Multi-Omics Unravels Metabolic Alterations in the Ileal Mucosa of Neonatal Piglets Receiving Total Parenteral Nutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. TPN Piglet Model
2.2. Intestinal Morphology Assessment
2.3. Metabolomic Profiles and Data Processing
2.4. Proteomic Profiles and Data Processing
2.5. Biochemical Measurement
2.6. Statistical Analysis
3. Results
3.1. General Status of TPN Piglets
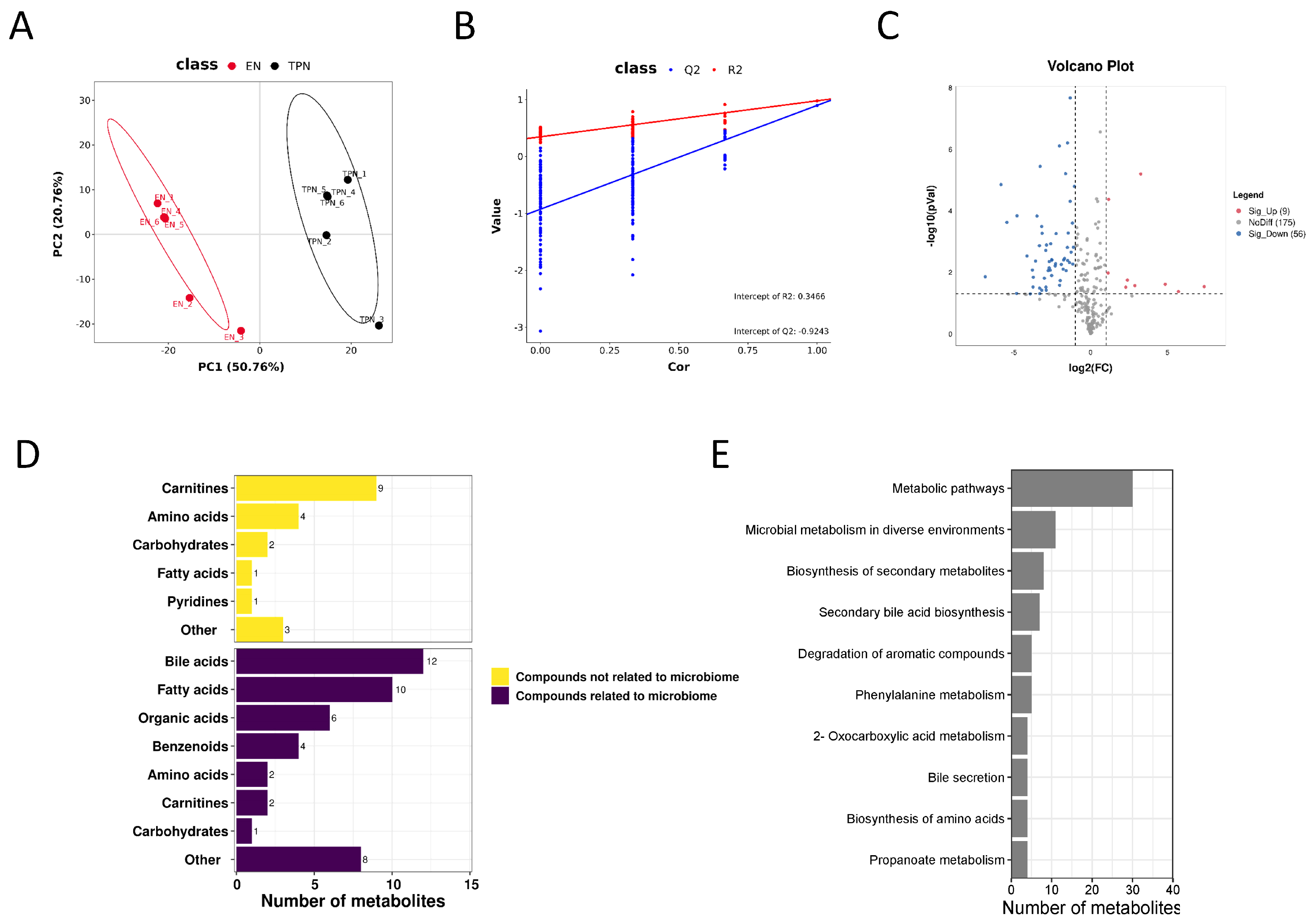
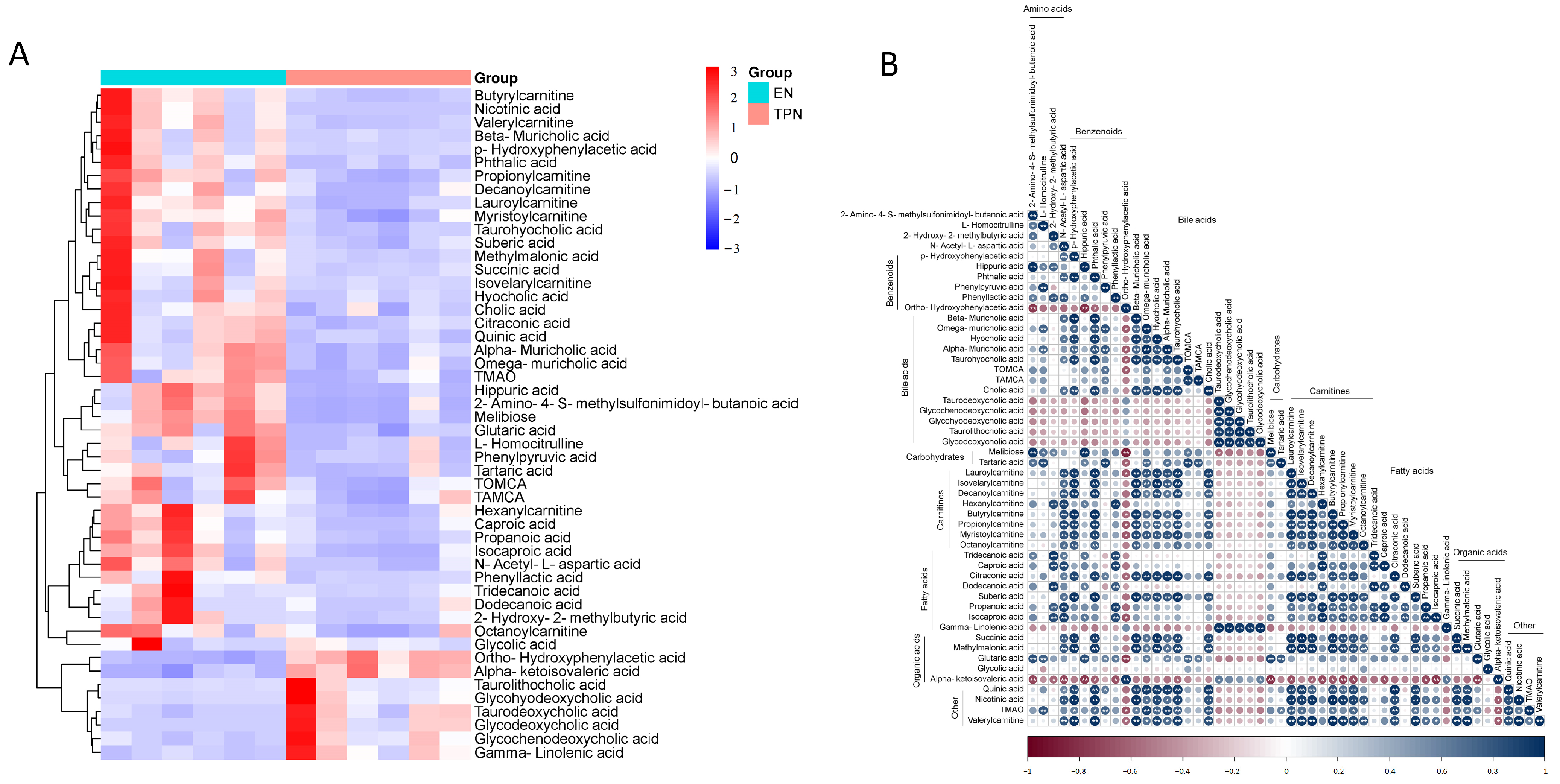
3.2. Metabolomic Profiles Altered by TPN
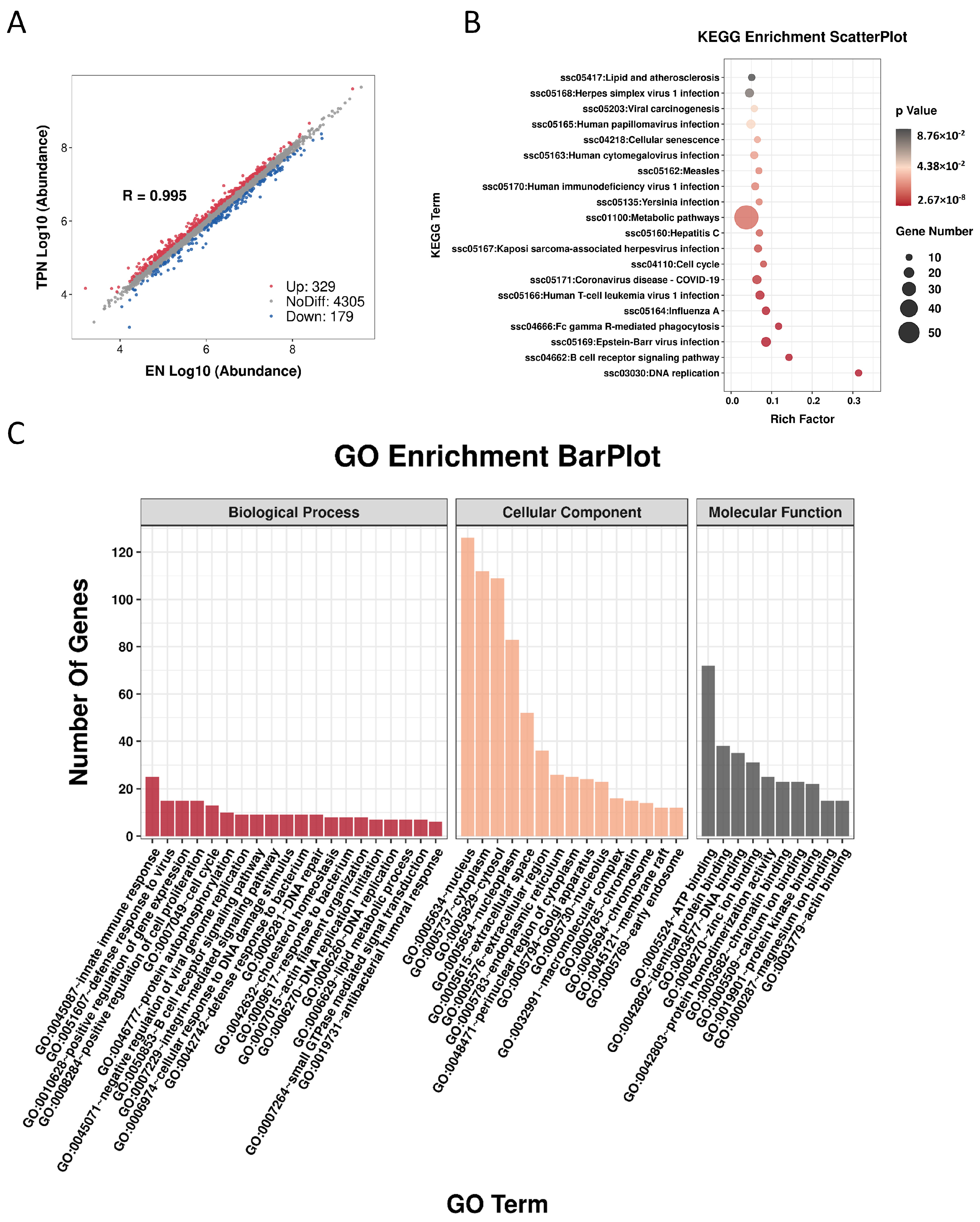
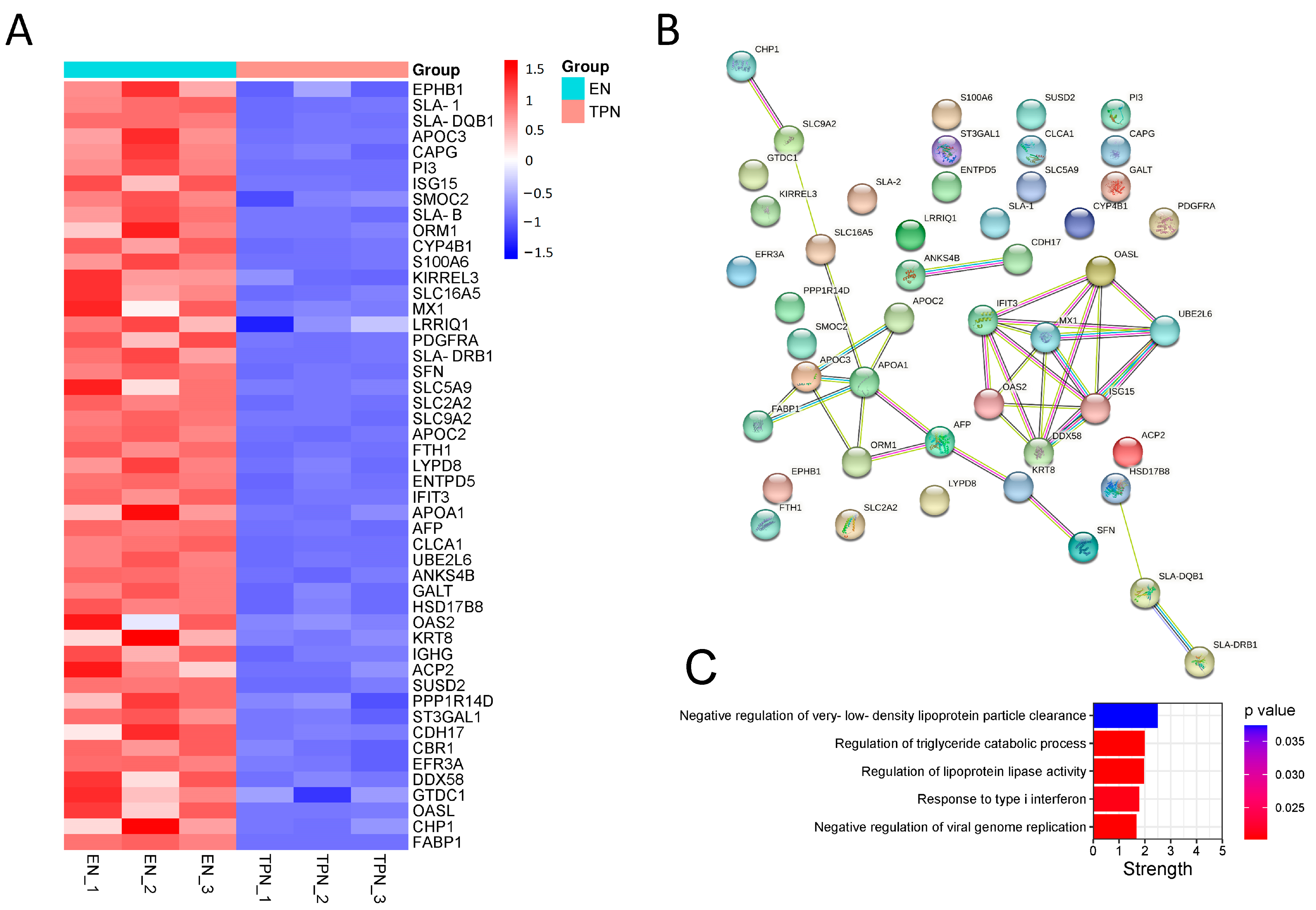
3.3. Proteomic Profiles Altered by TPN
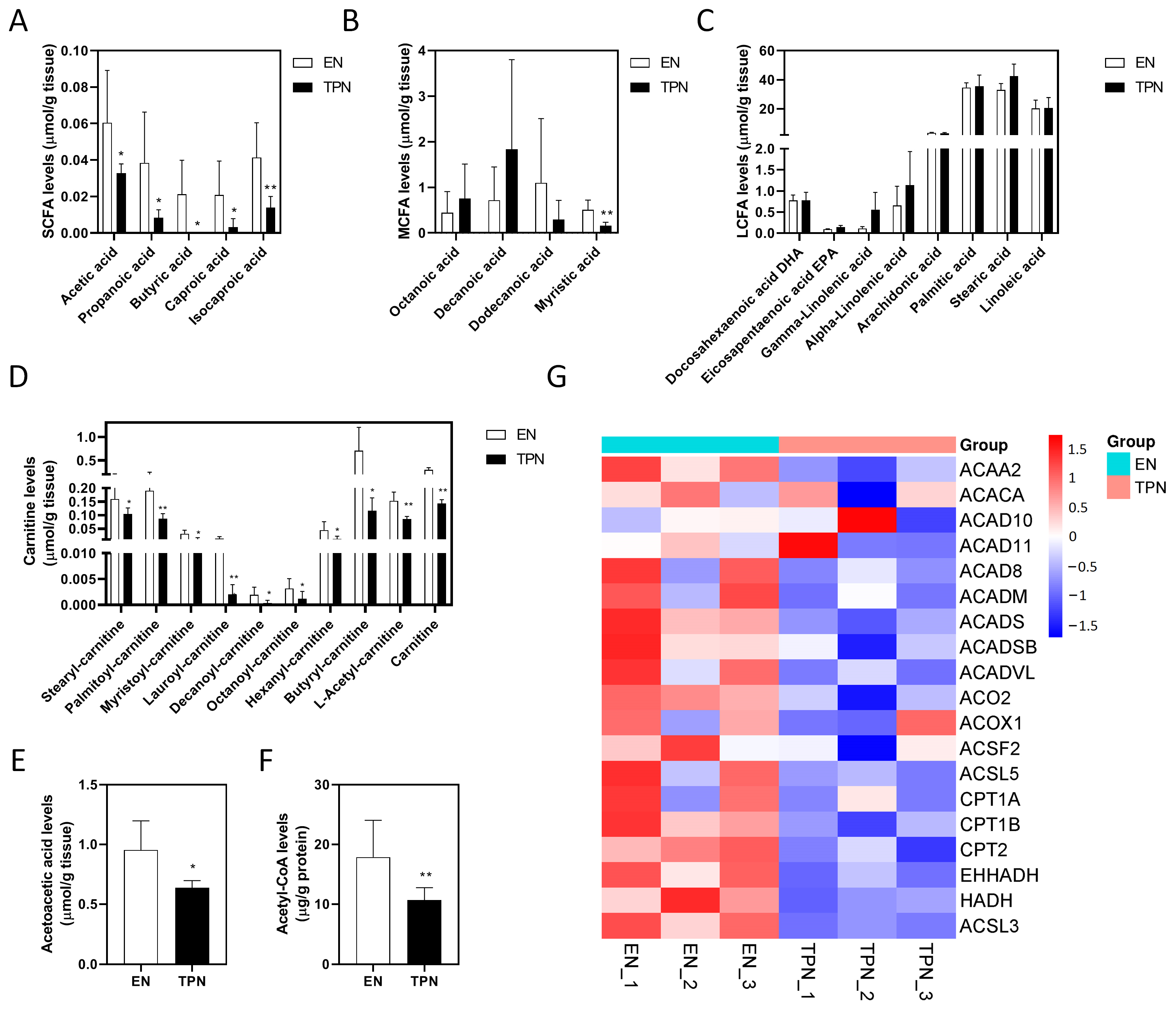
3.4. Disrupted Fatty Acid Oxidation (FAO) by TPN
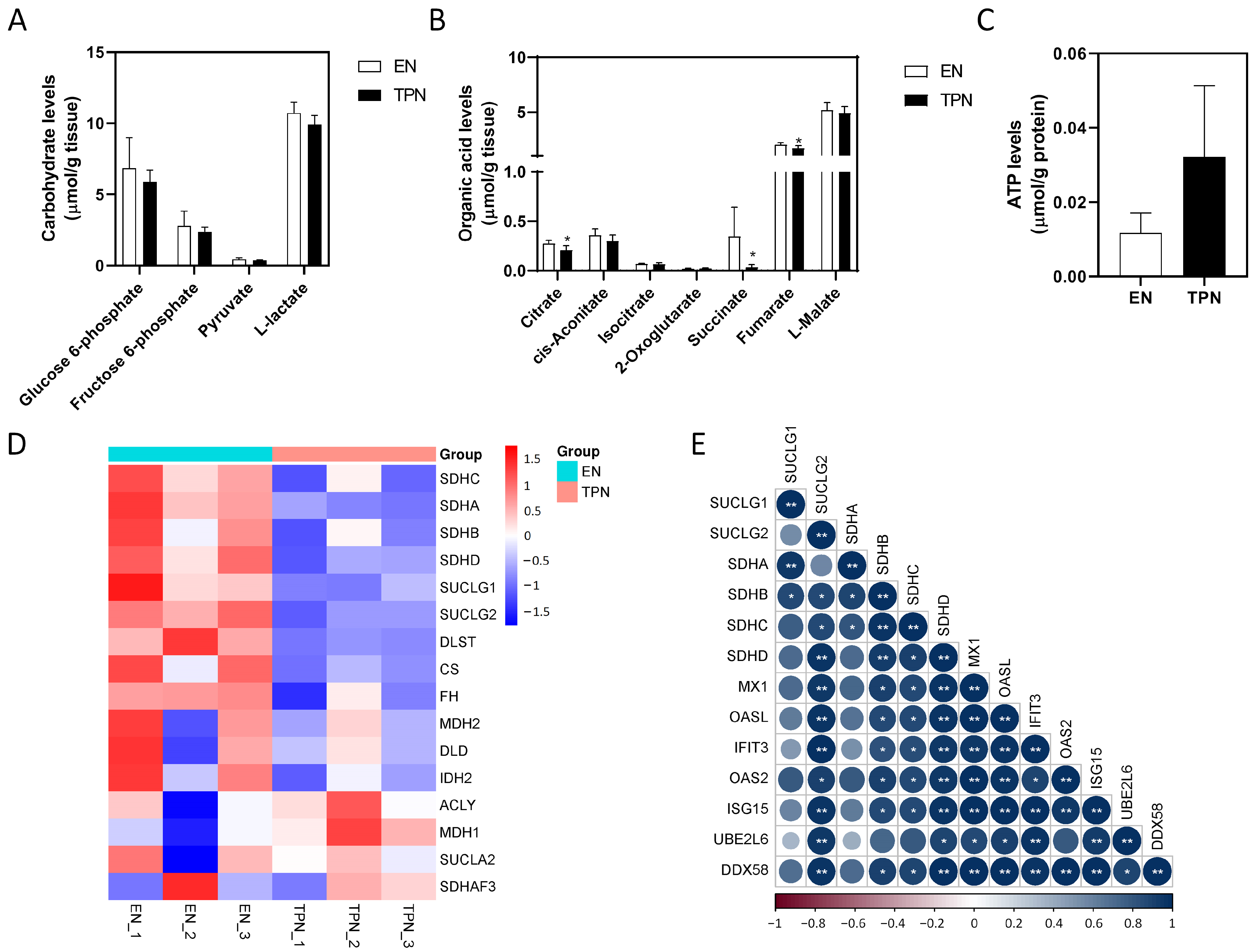
3.5. Disrupted Citrate Cycle by TPN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzo, V.; Capozza, M.; Panza, R.; Laforgia, N.; Baldassarre, M.E. Macronutrients and Micronutrients in Parenteral Nutrition for Preterm Newborns: A Narrative Review. Nutrients 2022, 14, 1530. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef]
- Bielawska, B.; Allard, J.P. Parenteral Nutrition and Intestinal Failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the European prospective survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, P.; Stewart, I.D.; Kelly, R.S.; Stav, M.; Mendez, K.; Dahlin, A.; Soeteman, D.I.; Chu, S.H.; Huang, M.; Cote, M.; et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 2022, 28, 814–822. [Google Scholar] [CrossRef]
