cereals remains a research hotspot.
The growth of molds and mycotoxin production cannot be separated from warm temperatures (28–31 °C) and high humidity (60–90%). The climate in China exhibits significant differences in rainfall, temperature, and humidity across its vast territory, accompanied by seasonality and an extreme climate, making cereals more susceptible to mold contamination [34,35]. Although established physical and chemical methods are widely used to decontaminate cereals, they often result in nutritional losses or chemical residues. Biological (e.g., microbial) decontamination methods present an alternative method for inhibiting the growth of foodborne pathogens and, importantly, keeping mycotoxin concentrations below the prescribed limits [36]. Some of these methods have the advantages of being efficient, economical, and environmentally friendly [37].
Recent developments in microbiology, such as the studies of Mannaa et al. and Simonetti et al. [38,39], which focus on important processes for the microbial decontamination of cereals outside China, have led to a renewed interest in microbial decontamination, indicating a growing trend toward replacing physical or chemical means with microbial methods. The reaction between the studied Stenotrophomonas species and eleven food and feed crops contaminated by trichothecene mycotoxins [40] triggered detoxification effects beyond the researchers’ expectations, highlighting the industrial potential of using such strains to reduce trichothecene contamination in food and feed and to minimize their cytotoxicity.
This review summarizes the status of mycotoxin contamination of major cereal crops, including rice, wheat, and maize, in 30 provincial areas of China between 2005 and 2021. Data were sourced from the Food and Agriculture Organization of the United Nations (FAO), the State Food and Material Reserve Administration, the State Administration of Market Supervision, and the World Health Organization (WHO) databases. The review also analyzes the persistent problems with the established physical and chemical detoxification methods. Against this background, our study proposes practical methods for inhibiting or detoxifying of mycotoxins using microorganisms or active microbial substances and systematically describes the mechanisms of different inhibition or detoxification methods. Finally, it discusses the opportunities and challenges in the practical application of various methods to solve the ongoing problem of mycotoxin contamination in China.
2. Cereal Contamination by Mycotoxins in China and the Existing Decontamination Methods
2.1. Impact of Climate Change on the Mycotoxin Contamination Rate of Cereals in China
China serves as a major global cultivator and preserver of numerous cereals, including maize, wheat, and rice [41]. Above 60% of consumers rely on cereals as their main energy source in this developing country [42]. Due to China’s distinct continental monsoon climate and complex geographical conditions, high temperature, high humidity, and drought are observed in many of its regions annually [43]. These conditions benefit the growth and metabolism of toxin-producing molds [44,45].
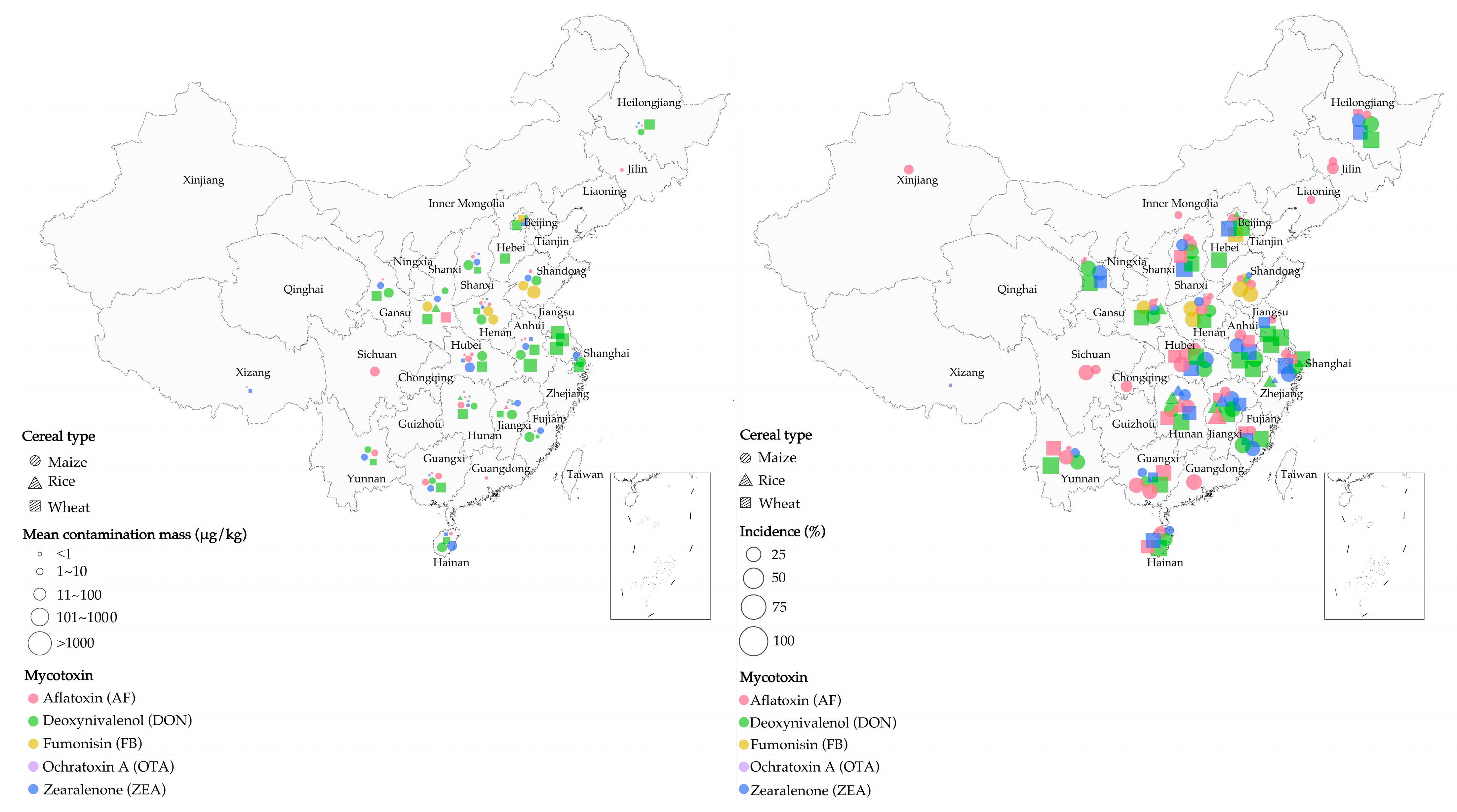
The amount of cereal contaminated by mycotoxins increased due to climate change in southeastern China from 2009 to 2015 [46,47,48]. DON is a mycotoxin secreted by Fusarium species, including F. culmorum, F. pseudograminearum, and F. graminearum, causing Fusarium head blight (FHB) in cereals [49,50]. Their production has been reported to be associated with increased rainfall and higher temperatures [45,46]. From 2010 to 2012, increased rainfall led to severe DON contamination of wheat in Jiangsu Province (Southeastern China) [51]. Additionally, an increasing trend in DON-contaminated maize (n = 50) was observed in Shanghai for 4 consecutive years (from 2009 to 2012), with an average contamination concentration of 130 μg/kg [52]. The above observations demonstrate the strong correlation between AF-contaminated cereals and temperature and humidity in China. Specifically, most molds can multiply at a relative humidity above 70% [53], with Aspergillus flavus being able to multiply consistently at water contents ranging from 94 g/kg to 175 g/kg and temperatures in the range of 30–40 °C [54]. A 2010 survey revealed that the better growth conditions for A. flavus and A. parasiticus Speare in Huaian and Fusui (Southeastern China), as compared to Huantai (Northeastern China), were caused by the lower annual rainfall and temperature in the former regions. This in turn explained the greater amount of aflatoxin B1 (AFB1)-contaminated maize in Huaian and Fusui [47], with >20 μg/kg AFB1 in ~35% of maize samples collected in Huaian [55]. Figure 1 illustrates our review of cereal contamination by mycotoxins in China as calculated by several surveys (a total of 8700 samples). Combined with other findings [56,57,58,59,60,61], this overview demonstrates that the humid and hot climate in southern China is favorable for mycotoxin production by fungi, resulting in more severe cereal contamination in southern China than in the north [41].
Figure 1.
Mycotoxin contamination of cereals (maize, rice, and wheat) in surveys across China. (Left) mean mass of cereal contamination in different regions; (right) incidence of cereal contamination in different regions. (Source: our own study.)
2.2. Correlation between Cereal Types and Mycotoxin Contaminants in China
It is common for specific mycotoxins to only be found in certain cereals in China. For example, among 151 rice samples tested in 3 northeastern provinces of China, the contamination rate of AF reached 63%, while the contamination rate of OTA was only 5.3% [62]. Fumonisin B1 (FB1), secreted by F. moniliforme, was mainly contaminated in maize and its products when the ambient humidity was 18–23%. Figure 1 shows the contamination of maize with FB1 in Henan and Shandong provinces. In warm and humid areas, Alternaria species produce tenuazonic acid (TeA), which is the main mycotoxin present in wheat samples [63,64]. Overall, DON and OTA were found in oat, barley, and gypsophila. AF and ZEN were frequently found in maize, while OTA, T-2, HT-2, and diacetoxyscirpenol (DAS) were not specific to contaminated cereals [60,65,66]. Figure 1 shows the cereals contaminated with different mycotoxins found in various regions of China.