- Masoodi, M.; Gastaldelli, A.; Hyotylainen, T.; Arretxe, E.; Alonso, C.; Gaggini, M.; Brosnan, J.; Anstee, Q.M.; Millet, O.; Ortiz, P.; et al. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Mokha, J.S.; Davidovics, Z.H.; Maas, K.; Caimano, M.J.; Matson, A. Fecal Microbiomes in Premature Infants With and Without Parenteral Nutrition-Associated Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 224–230. [Google Scholar] [CrossRef]
- Meessen, E.C.E.; Bakker, G.J.; Nieuwdorp, M.; Dallinga-Thie, G.M.; Kemper, E.M.; Olde Damink, S.W.; Romijn, J.A.; Hartmann, B.; Holst, J.J.; Knop, F.K.; et al. Parenteral nutrition impairs plasma bile acid and gut hormone responses to mixed meal testing in lean healthy men. Clin. Nutr. 2021, 40, 1013–1021. [Google Scholar] [CrossRef]
- Manithody, C.S.; Van Nispen, J.; Murali, V.; Jain, S.; Samaddar, A.; Armstrong, A.; Jain, A. Role of Bile Acids and Gut Microbiota in Parenteral Nutrition Associated Injury. J. Hum. Nutr. 2020, 4, 75–79. [Google Scholar] [CrossRef]
- Dai, L.N.; Zhao, Y.L.; Jiang, L.; Yan, J.K. Changes in the intestinal expression of drug metabolism-related genes in a piglet model of parenteral nutrition. Nutr. Metab. 2022, 19, 18. [Google Scholar] [CrossRef]