2.3. Existing Mycotoxin Detoxification Methods
In China, mycotoxins in food and feed are mainly removed using established physical and chemical methods. Specifically, physical methods combine light, ultrasound, or ultra-high temperatures with the sorting, washing, and milling process [67], or use UV/gamma radiation [68] during cereal processing to remove mycotoxins, but this may lead to nutrient loss in the food. Chemical methods involve the addition of substances that promote mycotoxin degradation during cereal processing, such as oxidants, ammonium, sodium hydroxide, and diatomite. However, these substances are hard to remove and can remain in cereals [69,70,71]. Moreover, the application of organic solvents for extraction generates wastes and is not environmentally friendly. The advantages and disadvantages of these two approaches for detoxification are analyzed in Table 2. Considering the chemical and thermal stability of most mycotoxins, complete detoxification (i.e., mitigation of their toxicity by altering or shifting the relevant structure) cannot be achieved using conventional measures. What is more, mycotoxin contamination is usually heterogeneous, which poses another challenge for the established treatment methods.

Table 2.
Physical and chemical detoxification methods with analysis.
During the storage of cereal, toxin-producing molds reproduce rapidly, allowing mycotoxins to accumulate in cereals; this causes significant potential economic losses. For example, one study tested 182 cereal samples from cereal silos in Hubei Province, China, and showed that the average content of FB1 was 12.55 mg/kg [79]. To avoid the huge losses caused by mycotoxins in cereals, the Chinese government has updated the thresholds for common mycotoxins in cereals and other agricultural commodities. Manufacturers in food and feed processing should follow the relevant standards in Table 3. In 2019–2020, the Ministry of Agriculture of China issued the National Agricultural Product Quality and Safety Risk Assessment Plan, which took the contamination of common mycotoxins in cereals in different regions of China as a key assessment item. These decisions indicate that mycotoxin contamination in the production and storage of cereals in China had not been effectively controlled previously. Furthermore, in consideration of the serious hazards caused by mycotoxins due to their high toxicity, scientists have recommended enhanced mold protection from early planting to storage and the adoption of more effective detoxification methods [43,80].

Table 3.
Maximum tolerable level of mycotoxins in cereals and other agricultural commodities in China.
3. Microbial Methods of Inhibiting Mycotoxin Growth and Detoxifying Mycotoxins
3.1. Decontamination Methods Based on Microorganisms
The most recent research focus in the field of cereal contamination is the use of microbial methods to inhibit the growth of toxin-producing molds and detoxify mycotoxins. The microorganisms employed include yeasts, lactic acid bacteria, bacilli, non-toxic molds, and marine microorganisms. The microorganisms that play a crucial role in the prevention and control of toxic mold and mycotoxin production in cereals are discussed here, and they are classified according to their different mechanisms.
3.1.1. Adsorption and Binding Using Microorganisms
The physisorption of some microorganisms, especially Saccharomyces cerevisiae strains, can reduce certain mycotoxins in feed and cereals. Stanley et al. [81] demonstrated improved poultry growth after the addition of yeast to AF-contaminated feed. Another study [82] pointed out that the active component of yeast for mycotoxin adsorption is the glucomannan in the yeast cell wall, so both yeast [83] and the isolated yeast cell wall can act as mycotoxin adsorbents. In addition, the modification of yeasts can increase the noncovalent interaction between the side chains of cell walls and toxin molecules. One study demonstrated that yeast modified with β-1,3-glucan adsorbed 81.6%, 27.8%, and 25.6% of AFB1, T-2, and OTA, respectively [84]. Yiannikouris et al. [85] extracted yeast cell walls and tested their adsorption capacity using S. cerevisiae as the raw material. They observed that the affinity of β-D-glucan for different toxin molecules was in the order of AFB1, DON, and OTA from high to low. Similarly, some yeasts modified by the crosslinking-esterification of alkyl ammonium ion glucan compounds had a high adsorption capacity for ZEN (183 mg/g) and T-2 (10 mg/g) [86]. Accordingly, various mycotoxin adsorbents have been developed using yeast cells to reduce the harmful effects of mycotoxins.
Scientists have conducted many studies on the binding sites of lactic acid bacteria and probiotics to mycotoxins, as lactic acid bacteria and several probiotics can be powdered and added to mycotoxin-contaminated cereals to remove mycotoxins [87]. An in vitro comparison of AFB1 binding to Lactobacillus and Propionibacterium revealed that Lacticaseibacillus rhamnosus GG and L. rhamnosus LC705 could bind nearly 80% of AFB1 (5 μg/mL) within one hour at 1010 cfu/mL [88]. After heat inactivation and acid treatment, the two Lactobacillus strains are more effective in reducing AFB1 and exhibit a better binding capacity for ZEN and its derivative α-ZOL [89]. Accordingly, heating and acid treatment may affect the binding sites of probiotics, thereby increasing their binding affinities for AFB1 and reducing its accumulation. Niderkorn et al. [90] found that L. rhamnosus GG, L. delbruekii ssp. bulgaricus R0149, and Leuconostoc mesenteroides R1107 could detoxify ZEN, FB, and several trichothecenes (DON, nivalenol, and T-2), revealing that peptidoglycans in the bacterial cell wall are binding sites for Aspergillus toxins. Other researchers demonstrated that the peptidoglycans of L. rhamnosus GG [91] and the teichoic acid of the Lacticaseibacillus casei strain Shirota [92] are indispensable components for their cell walls binding to AFB1. They are involved in the formation of a reversible complex between the mycotoxin and the microbial surface, and participate in the process of mycotoxin binding and release [93].
Although the addition of appropriate probiotics to cereals can reduce the number of mycotoxins [94], the application of this approach in food and other commodities is limited. This is because many lactic acid bacteria that are generally regarded as safe (GRAS) as bioconjugates require strict anaerobic characteristics and abnormal culture conditions. It is clear that the future development of multiple safe strains for incorporation into contaminated cereals remains challenging. Additionally, more microbial adsorption and binding mechanisms need to be investigated to enable the further exploration of more efficient microbial detoxification pathways.
3.1.2. Biocompetitive Inhibition Using Microorganisms
Biocompetitive inhibition is based on the inoculation of highly competitive non-toxic strains in the soil where the cereal grows, competing with the toxin-producing molds. The non-toxic strains, which are generally from the same species as the toxic strains, are able to reduce or inhibit mycotoxin production to some extent, thus reducing the probability of cereal infection. Such biocompetitive methods were first used by the ARS laboratories in the USA to reduce AFs in cereals [95]. Since then, numerous studies have also demonstrated that non-toxic Aspergillus spp. can be used to reduce Afs in cereals and crops by competing biologically with AF-producing species and inhibiting the latter’s metabolism. Dorner et al. [80] used competition among microorganisms to inoculate the non-toxic A. parasiticus into peanut-producing land to reduce AFB1 content in edible peanuts by 83–98%. Considering the biocompetitive relationships among microbes, another study [96] introduced the Aspergillus strain (atoxigenic) into the soil, which decreased AFB1 content in cotton seeds compared to the control group. The inoculation of corn with non-toxigenic A. flavus K49 and CT3 reduced AFs by 65–94% [97]. Additionally, non-toxic A. flavus NRRL 18543 and NRRL 21882 are also commercially produced nowadays, due to their highly effective removal of mycotoxins from cereals including corn [97,98,99]. Moreover, antagonistic bacteria can inhibit the growth and infection of toxic fungi. For example, Bacillus subtilis and some species of the genus Streptococcus were employed to control wheat scab, and Bacillus polymyxa AFR0406 was shown to prevent wheat scab and sheath blight [100]. Such strains are highly effective in inhibiting mycotoxin production. Although they are able to inhibit the growth of toxin-producing molds under laboratory conditions, their ability to achieve good results in cereals and foods is limited due to the difficulty of bringing bacterial cells to the site of infection. On the other hand, although the purpose of using the biocompetitive method is to find more favorable strains than toxigenic molds, the competition mechanism is still unclear, making it impossible to determine the amount of inoculum required and the suitable treatment conditions.
3.2. Microbial Active Substance Decontamination Methods
Microorganisms can purify cereals by generating active substances or degrading mycotoxins into less toxic or non-toxic substances. The following sections describe these two processes, providing theoretical support for this approach to reducing mycotoxins in cereals.