- Stoll, B.; Horst, D.A.; Cui, L.; Chang, X.; Ellis, K.J.; Hadsell, D.L.; Suryawan, A.; Kurundkar, A.; Maheshwari, A.; Davis, T.A.; et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J. Nutr. 2010, 140, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, Y.; Chen, S.; Tian, X.; Wang, W.; Wang, Y.; Cai, W. The Farnesoid X Receptor Agonist Tropifexor Prevents Liver Damage in Parenteral Nutrition-fed Neonatal Piglets. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e11–e19. [Google Scholar] [CrossRef] [PubMed]
- Wiskin, A.E.; Russell, R.; Barclay, A.R.; Thomas, J.; Batra, A.; on behalf of BANS Committee of BAPEN. Prevalence of home parenteral nutrition in children. Clin. Nutr. ESPEN 2021, 42, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Breton, A.; Coste, M.E.; Dubern, B.; Ecochard-Dugelay, E.; Guimber, D.; Loras-Duclaux, I.; Abi Nader, E.; Marinier, E.; Peretti, N.; et al. Pediatric Home Parenteral Nutrition in France: A six years national survey. Clin. Nutr. 2021, 40, 5278–5287. [Google Scholar] [CrossRef]
- Yan, J.K.; Zhang, T.; Dai, L.N.; Gu, B.L.; Zhu, J.; Yan, W.H.; Cai, W.; Wang, Y. CELF1/p53 axis: A sustained antiproliferative signal leading to villus atrophy under total parenteral nutrition. FASEB J. 2019, 33, 3378–3391. [Google Scholar] [CrossRef]