3.2.1. Inhibition Using Microbial Active Substances
Microorganisms inhibit the growth of toxin-producing molds, and mycotoxins exert toxicity by producing active substances such as secondary metabolites. Table S1 lists many active substances and their action conditions, including polypeptides, small molecular substances, enzymes, and organic acids [101]. Some metabolites of yeast and lactic acid bacteria (e.g., 2-phenylethanol, phenyllactic acid, and indole lactic acid) have antagonistic effects [102,103,104,105,106,107,108]. Multiple antifungal compounds contained in the supernatant of Lactiplantibacillus plantarum K35 completely inhibit the growth and AF production of A. flavus TISTR3041 and A. parasiticus TISTR3276 [108]. Munimbazi et al. [109] found that the fermentation broth of B. pumilus inhibited the production of AF. Later, the same authors identified cyclic polypeptides or nonpeptide compounds in the broth as the active ingredient isolated from B. pumilus [110]. When the concentration of the active substance was 0.2 mg/mL, its inhibition rate of OTA and A. ochraceus NRRL 3174 hyphae was approximately 71% and 76%, respectively. Futhermore, aflastatin, an active substance with antibiotic effects extracted from Streptomyces, was able to prevent the production of Afs by A. parasiticus at a concentration of 0.5 μg/mL [111]. In addition to the active substances extracted from microbial cultures, volatile compounds produced during the metabolism of certain microorganisms can also purify cereals. For example, phenyl ethanol and 1-pentanol, produced by Enterobacter asburiae Vt-7, have been shown to downregulate the expression of AF genes and thus significantly reduce A. flavu contamination of cereals [112]. Studies have also demonstrated that the main volatile compound produced by the four yeasts (Cyberlindnera jadinii 273, Candida friedrichii 778, Candida intermedia 235, and Lachancea thermotolerans 751) is 2-phenylethanol, which makes a significant contribution to the inhibition of growth and OTA production by A. ochraceus and A. carbonarius [102]. Interestingly, the mechanism of inhibition by 2-phenylethanol is similar to that of some Streptomycete spp. in that it inhibits the spore production of toxin-producing molds [107]. Although various microorganisms produce active substances that can inhibit the growth of toxin-producing molds and halt mycotoxin production, the conditions of action for these active substances are complex and varied, posing a significant challenge to the practical application of such substances.
3.2.2. Detoxification Using Microbial Active Substances
In addition to inhibition, detoxification with microbial active substances is one of the main strategies for reducing the contamination of cereals with mycotoxins [113,114,115,116,117,118,119,120,121,122]. Table 4 lists the detoxification conditions and products and detoxification efficiency of various microorganisms. According to the study conducted by Takahashi-Ando et al. [123], the lactone hydrolase ZHD101 produced by Clonostachys rosea IFO 7063 can bind and break the lactone ring of zearalenone. Meanwhile, monooxygenases can oxidize the 12,13-epoxy group of deoxynivalenol. The functional group of mycotoxins are cleaved to form new metabolites that are more easily excreted by the digestive system, which significantly reduces their toxic effects. The active substances produced by specific microorganisms, such as epoxidase, extracellular xylanase, proteases, and esterases, can detoxify DON and ZEN [124,125]. Other active components in cell-free cultures have also been confirmed to detoxify mycotoxins [126,127,128]. According to Teniola et al. [103], cell-free extracts of four species of bacteria (Rhodococcus erythropolis DSM 14303, Nocardia corynebacterioides DSM 12676, Nocardia corynebacterioides DSM 20151, and Mycobacterium fluoranthenivorans sp. nov. DSM 14304) substantially detoxified AFB1 at 30 °C, pH = 7.0. There are relatively few studies on the use of active microbial substances for detoxification, leading to limitations in the further development of efficient detoxification procedures using this approach.

Table 4.
Sources of active microbial substances that detoxify mycotoxins and their conditions of detoxification.
3.3. Other Microbial Inhibition Methods
A great leap in molecular biology relates to the uncovering of the whole genome sequences of ten important Aspergillus species: A. flavus, A. parasiticus, A. fumigatus, A. sojae, A. nomius, A. tamarii, A. pseudotamarii, A. bombycis, A. oryzae, and A. sojae [153], which allowed scientists to explore the biochemical mechanisms of mycotoxin synthesis. A comparison of the genomes of toxin-producing molds with those of similar strains without toxin-producing activity will help to identify the key toxin-producing genes and corresponding enzymes and proteins. Then, the key genes involved in mycotoxin synthesis can be removed by gene knockout, and the resulting non-toxic mold can replace the original toxin-producing strain and alleviate mycotoxin contamination in cereals [154]. Cereals can be genetically modified to enhance the expression of endogenous genes that resist toxin-producing molds and mycotoxin contamination. Additionally, transgenic technology can be used to introduce exogenous genes, thus equipping cereals with antimicrobial properties and reducing mycotoxin contamination [155].
The practical production of the enzyme is difficult due to the complex separation and purification process, its unstable activity, and the harsh survival conditions. This limitation can be overcome by cloning detoxification genes with high activity and by achieving the heterologous expressions of degrading enzyme genes in prokaryotic or eukaryotic engineered strains [156,157] (more information on mechanism (5) is provided in Section 3.4).
Combination enzymes with microorganisms antagonistic to the toxic strain can also increase enzymatic activity and improve the decontamination of mycotoxins [158]. Zuo’s research demonstrates that the addition to chicken feed of probiotics and the AFBl degrading enzyme conjugates resulted in a reduction in toxic substances and a significant improvement in the antioxidant capacity of chicken liver cells and the chicken production performance [159]. However, only a few degradation enzymes have been found to be efficient in degrading mycotoxins. In the future, a larger number of strains need to be screened and studied in depth for their degradation ability, enabling scientists to isolate and purify their degradation enzymes and find the genes that regulate these enzymes. Then, these enzymes will be cloned into heterologous expression vectors so that they can be efficiently used in the actual production of cereals and feed. Furthermore, since bioactive enzymes are highly specific for mycotoxins, efforts should be made to explore appropriate degrading enzymes for rare or even unknown mycotoxins [160,161].
These studies reveal that molecular biotechnology can identify the genes and enzymes responsible for mycotoxin synthesis and transfer genes encoding good mycotoxin degradation properties. Further understanding of the mechanisms of and interactions between toxins and microorganisms will provide a significant theoretical and practical basis for controlling mycotoxins in cereals using biotechnology.
3.4. Mechanism of Mycotoxin Reduction Using Microbiological Methods
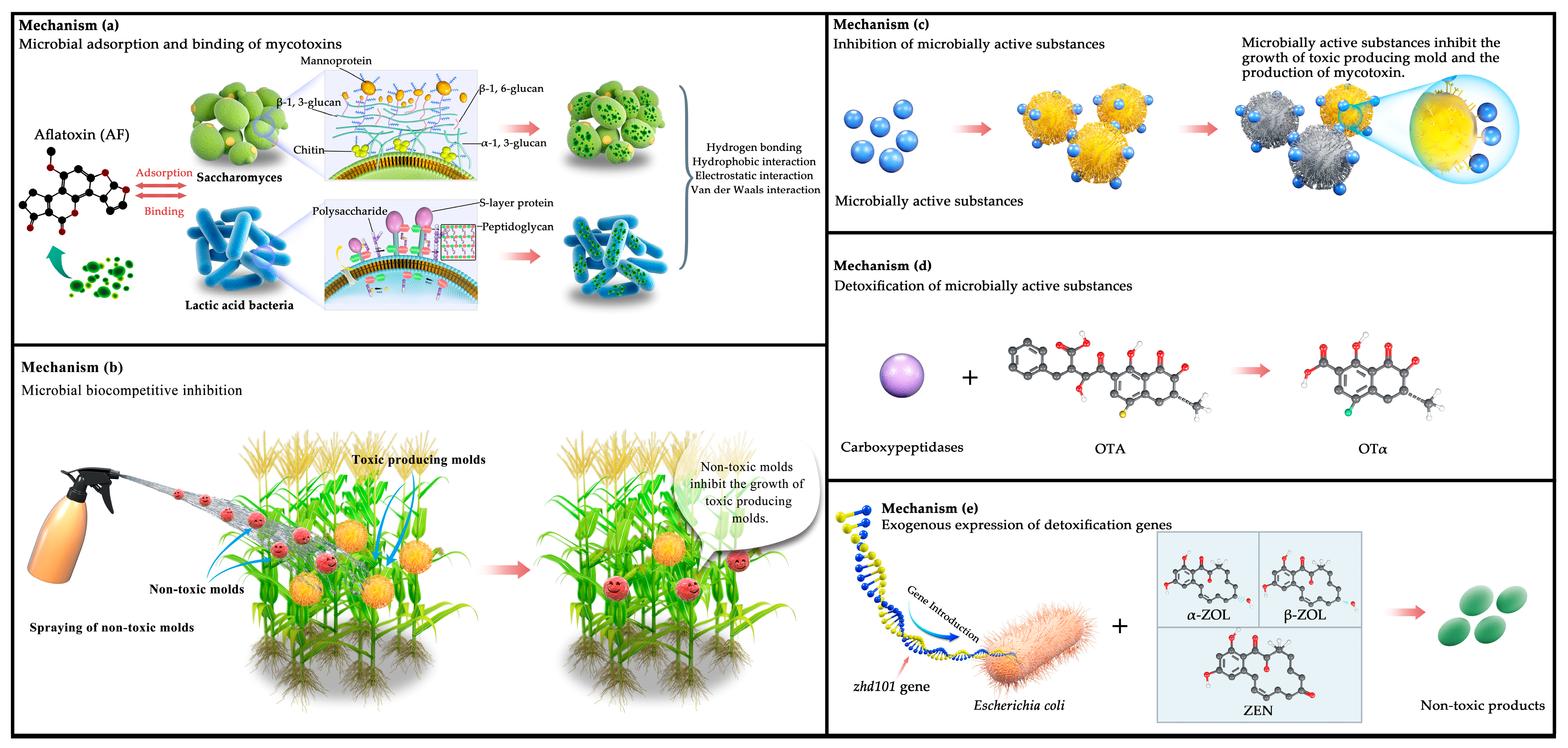
Figure 2 illustrates five mechanisms of mycotoxin reduction: (a) previous studies have demonstrated a direct relationship between the cell walls of beneficial microorganisms (e.g., yeast and lactic acid bacteria) and their adsorption and binding of mycotoxins [162,163]. Specifically, the yeast cell wall consists of three layers: the outer layer with mannose oligosaccharides and protein complexes, the middle layer with dextran, and the inner layer with chitin. Luo et al. [164] reported that the structural framework formed by β-1,3-glucan and chitin in the yeast cell wall provides more meshes for the adsorption of mycotoxins. The higher the density of the meshes, the stronger the adsorption capacity. This structure provides various hydrogen bonding, electrostatic, and hydrophobic interaction sites for mycotoxin adsorption, which enhances the adsorption capacity, as well as contributing to the purification of cereals and feeds. Similarly, peptidoglycans, polysaccharides, and S-layer proteins in the lactic acid bacteria cell wall contribute to the mycotoxin-binding process [91,165], and they generate chemical complexes with mycotoxins via hydrogen bonds, van der Waals forces, and hydrophobic interactions [166,167]. (b) Biocompetition using non-toxic molds is shown in the figure. The atoxigenic strains compete against the toxin-producing molds for nutrition, limiting the latter’s access to appropriate spaces and exhausting nutrients in the wound site. This causes the toxin-producing molds to stop growing due to a lack of nutrients. (c) Microbial active substances can specifically bind to toxin-producing molds, induce oxidative stress, break biochemical reactions, and block the physiological metabolism of certain sensitive fungi, thereby inhibiting the conidial growth and mycotoxin production of these molds. (d) Detoxifying mycotoxins using active microbial substances involves specific, affinity, and high-catalytic enzymes that convert mycotoxins into small non-toxic or less toxic molecules. It involves acetylation, ring cleavage, hydrolysis, glycosylation, deamination, and decarboxylation [143,144,151,152]. For example, Yarrowia lipolytica Y-2, Acinetobacter sp. neg1 ITEM 17016, and B. amyloliquefaciens ASAG1 produce carboxypeptidase, detoxifying ochratoxin under appropriate conditions and producing the less-toxic OTα [146,147,148]. (e) The removal of key genes for mycotoxin synthesis or the enhanced expression of degradation enzyme genes is another method for decreasing mycotoxin levels in cereals. Zhd101 is a new gene encoding a hydrolase of inhibitory metabolites for ZEN [168]; it can be introduced into Escherichia coli and S. cerevisiae to break down ZEN, α-zearalol, and β-zearalol into non-toxic products within 24 h.
Figure 2.
Mechanisms of mycotoxin reduction. (a) Microbial adsorption and binding of mycotoxins; (b) microbial biocompetitive inhibition; (c) inhibition of microbially active substances; (d) detoxification of microbially active substances; (e) exogenous expression of detoxification genes. (Source: our own study).
4. Conclusions and Prospects
In recent years, an increasing amount of research has explored the methods for the mycotoxin decontamination of Chinese cereals and agricultural products [169,170], indicating that the problem still needs to be solved. This review discussed the status of cereal contamination in China. The collected data from 2005 to 2021 show that mycotoxin levels in many cereals still exceed national standards. This is due to the temperature and humidity in China being favorable for the growth of toxin-producing molds, especially in southern China, which has a variable climate and complex geography. Physical and chemical decontamination methods have certain limitations, such as insufficient cereal nutrition, poor safety, and high energy consumption. To further reduce the content of mycotoxins in cereals and avoid the shortcomings of the established methods, it is imperative to develop new methods. This paper presents a variety of practical approaches to biotechnological detoxification, including the microbial adsorption and binding of mycotoxins, the competitive inhibition of the growth of toxin-producing molds or mycotoxin production, or the specific screening of certain microorganisms or enzymes to detoxify or produce non-toxic degradation products by destroying or modifying mycotoxins with the participation of their secondary metabolites or secreted intracellular and extracellular enzymes [171,172]. Furthermore, some previous studies were used to systematically summarize the mechanisms of various biotechnological detoxification methods, providing a more intuitive reference for preventing and controlling cereal contamination in China. Combining these findings with the specific conditions of the Chinese environment is expected to reduce mycotoxin contamination in grains during processing, transportation, and storage. This will reduce the health risks to consumers and the economic losses to the feed industry and animal husbandry.
Based on the expectations outlined in the previous section, the following recommendations are made to ensure that the detoxification process becomes more effective and environmentally friendly: (1) the summer season in southern China is characterized by a temperature of ~30 °C and relatively high humidity, creating the right conditions for active substances. Therefore, depending on the temperature and humidity of the environment, different kinds of microorganisms can be cultivated, and many active substances are produced, which in turn detoxify or inhibit the production of mycotoxins, thus reducing the contamination of cereals. (2) Northern regions of China such as the Liaoning and Jilin Provinces have a dry climate and low temperatures. In these regions, more Spirulina sp. and B. pumilus can be used for cereal detoxification, considering the inhibition conditions of microorganisms presented in Table S1 and Table 4; they will have a good detoxification effect on the main mycotoxins. (3) The typical climatic characteristics of the area north and east of the Qinling–Huaihe line limit the main cereals grown to wheat and corn, which are susceptible to AF and DON infections, respectively. Therefore, spraying active microbial substances produced by Bacillus sp. and Spirulina sp. on cereals will likely yield more effective detoxification effects. (4) Given that the southern region is contaminated with multiple mycotoxins due to the diversity of cereal varieties and frequent rainy seasons, it may be impractical to utilize active substances that specifically degrade one mycotoxin. This prompts the use of multiple probiotic microorganisms to achieve detoxification by the biocompetitive inhibition of toxin-producing molds or adsorption of mycotoxins by beneficial bacteria, such as by using yeast in agricultural fields or barns.
The development of mycotoxin biodegradation and the inhibition of toxin-producing molds has positive effects on the detoxification of cereals and other agricultural commodities. However, several challenges and limitations remain: (1) the current research on microorganisms for adsorption and combined detoxification mainly focuses on yeast and its cell wall, lactic acid bacteria, and other strains, which still cannot meet the needs of cereal food safety. (2) The mode of action of antagonistic microorganisms is unknown, limiting the use of these organisms in field conditions. (3) The complex and diverse conditions of the inhibition of active substances produced by various microorganisms and the relatively few studies that use them for detoxification pose challenges to the effectiveness of cereal detoxification and the further development of efficient detoxification products. (4) There are few studies on degradation enzymes that effectively degrade mycotoxins, posing a challenge to the isolation and purification of degradation enzymes released by microorganisms. In conclusion, much work needs be done to address the current limitations and challenges so that microbial detoxification techniques can be fully used to detoxify cereals and agricultural products. First, since strains have different detoxification effects on different mycotoxins, using a single strain often does not achieve the desired effect. Therefore, it is possible to further improve the detoxification effect by combining multiple strain detoxification methods in future research. Second, most strains currently screened in the natural environment have detoxification effects on cereals but still do not meet the GRAS Act standards. Scientific safety studies could be conducted on the strains known to have detoxification effects so that most strains can be legally added to cereals as soon as possible. Finally, a large number of strains with detoxification abilities can be further screened, the mechanism can be studied in depth, the degradation enzymes released by the strains can be further isolated and purified, the genes regulating the degradation enzymes can be searched for, and then further cloning can be performed to finally achieve efficient expression in vector. In the future, we hope to combine scientific research with Chinese environmental conditions and cereal storage methods to solve the problem of the mycotoxin contamination of cereals and crops as early as possible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13040551/s1, Table S1: Sources of active microbial substances inhibiting toxin-producing molds and mycotoxins and their conditions of action [102,103,104,105,107,108,109,110,111,112]. References [173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197] are cited in the supplementary materials.