- Xiao, W.; Feng, Y.; Holst, J.J.; Hartmann, B.; Yang, H.; Teitelbaum, D.H. Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J. 2014, 28, 2073–2087. [Google Scholar] [CrossRef]
- Lei, Q.; Bi, J.; Chen, H.; Tian, F.; Gao, X.; Li, N.; Wang, X. Glucagon-like peptide-2 improves intestinal immune function and diminishes bacterial translocation in a mouse model of parenteral nutrition. Nutr. Res. 2018, 49, 56–66. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Gosain, A.; Kudsk, K.A. IL-25 improves luminal innate immunity and barrier function during parenteral nutrition. Ann. Surg. 2014, 259, 394–400. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Yan, J.K.; Cai, W. Early downregulation of P-glycoprotein facilitates bacterial attachment to intestinal epithelial cells and thereby triggers barrier dysfunction in a rodent model of total parenteral nutrition. FASEB J. 2020, 34, 4670–4683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Z.; Zhou, J.; Yan, J.; Cai, W. Lin 28A/Occludin axis: An aberrantly activated pathway in intestinal epithelial cells leading to impaired barrier function under total parenteral nutrition. FASEB J. 2021, 35, e21189. [Google Scholar] [CrossRef]
- Feng, Y.; Demehri, F.R.; Xiao, W.; Tsai, Y.H.; Jones, J.C.; Brindley, C.D.; Threadgill, D.W.; Holst, J.J.; Hartmann, B.; Barrett, T.A.; et al. Interdependency of EGF and GLP-2 Signaling in Attenuating Mucosal Atrophy in a Mouse Model of Parenteral Nutrition. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 447–468. [Google Scholar] [CrossRef]
- Freeman, J.J.; Feng, Y.; Demehri, F.R.; Dempsey, P.J.; Teitelbaum, D.H. TPN-associated intestinal epithelial cell atrophy is modulated by TLR4/EGF signaling pathways. FASEB J. 2015, 29, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Teitelbaum, D.H. Tumour necrosis factor--induced loss of intestinal barrier function requires TNFR1 and TNFR2 signalling in a mouse model of total parenteral nutrition. J. Physiol. 2013, 591, 3709–3723. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B. Animal models to study neonatal nutrition in humans. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.; Sangild, P.T.; Stoll, B.; Thymann, T.; Buddington, R.; Marini, J.; Olutoye, O.; Shulman, R.J. Translational Advances in Pediatric Nutrition and Gastroenterology: New Insights from Pig Models. Annu. Rev. Anim. Biosci. 2020, 8, 321–354. [Google Scholar] [CrossRef]
- Moutinho, T.J.; Powers, D.A.; Hanson, G.F.; Levy, S.; Baveja, R.; Hefner, I.; Mohamed, M.; Abdelghani, A.; Baker, R.L.; Papin, J.A.; et al. Fecal sphingolipids predict parenteral nutrition-associated cholestasis in the neonatal intensive care unit. JPEN J. Parenter Enter. Nutr. 2022, 46, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Liu, Y.; Tian, X.; Chen, S.; Lu, Y.; Wu, B.; Xiao, Y.; Cai, W. Lactobacillus plantarum supplementation alleviates liver and intestinal injury in parenteral nutrition-fed piglets. JPEN J. Parenter Enter. Nutr. 2022, 46, 1932–1943. [Google Scholar] [CrossRef]
- Dowhaniuk, J.K.; Szamosi, J.; Chorlton, S.; Owens, J.; Mileski, H.; Clause, R.F.; Pernica, J.M.; Bowdish, D.M.E.; Surette, M.G.; Ratcliffe, E.M. Starving the Gut: A Deficit of Butyrate in the Intestinal Ecosystem of Children With Intestinal Failure. JPEN J. Parenter Enter. Nutr. 2020, 44, 1112–1123. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Xiao, Y.; Wang, Y.; Yan, J.; Schnabl, B.; Cai, W. Role of the Gut Microbiota in Parenteral Nutrition-Associated Liver Disease: From Current Knowledge to Future Opportunities. J. Nutr. 2022, 152, 377–385. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Browner, P.; Teitelbaum, D.H. Effects on varying intravenous lipid emulsions on the small bowel epithelium in a mouse model of parenteral nutrition. JPEN J. Parenter Enter. Nutr. 2013, 37, 775–786. [Google Scholar] [CrossRef]