Author Contributions
Investigation and data curation, J.Z. and X.T.; resources, W.-W.Z.; writing—original draft preparation, X.T. and J.Z.; writing—review and editing, Y.C. and W.-W.Z.; supervision, W.-W.Z.; funding acquisition, W.-W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Zhejiang Provincial Natural Science Foundation of China (LTGN23C200014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to fork risk assessment of mycotoxins under climate change influences—Recent developments. Trends Food Sci. Technol. 2022, 126, 126–141. [Google Scholar] [CrossRef]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.; Wild, C.P. IARC activities in mycotoxin research. Nat. Toxins 1995, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Q.; Yoshizawa, T.; Kawamura, O.; Luo, X.-Y.; Li, Y.-W. Aflatoxins and Fumonisins in Corn from the High-Incidence Area for Human Hepatocellular Carcinoma in Guangxi, China. J. Agric. Food Chem. 2001, 49, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N. Aflatoxin as a human carcinogen. Hepatology 1999, 30, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Abbitt, B.; Cotter, S.; Murphy, M.; Reagor, J.; Robinson, R.; West, J.; Whitford, H. Bovine abortion and death associated with consumption of aflatoxin-contaminated peanuts. J. Am. Vet. Med. Assoc. 1986, 188, 1187–1188. [Google Scholar]
- Lombard, M.J. Mycotoxin Exposure and Infant and Young Child Growth in Africa: What Do We Know? Ann. Nutr. Metab. 2014, 64 (Suppl. 2), 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Bocharova, T.; Chernozemsky, I.N.; Castegnaro, M. Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary system tumours in Bulgaria. Food Addit. Contam. 1988, 5, 299–301. [Google Scholar] [CrossRef]
- Bbosa, G.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.; Kyegombe, D. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. In Aflatoxins: Recent Advances and Future Prospects; Razzaghi-Abyaneh, M., Ed.; InTech: Rijeka, Croatia, 2013; Volume 3, pp. 239–265. [Google Scholar]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus flavus and Aspergillus parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, R.A., Jr.; Sharma, R.P. Effect of repeated dietary exposure of aflatoxin B1 on brain biogenic amines and metabolites in the rat. Toxicol. Appl. Pharmacol. 1985, 80, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in Dairy Cows: Toxicity, Occurrence in Feedstuffs and Milk and Dietary Mitigation Strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Hernandez, M.; Verde, M.; Sanz, M. Effect of aflatoxin on performance, hematology, and clinical immunology in lambs. Can. J. Vet. Res. 2000, 64, 53–58. [Google Scholar] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Denli, M.; Perez, J.F. Ochratoxins in Feed, a Risk for Animal and Human Health: Control Strategies. Toxins 2010, 2, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Bucci, T.J.; Howard, P.C. Effect of Fumonisin Mycotoxins in Animals. J. Toxicol. Toxin Rev. 1996, 15, 293–302. [Google Scholar] [CrossRef]
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 Promotes Aflatoxin B1 and N-Methyl-N′-nitro-nitrosoguanidine-Initiated Liver Tumors in Rainbow Trout. Toxicol. Appl. Pharmacol. 2001, 172, 29–36. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Wu, D. Effects of zearalenone on proliferation and apoptosis in MCF-7 cells. Zhonghua Yu Fang Yi Xue Za Zhi 2005, 39, 328–331. [Google Scholar] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Dalefield, R. Chapter 21—Mycotoxins and Mushrooms. In Veterinary Toxicology for Australia and New Zealand; Dalefield, R., Ed.; Elsevier: Oxford, UK, 2017; pp. 373–419. [Google Scholar]
- Ahamad, D.B. Toxic Effects of Citrinin in Animals and Poultry. Shanlax Int. J. Vet. Sci. 2019, 5, 12–31. [Google Scholar]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turna, N.S.; Wu, F. Risk assessment of dietary deoxynivalenol exposure in wheat products worldwide: Are new codex DON guidelines adequately protective? Trends Food Sci. Technol. 2019, 89, 11–25. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; de Souza Garcia, F.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, S.; Hu, B.; Zhou, Y.; Liang, Z.; Jia, X.; Huang, M.; Wei, J.; Shi, Z. A comprehensive framework for assessing the impact of potential agricultural pollution on grain security and human health in economically developed areas. Environ. Pollut. 2020, 263, 114653. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Thermophilic Fungi to Dominate Aflatoxigenic/Mycotoxigenic Fungi on Food under Global Warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Kagot, V.; Okoth, S.; De Boevre, M.; De Saeger, S. Biocontrol of Aspergillus and Fusarium Mycotoxins in Africa: Benefits and Limitations. Toxins 2019, 11, 109. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Microbe-Mediated Control of Mycotoxigenic Grain Fungi in Stored Rice with Focus on Aflatoxin Biodegradation and Biosynthesis Inhibition. Mycobiology 2016, 44, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, E.; Roberts, I.N.; Montecchia, M.S.; Gutierrez-Boem, F.H.; Gomez, F.M.; Ruiz, J.A. A novel Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium spp. growth. Microbiol. Res. 2018, 206, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ahad, R.; Zhou, T.; Lepp, D.; Pauls, K.P. Microbial detoxification of eleven food and feed contaminating trichothecene mycotoxins. BMC Biotechnol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Halder, J.; White, R.P.; Hughes, D.J.; Ye, Z.; Wang, C.; Xu, R.; Gan, B.; Fitt, B.D.L. Climate change increases risk of fusarium ear blight on wheat in central China. Ann. Appl. Biol. 2014, 164, 384–395. [Google Scholar] [CrossRef]
- Abate, D.; Mitiku, F.; Negash, R. Commercialization level and determinants of market participation of smallholder wheat farmers in northern Ethiopia. Afr. J. Sci. Technol. Innov. Dev. 2022, 14, 428–439. [Google Scholar] [CrossRef]
- Fei, L.; Meijun, Z.; Jiaqi, S.; Zehui, C.; Xiaoli, W.; Jiuchun, Y. Maize, wheat and rice production potential changes in China under the background of climate change. Agric. Sys. 2020, 182, 102853. [Google Scholar] [CrossRef]
- WHO; FAO. Report of the Expert Meeting on Ciguatera Poisoning: Rome, 19–23 November 2018; Food Safaty and Quality Series No. 9. WHO: Rome, Italy, 2020. [Google Scholar]
- Liu, C.; Van der Fels-Klerx, H.J. Quantitative Modeling of Climate Change Impacts on Mycotoxins in Cereals: A Review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Wang, Y.; Zhou, L.; Zhao, Y.; Xing, F.; Dai, X.; Liu, Y. Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, W.; Zhang, P.; Sun, H.Y.; Zhang, X.X.; Zhang, A.X.; Chen, H.G. Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in eastern China. Plant Pathol. 2020, 69, 240–248. [Google Scholar] [CrossRef]
- Cui, L.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Zhou, L.; Liu, Y. A minor survey of deoxynivalenol in Fusarium infected wheat from Yangtze–Huaihe river basin region in China. Food Control 2013, 30, 469–473. [Google Scholar] [CrossRef]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.; De Saeger, S.; Wu, A. Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: Case study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef]
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Saïd, S.; Sanchis, V. Influence of temperature, water activity and incubation time on fungal growth and production of ochratoxin A and zearalenone by toxigenic Aspergillus tubingensis and Fusarium incarnatum isolates in sorghum seeds. Int. J. Food Microbiol. 2017, 242, 53–60. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus flavus: A Literature Review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.-S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, S.; Yu, M.; Sun, Y.; Xu, J.; Shi, J. Natural occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in various wheat cultivars grown in Jiangsu province, China. World Mycotoxin J. 2017, 10, 285–293. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin Contamination of Maize in China. Compr. Rev. Food Sci. Food Saf. 2017, 16, 835–849. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.M. Contamination of aflatoxins in different kinds of foods in China. Biomed. Environ. Sci. 2007, 20, 483–487. [Google Scholar]