- Chen, L.; Vasoya, R.P.; Toke, N.H.; Parthasarathy, A.; Luo, S.; Chiles, E.; Flores, J.; Gao, N.; Bonder, E.M.; Su, X.; et al. HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice. Gastroenterology 2020, 158, 985–999. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Cheng, C.W.; Cao, A.Q.; Tripathi, S.; Mana, M.D.; Bauer-Rowe, K.E.; Abu-Remaileh, M.; Clavain, L.; Erdemir, A.; Lewis, C.A.; et al. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 2018, 22, 769–778.e4. [Google Scholar] [CrossRef]
- Esturau-Escofet, N.; Rodriguez de San Miguel, E.; Vela-Amieva, M.; Garcia-Aguilera, M.E.; Hernandez-Espino, C.C.; Macias-Kauffer, L.; Lopez-Candiani, C.; Naveja, J.J.; Ibarra-Gonzalez, I. A Longitudinal (1)H NMR-Based Metabolic Profile Analysis of Urine from Hospitalized Premature Newborns Receiving Enteral and Parenteral Nutrition. Metabolites 2022, 12, 255. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed]
- Grimolizzi, F.; Arranz, L. Multiple faces of succinate beyond metabolism in blood. Haematologica 2018, 103, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F., Jr.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef]
| Component | Volume (mL) |
|---|---|
| 50% dextrose | 50 |
| 8.5% amino acids | 153 |
| 20% SOLE | 25 |
| 10% sodium chloride | 2.4 |
| 10% potassium chloride | 2.4 |
| Trace elements mix | 0.5 |
| Water-soluble vitamins mix | 0.5 |
| Fat-soluble vitamins mix | 0.5 |
| 10% calcium gluconate | 4.6 |
| Sodium glycerophophate | 4.6 |
| magnesium sulfate | 0.33 |
| Total | 243.83 |
| Indicators | EN | TPN |
|---|---|---|
| Body weight (onset, kg) | 1.09 ± 0.24 | 0.92 ± 0.21 |
| Body weight (sacrifice, kg) | 2.36 ± 0.12 | 1.59 ± 0.47 |
| Villus height (μm) | 256.33 ± 45.01 | 191.76 ± 28.56 ** |
| Crypt depth (μm) | 123.41 ± 29.38 | 83.77 ± 27.69 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhao, Y.; Jiang, L.; Wang, Y.; Cai, W. Multi-Omics Unravels Metabolic Alterations in the Ileal Mucosa of Neonatal Piglets Receiving Total Parenteral Nutrition. Metabolites 2023, 13, 555. https://doi.org/10.3390/metabo13040555
Yan J, Zhao Y, Jiang L, Wang Y, Cai W. Multi-Omics Unravels Metabolic Alterations in the Ileal Mucosa of Neonatal Piglets Receiving Total Parenteral Nutrition. Metabolites. 2023; 13(4):555. https://doi.org/10.3390/metabo13040555
Chicago/Turabian StyleYan, Junkai, Yuling Zhao, Lu Jiang, Ying Wang, and Wei Cai. 2023. "Multi-Omics Unravels Metabolic Alterations in the Ileal Mucosa of Neonatal Piglets Receiving Total Parenteral Nutrition" Metabolites 13, no. 4: 555. https://doi.org/10.3390/metabo13040555
APA StyleYan, J., Zhao, Y., Jiang, L., Wang, Y., & Cai, W. (2023). Multi-Omics Unravels Metabolic Alterations in the Ileal Mucosa of Neonatal Piglets Receiving Total Parenteral Nutrition. Metabolites, 13(4), 555. https://doi.org/10.3390/metabo13040555