- Zhu, F.; Wang, L.; Lin, Y. Investigation of mycotoxin contamination on common sheep feed in Shandong province. Chin. J. Anim. Sci. 2014, 10, 72–76. [Google Scholar]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Shi, H.; Li, S.; Bai, Y.; Prates, L.L.; Lei, Y.; Yu, P. Mycotoxin contamination of food and feed in China: Occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Zhao, K.; Shao, B.; Yang, D.; Li, F.; Zhu, J. Natural Occurrence of Alternaria Toxins in Wheat-Based Products and Their Dietary Exposure in China. PLoS ONE 2015, 10, e0132019. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, K.; Fan, Z.; Diana Di Mavungu, J.; Dong, M.; Guo, W.; Fan, K.; Campbell, K.; Zhao, Z.; Wu, Y. Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control 2017, 79, 177–184. [Google Scholar] [CrossRef]
- Xing, F.; Liu, X.; Wang, L.; Selvaraj, J.N.; Jin, N.; Wang, Y.; Zhao, Y.; Liu, Y. Distribution and variation of fungi and major mycotoxins in pre- and post-nature drying maize in North China Plain. Food Control 2017, 80, 244–251. [Google Scholar] [CrossRef]
- Temba, M.C.; Njobeh, P.B.; Kayitesi, E. Storage stability of maize-groundnut composite flours and an assessment of aflatoxin B1 and ochratoxin A contamination in flours and porridges. Food Control 2017, 71, 178–186. [Google Scholar] [CrossRef]
- Albuzaudi, M.; Eerikäinen, T.; Turunen, O.; Ghelawi, M.; El Haj Assad, M.; Tawalbeh, M.; Bedade, D.; Shamekh, S. Effect of gamma irradiation and heat treatment on the artificial contamination of maize grains by Aspergillus flavus Link NRRL 5906. J. Stored Prod. Res. 2017, 71, 57–63. [Google Scholar] [CrossRef]
- Basappa, S.; Shantha, T. Methods for detoxification of aflatoxins in foods and feeds-a critical appraisal. J. Food Sci. Technol. 1996, 33, 95–107. [Google Scholar]
- Bata, Á.; Lásztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar] [CrossRef]
- Womack, E.D.; Brown, A.E.; Sparks, D.L. A recent review of non-biological remediation of aflatoxin-contaminated crops. J. Sci. Food Agric. 2014, 94, 1706–1714. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, D.; Basílico, J.C.; González, R.J.; Torres, R.L.; De Greef, D.M. Mycotoxins inactivation by extrusion cooking of corn flour. Lett. Appl. Microbiol. 2001, 33, 144–147. [Google Scholar] [CrossRef]
- Samarajeewa, U.; Sen, A.C.; Cohen, M.D.; Wei, C.I. Detoxification of Aflatoxins in Foods and Feeds by Physical and Chemical Methods 1. J. Food Prot. 1990, 53, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, B.N.; Shah, M.A.; Sofi, S.A.; Hamdani, A.M.; Oliveira, C.A.F.; Hashemi Moosavi, M.; Mousavi Khaneghah, A.; Sant’Ana, A.S. Application of new technologies in decontamination of mycotoxins in cereal grains: Challenges, and perspectives. Food Chem. Toxicol. 2021, 148, 111976. [Google Scholar] [CrossRef]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Int. 2020, 36, 193–214. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, O.; Venâncio, A. Ozone applications to prevent and degrade mycotoxins: A review. Drug Metab. Rev. 2010, 42, 612–620. [Google Scholar] [CrossRef]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin Contamination of Rice in China. J. Food Sci. 2017, 82, 573–584. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Blankenship, P.D. Use of a Biocompetitive Agent to Control Preharvest Aflatoxin in Drought Stressed Peanuts. J. Food Prot. 1992, 55, 888–892. [Google Scholar] [CrossRef]
- Stanley, V.G.; Ojo, R.; Woldesenbet, S.; Hutchinson, D.H.; Kubena, L.F. The use of Saccharomyces cerevisiae to suppress the effects of aflatoxicosis in broiler chicks. Poult. Sci. 1993, 72, 1867–1872. [Google Scholar] [CrossRef]
- Dvegowda, G.; Raju, M.V.L.N.; Swamy, H.D.N. Mycotoxins: Novel solutions for their counteraction. Feedstuffs 1998, 70, 12–17. [Google Scholar]
- Armando, M.; Dogi, C.; Pizzolitto, R.; Escobar, F.; Peirano, M.; Salvano, M.; Sabini, L.; Combina, M.; Dalcero, A.; Cavaglieri, L. Saccharomyces cerevisiae strains from animal environment with in vitro aflatoxin B1 binding ability and anti-pathogenic bacterial influence. World Mycotoxin J. 2011, 4, 59–68. [Google Scholar] [CrossRef]
- Raju, M.V.L.N.; Devegowda, G. Esterified-Glucomannan in Broiler Chicken Diets-Contaminated with Aflatoxin, Ochratoxin and T-2 Toxin: Evaluation of its Binding Ability (in vitro) and Efficacy as Immunomodulator. Asian-Australas. J. Anim. Sci. 2002, 15, 1051–1056. [Google Scholar] [CrossRef]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.-G.; Jeminet, G.; Bertin, G.; Jouany, J.-P. Chemical and Conformational Study of the Interactions Involved in Mycotoxin Complexation with β-d-Glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Freimund, S.; Sauter, M.; Rys, P. Efficient Adsorption of the Mycotoxins Zearalenone and T-2 Toxin on a Modified Yeast Glucan. J. Environ. Sci. Health Part B 2003, 38, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef] [PubMed]
- Haskard Carolyn, A.; El-Nezami Hani, S.; Kankaanpää Pasi, E.; Salminen, S.; Ahokas Jorma, T. Surface Binding of Aflatoxin B1 by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef]
- El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkänen, H. Binding Rather Than Metabolism May Explain the Interaction of Two Food-Grade Lactobacillus Strains with Zearalenone and Its Derivative ά-Zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [Google Scholar] [CrossRef]
- Niderkorn, V.; Boudra, H.; Morgavi, D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006, 101, 849–856. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Guzman-de-Peña, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical Adsorption of Aflatoxin B1 by Lactic Acid Bacteria and Saccharomyces cerevisiae: A Theoretical Model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef]
- Oluwafemi, F.; Silva, F.A.d. Removal of aflatoxins by viable and heat-killed Lactobacillus species isolated from fermented maize. J. Appl. Biosci. 2009, 16, 871–876. [Google Scholar]
- Cleveland, T.E.; Dowd, P.F.; Desjardins, A.E.; Bhatnagar, D.; Cotty, P.J. United States Department of Agriculture—Agricultural Research Service research on pre-harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 2003, 59, 629–642. [Google Scholar]
- Cotty, P. Influence of field application of an atoxigenic strain of Aspergillus flavus on the populations of A. flavus infecting cotton bolls and on the aflatoxin content of cottonseed. Phytopathology 1994, 84, 1270–1277. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Bruns, H.A.; Abel, C.A. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2006, 16, 437–449. [Google Scholar] [CrossRef]
- Probst, C.; Bandyopadhyay, R.; Price, L.E.; Cotty, P.J. Identification of Atoxigenic Aspergillus flavus Isolates to Reduce Aflatoxin Contamination of Maize in Kenya. Plant Dis. 2010, 95, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J.; Connick, W.J.; Daigle, D.J.; McGuire, M.R.; Shasha, B.S. Evaluation of biological control formulations to reduce aflatoxin contamination in peanuts. Biol. Control 2003, 26, 318–324. [Google Scholar] [CrossRef]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Lipps, P.E.; Slininger, P.J. Field testing of antagonists of Fusarium head blight incited by Gibberella zeae. Biol. Control 2004, 29, 245–255. [Google Scholar] [CrossRef]
- Leite de Souza, E. Insights into the current evidence on the effects of essential oils toward beneficial microorganisms in foods with a special emphasis to lactic acid bacteria—A review. Trends Food Sci. Technol. 2021, 114, 333–341. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z.; Bakar, F.A.; Saari, N. Identification of antifungal peptides produced by Lactobacillus plantarum IS10 grown in the MRS broth. Food Control 2016, 59, 27–30. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, L.; Pang, J.; Hu, Y.; Guo, Q.; Li, Z.; Wu, S.; Liu, H.; Wang, C. Biocontrol activity of volatile organic compounds from Streptomyces alboflavus TD-1 against Aspergillus flavus growth and aflatoxin production. J. Microbiol. 2019, 57, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sangmanee, P.; Hongpattarakere, T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control 2014, 40, 224–233. [Google Scholar] [CrossRef]
- Munimbazi, C.; Bullerman, L.B. Inhibition of aflatoxin production of Aspergillus parasiticus NRRL 2999 by Bacillus pumilus. Mycopathologia 1997, 140, 163–169. [Google Scholar] [CrossRef]
- Munimbazi, C.; Bullerman, L.B. Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J. Appl. Microbiol. 1998, 84, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sakuda, S.; Suzuki, A.; Isogai, A. Aflastatin A, a novel inhibitor of aflatoxin production by aflatoxigenic fungi. J. Antibiot. 1997, 50, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.-D.; Dong, F.-Y.; Hu, M.-J.; Kong, X.-W.; Wei, F.-F.; Gong, S.-J.; Zhang, Y.-M.; Zhang, J.-B.; Wu, A.-B.; Liao, Y.-C. Antifungal activity of volatile emitted from Enterobacter asburiae Vt-7 against Aspergillus flavus and aflatoxins in peanuts during storage. Food Control 2019, 106, 106718. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Jin, Y.; Wu, C.; Shen, D.; Zhang, S.; Zhou, R. Aflatoxin B1 degradation by salt tolerant Tetragenococcus halophilus CGMCC 3792. Food Chem. Toxicol. 2018, 121, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Jin, Y.; Wu, C.; Shen, D.; Zhang, S.; Zhou, R. Mechanism and kinetics of degrading aflatoxin B1 by salt tolerant Candida versatilis CGMCC 3790. J. Hazard. Mater. 2018, 359, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Stander, M.A.; Bornscheuer, U.T.; Henke, E.; Steyn, P.S. Screening of Commercial Hydrolases for the Degradation of Ochratoxin A. J. Agric. Food Chem. 2000, 48, 5736–5739. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, M.; Dong, F.; Shi, J.; Xu, J. Esterase activity inspired selection and characterization of zearalenone degrading bacteria Bacillus pumilus ES-21. Food Control 2017, 77, 57–64. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2011, 314, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.; Liu, Z.; Shi, Y.; Liu, J.; Xu, X.; Hao, S.; Mu, P.; Deng, F.; Deng, Y. Aflatoxin B1 Degradation and Detoxification by Escherichia coli CG1061 Isolated From Chicken Cecum. Front. Pharmacol. 2019, 9, 130. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Gong, A.; Liu, N.; Chen, S.; Zhao, X.; Li, X.; Chen, L.; Zhou, C.; Wang, J. Biodegradation of mycotoxin fumonisin B1 by a novel bacterial consortium SAAS79. Appl. Microbiol. Biotechnol. 2019, 103, 7129–7140. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Tang, X.; Zheng, X.; Zhang, Y.; Liu, S. Screening and Application of Bacillus Strains to Degrade Aflatoxin B1. Patent No. CN108102963A, 1 June 2018. [Google Scholar]
- Takahashi-Ando, N.; Kimura, M.; Kakeya, H.; Osada, H.; Yamaguchi, I. A novel lactonohydrolase responsible for the detoxification of zearalenone: Enzyme purification and gene cloning. Biochem. J. 2002, 365, 1–6. [Google Scholar] [CrossRef]
- Yeong-Hsiang, C.; Ching-Feng, W.; Bao-Ji, C.; Ming-Huang, C. Toxicity of different Fusarium mycotoxins on growth performance, immune responses and efficacy of a mycotoxin degrading enzyme in pigs. Anim. Res. 2006, 55, 579–590. [Google Scholar]
- Utermark, J.; Karlovsky, P. Role of Zearalenone Lactonase in Protection of Gliocladium roseum from Fungitoxic Effects of the Mycotoxin Zearalenone. Appl. Environ. Microbiol. 2007, 73, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Altalhi, A.D.; El-Deeb, B. Localization of zearalenone detoxification gene(s) in pZEA-1 plasmid of Pseudomonas putida ZEA-1 and expressed in Escherichia coli. J. Hazard. Mater. 2009, 161, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Teniola, O.D.; Addo, P.A.; Brost, I.M.; Färber, P.; Jany, K.D.; Alberts, J.F.; van Zyl, W.H.; Steyn, P.S.; Holzapfel, W.H. Degradation of aflatoxin B1 by cell-free extracts of Rhodococcus erythropolis and Mycobacterium fluoranthenivorans sp. nov. DSM44556T. Int. J. Food Microbiol. 2005, 105, 111–117. [Google Scholar] [CrossRef]
- Tinyiro, S.E.; Wokadala, C.; Xu, D.; Yao, W. Adsorption and degradation of zearalenone by bacillus strains. Folia Microbiol. 2011, 56, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Eisa Ahmed, M.F.; Sangare, L.; Zhao, Y.; Selvaraj, J.N.; Xing, F.; Wang, Y.; Yang, H.; Liu, Y. Novel Aflatoxin-Degrading Enzyme from Bacillus shackletonii L7. Toxins 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, M.; Shi, Z.-Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control 2012, 23, 100–106. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Wei, X.; Wang, S.; Qian, C. High-Efficiency Fermentation Method of Aflatoxin-Degrading Bacteria. Patent No. CN111154679A, 15 May 2020. [Google Scholar]
- Wang, Y.; Zhang, H.; Yan, H.; Yin, C.; Liu, Y.; Xu, Q.; Liu, X.; Zhang, Z. Effective Biodegradation of Aflatoxin B1 Using the Bacillus licheniformis (BL010) Strain. Toxins 2018, 10, 497. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Zhou, Q.; Li, M.; Hu, H.; Ma, Y.; Chen, X.; Ni, J.; Zhao, W.; Huang, S.; et al. Biological Degradation of Aflatoxin B1 by Cell-Free Extracts of Bacillus velezensis DY3108 with Broad PH Stability and Excellent Thermostability. Toxins 2018, 10, 330. [Google Scholar] [CrossRef]
- Gu, X.; Sun, J.; Cui, Y.; Wang, X.; Sang, Y. Biological degradation of aflatoxin M1 by Bacillus pumilus E-1-1-1. MicrobiologyOpen 2019, 8, e00663. [Google Scholar] [CrossRef]
- Shao, S.; Cai, J.; Du, X.; Wang, C.; Lin, J.; Dai, J. Biotransformation and detoxification of aflatoxin B1 by extracellular extract of Cladosporium uredinicola. Food Sci. Biotechnol. 2016, 25, 1789–1794. [Google Scholar] [CrossRef]
- Sangare, L.; Zhao, Y.; Folly, Y.M.; Chang, J.; Li, J.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Liu, Y. Aflatoxin B1 Degradation by a Pseudomonas Strain. Toxins 2014, 6, 3028–3040. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Mehta, A. Protein-mediated degradation of aflatoxin B1 by Pseudomonas putida. Braz. J. Microbiol. 2019, 50, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Li, W.-Y.; Hsu, I.N.; Liao, Y.-Y.; Yang, C.-Y.; Taylor, M.C.; Liu, Y.-F.; Huang, W.-H.; Chang, H.-H.; Huang, H.-L.; et al. Recombinant Aflatoxin-Degrading F420H2-Dependent Reductase from Mycobacterium smegmatis Protects Mammalian Cells from Aflatoxin Toxicity. Toxins 2019, 11, 259. [Google Scholar] [CrossRef]
- Elaasser, M.; El Kassas, R. Detoxification of aflatoxin B1 by certain bacterial species isolated from Egyptian soil. World Mycotoxin J. 2011, 4, 169–176. [Google Scholar] [CrossRef]
- Sokoutifar, R.; Razavilar, V.; Anvar, A.A.; Shoeiby, S. Degraded aflatoxin M1 in artificially contaminated fermented milk using Lactobacillus acidophilus and Lactobacillus plantarum affected by some bio-physical factors. J. Food Saf. 2018, 38, e12544. [Google Scholar] [CrossRef]
- Smiley, R.D.; Draughon, F.A. Preliminary evidence that degradation of aflatoxin B1 by Flavobacterium aurantiacum is enzymatic. J. Food Prot. 2000, 63, 415–418. [Google Scholar] [CrossRef]
- Guan, S.; Ji, C.; Zhou, T.; Li, J.; Ma, Q.; Niu, T. Aflatoxin B1 Degradation by Stenotrophomonas Maltophilia and Other Microbes Selected Using Coumarin Medium. Int. J. Mol. Sci. 2008, 9, 1489–1503. [Google Scholar] [CrossRef]
- Yi, P.-J.; Pai, C.-K.; Liu, J.-R. Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J. Microbiol. Biotechnol. 2011, 27, 1035–1043. [Google Scholar] [CrossRef]
- He, X.; Li, S.; Li, Y.; Gu, W.; Sun, Y.; Sun, X. Evaluation of reduced toxicity of zearalenone as measured by the Hep G2 cell assay on degradation enzymes. Food Control 2015, 57, 161–168. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Venâncio, A. Isolation and purification of an enzyme hydrolyzing ochratoxin A from Aspergillus niger. Biotechnol. Lett. 2007, 29, 1909–1914. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Apaliya, M.T.; Zhao, L.; Gu, X.; Zheng, X.; Hu, W.; Zhang, H. The mechanisms involved in ochratoxin A elimination by Yarrowia lipolytica Y-2. Ann. Appl. Biol. 2018, 173, 164–174. [Google Scholar] [CrossRef]
- Liuzzi, V.C.; Fanelli, F.; Tristezza, M.; Haidukowski, M.; Picardi, E.; Manzari, C.; Lionetti, C.; Grieco, F.; Logrieco, A.F.; Thon, M.R.; et al. Transcriptional Analysis of Acinetobacter sp. neg1 Capable of Degrading Ochratoxin A. Front. Microbiol. 2017, 7, 2162. [Google Scholar]
- Chang, X.; Wu, Z.; Wu, S.; Dai, Y.; Sun, C. Degradation of ochratoxin A by Bacillus amyloliquefaciens ASAG1. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 564–571. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.Y.; Xu, S.H.; Yuan, G.Q.; Shi, X.J.; Liang, Z.H. Degradation of ochratoxin A by supernatant and ochratoxinase of Aspergillus niger W-35 isolated from cereals. World Mycotoxin J. 2020, 13, 287–298. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Yin, T.; Wang, J.; Zhang, X. Heterologous Expression and Characterization of A Novel Ochratoxin A Degrading Enzyme, N-acyl-L-amino Acid Amidohydrolase, from Alcaligenes faecalis. Toxins 2019, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.D.; Grabherr, R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Schatzmayr, G.; Moll, W.D.; Grabherr, R. An aminotransferase from bacterium ATCC 55552 deaminates hydrolyzed fumonisin B1. Biodegradation 2011, 22, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Matsushima, K.; Takahashi, T.; Yu, J.; Abe, K.; Bhatnagar, D.; Yuan, G.-F.; Koyama, Y.; Cleveland, T.E. Understanding nonaflatoxigenicity of Aspergillus sojae: A windfall of aflatoxin biosynthesis research. Appl. Microbiol. Biotechnol. 2007, 76, 977–984. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pusztahelyi, T.; Pócsi, I. Mycotoxins—Prevention and decontamination by yeasts. J. Basic Microbiol. 2015, 55, 805–818. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Dou, K.; Lu, Z.; Wang, X.; Li, Y.; Chen, J. Enhanced biocontrol activity of cellulase from Trichoderma harzianum against Fusarium graminearum through activation of defense-related genes in maize. Physiol. Mol. Plant Pathol. 2018, 103, 130–136. [Google Scholar] [CrossRef]
- Ece, S.; Lambertz, C.; Fischer, R.; Commandeur, U. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. AMB Express 2017, 7, 86. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H.; Tang, Y.; Qiu, L. Cloning, expression of a peroxiredoxin gene from Acinetobacter sp. SM04 and characterization of its recombinant protein for zearalenone detoxification. Microbiol. Res. 2012, 167, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Zhu, Q.; Lu, F.; Gao, T. Effect of the combined compound probiotics with mycotoxin-degradation enzyme on detoxifying aflatoxin B1 and zearalenone. J. Toxicol. Sci. 2018, 43, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.Y.; Chang, J.; Yin, Q.Q.; Wang, P.; Yang, Y.R.; Wang, X.; Wang, G.Q.; Zheng, Q.H. Effect of the combined probiotics with aflatoxin B₁-degrading enzyme on aflatoxin detoxification, broiler production performance and hepatic enzyme gene expression. Food Chem. Toxicol. 2013, 59, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Yu, D.; Liu, N.; Wu, A. Degrading Ochratoxin A and Zearalenone Mycotoxins Using a Multifunctional Recombinant Enzyme. Toxins 2019, 11, 301. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of Four Major Mycotoxins by Eight Manganese Peroxidases in Presence of a Dicarboxylic Acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef]
- Armando, M.R.; Pizzolitto, R.P.; Dogi, C.A.; Cristofolini, A.; Merkis, C.; Poloni, V.; Dalcero, A.M.; Cavaglieri, L.R. Adsorption of ochratoxin A and zearalenone by potential probiotic Saccharomyces cerevisiae strains and its relation with cell wall thickness: Ochratoxin A and zearalenone adsorption by yeasts. J. Appl. Microbiol. 2012, 113, 256–264. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, Y.; Yue, T.; Hatab, S.; Wang, Z. Binding mechanism of patulin to heat-treated yeast cell. Lett. Appl. Microbiol. 2012, 55, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, J.; Liu, B.; Wang, Z.; Yuan, Y.; Yue, T. Effect of Yeast Cell Morphology, Cell Wall Physical Structure and Chemical Composition on Patulin Adsorption. PLoS ONE 2015, 10, e0136045. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Haskard, C.; Binnion, C.; Ahokas, J. Factors affecting the sequestration of aflatoxin by Lactobacillusrhamnosus strain GG. Chem. Biol. Interact. 2000, 128, 39–49. [Google Scholar] [CrossRef]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 Binding by Dairy Strains of Lactic Acid Bacteria and Bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Ohsato, S.; Shibata, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Metabolism of Zearalenone by Genetically Modified Organisms Expressing the Detoxification Gene from Clonostachys rosea. Appl. Environ. Microbiol. 2004, 70, 3239–3245. [Google Scholar] [PubMed]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural Occurrence of Alternaria Toxins in the 2015 Wheat from Anhui Province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Feng, Y.; He, X.; Zhang, D.; Wang, W.; Liu, D. Mycotoxins in livestock feed in China—Current status and future challenges. Toxicon 2022, 214, 112–120. [Google Scholar] [CrossRef]
- Abdi, M.; Asadi, A.; Maleki, F.; Kouhsari, E.; Fattahi, A.; Ohadi, E.; Lotfali, E.; Ahmadi, A.; Ghafouri, Z. Microbiological Detoxification of Mycotoxins: Focus on Mechanisms and Advances. Infect. Disord. Drug Targets 2021, 21, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, L.; Sun, J.; Wang, L.; Guo, H.; Ye, Y.; Sun, X. Microbial detoxification of mycotoxins in food and feed. Crit. Rev. Food Sci. Nutr. 2022, 62, 4951–4969. [Google Scholar] [CrossRef]
- Siahmoshteh, F.; Hamidi-Esfahani, Z.; Spadaro, D.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 2018, 89, 300–307. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, Q.; Liang, Y. Three newly identified peptides from Bacillus megaterium strongly inhibit the growth and aflatoxin B1 production of Aspergillus flavus. Food Control 2019, 95, 41–49. [Google Scholar] [CrossRef]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. MicrobiologyOpen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63. [Google Scholar] [CrossRef]
- Abdel-Kareem, M.M.; Rasmey, A.M.; Zohri, A.A. The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Lett. Appl. Microbiol. 2019, 68, 104–111. [Google Scholar] [CrossRef]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.M.d.; Prietto, L.; Ribeiro, A.C.; Souza, T.D.d.; Badiale-Furlong, E. Assessment of the antifungal activity of Spirulina platensis phenolic extract against Aspergillus flavus. Ciênc. Agrotecnol. 2011, 35, 1050–1058. [Google Scholar]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.H.; El-Agamy, M.A. Antifungal action of Pichia anomala against aflatoxigenic Aspergillus flavus and its application as a feed supplement. J. Sci. Food Agric. 2013, 93, 3259–3263. [Google Scholar] [CrossRef]
- Yoshinari, T.; Akiyama, T.; Nakamura, K.; Kondo, T.; Takahashi, Y.; Muraoka, Y.; Nonomura, Y.; Nagasawa, H.; Sakuda, S. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology 2007, 153, 2774–2780. [Google Scholar] [CrossRef]
- Shakeel, Q.; Lyu, A.; Zhang, J.; Wu, M.; Li, G.; Hsiang, T.; Yang, L. Biocontrol of Aspergillus flavus on Peanut Kernels Using Streptomyces yanglinensis 3-10. Front. Microbiol. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Huang, W.Q.; Li, Z.W.; Lu, D.L.; Zhang, Y.; Luo, X.C. Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme Microb. Technol. 2018, 112, 35–42. [Google Scholar] [CrossRef]
- Akocak, P.B.; Churey, J.J.; Worobo, R.W. Antagonistic effect of chitinolytic Pseudomonas and Bacillus on growth of fungal hyphae and spores of aflatoxigenic Aspergillus flavus. Food Biosci. 2015, 10, 48–58. [Google Scholar] [CrossRef]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. BioControl 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: Identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 1998, 50, 253–256. [Google Scholar] [CrossRef]
- Klich, M.A.; Lax, A.R.; Bland, J.M. Inhibition of some mycotoxigenic fungi by iturin A, a peptidolipid produced by Bacillus subtilis. Mycopathologia 1991, 116, 77–80. [Google Scholar] [CrossRef]
- Medina, Á.; Jiménez, M.; Mateo, R.; Magan, N. Efficacy of natamycin for control of growth and ochratoxin A production by Aspergillus carbonarius strains under different environmental conditions. J. Appl. Microbiol. 2007, 103, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Masoud, W.; Poll, L.; Jakobsen, M. Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 2005, 22, 1133–1142. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Investigation and Application of Bacillus licheniformis Volatile Compounds for the Biological Control of Toxigenic Aspergillus and Penicillium spp. ACS Omega 2019, 4, 17186–17193. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; de Oliveira Garcia, S.; Badiale-Furlong, E. Inhibition of in vitro trichothecenes production by microalgae phenolic extracts. Food Res. Int. 2019, 124, 175–180. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Wang, K.; Brian, K.; Liu, C.; Gu, Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010, 101, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Shatha, A.; Shafiq, S. Antagonistic activity of probiotic and sea weed extract against vegetative growth for some fungi and Zearalenone production. World J. Pharm. Res. 2015, 4, 1577–1585. [Google Scholar]
- Mahdi, L.H.; Shafiq, S.A.; Ajaa, H. Effects of crude and purified bacteriocin of Pediococcus pentosaceus on the growth and zearalenone production by Fusarium graminearum. Int. J. Curr Eng. Technol. 2013, 4, 2277–4106. [Google Scholar]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.-Y.; Jung, K.S.; Jang, J.Y.; Park, J.-C.; et al. Characterization of Bacillus amyloliquefaciens DA12 Showing Potent Antifungal Activity against Mycotoxigenic Fusarium Species. Plant Pathol. J. 2017, 33, 499–507. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76-3 from Wheat Spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).