Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives
Abstract
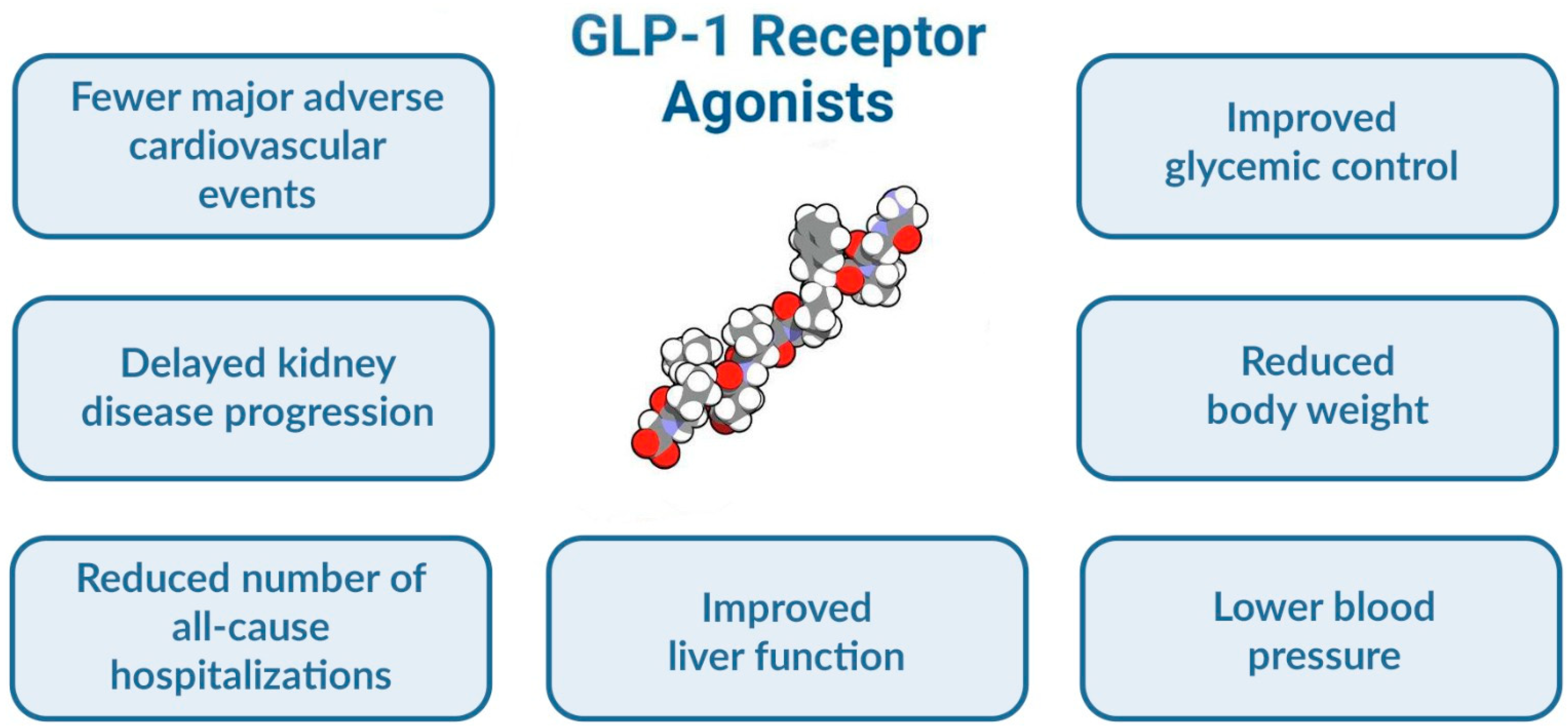
1. Introduction
2. Basic Concepts in the Management of Patients with T2DM and NAFLD
3. Search Methods
4. Search Results
4.1. Effects of GLP-1RAs on Diabetes-Related Liver Disease
4.1.1. Changes in Serum Liver Enzymes
4.1.2. Effects on Composite Indices of Hepatic Steatosis and Fibrosis
4.1.3. Changes in Liver Fat Content or Fibrosis Evaluated by Imaging Techniques
4.1.4. Effects on Biopsy-Proven Histopathological Modifications
4.1.5. Effects Assessed by Combined Investigations
5. Potential Beneficial Mechanisms Underlying the Effects of GLP-1RAs in Diabetes-Related Liver Disease
5.1. Indirect Effects That Promote NAFLD Improvement
5.2. Direct Mechanisms That Promote NAFLD Improvement
6. Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas. Available online: https://idf.org/e-library/epidemiology-research/diabetes-atlas.html?id=171 (accessed on 20 March 2023).
- Kotronen, A.; Yki-Järvinen, H. Fatty Liver: A Novel Component of the Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Henry, L. Epidemiology of Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. JHEP Rep. 2021, 3, 100305. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 Global Prevalence of Nonalcoholic Fatty Liver Disease Using Hierarchical Bayesian Approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of NASH in Endocrine Diseases. Endocr. Connect. 2021, 10, R52–R65. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and Diabetes Mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-Alcoholic Fatty Liver Disease and Diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Francque, S.M.; Marchesini, G.; Kautz, A.; Walmsley, M.; Dorner, R.; Lazarus, J.V.; Zelber-Sagi, S.; Hallsworth, K.; Busetto, L.; Frühbeck, G.; et al. Non-Alcoholic Fatty Liver Disease: A Patient Guideline. JHEP Rep. 2021, 3, 100322. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, M.; Christensen, A.S.; Andersen, A.; Solini, A.; Knop, F.K.; Vilsbøll, T. Future Perspectives on GLP-1 Receptor Agonists and GLP-1/Glucagon Receptor Co-Agonists in the Treatment of NAFLD. Front. Endocrinol. 2018, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The Impact of Macronutrient Intake on Non-Alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Therapeutic Landscape for NAFLD in 2020. Gastroenterology 2020, 158, 1984–1998.e3. [Google Scholar] [CrossRef]
- Diaconu, C.-T.; Guja, C. Nonalcoholic Fatty Liver Disease and Its Complex Relation with Type 2 Diabetes Mellitus-From Prevalence to Diagnostic Approach and Treatment Strategies. J. Clin. Med. 2022, 11, 5144. [Google Scholar] [CrossRef]
- Colosimo, S.; Tan, G.D.; Petroni, M.L.; Marchesini, G.; Tomlinson, J.W. Improved Glycaemic Control in Patients with Type 2 Diabetes Has a Beneficial Impact on NAFLD, Independent of Change in BMI or Glucose Lowering Agent. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 640–648. [Google Scholar] [CrossRef]
- Binet, Q.; Loumaye, A.; Preumont, V.; Thissen, J.P.; Hermans, M.P.; Lanthier, N. Non-Invasive Screening, Staging and Management of Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Type 2 Diabetes Mellitus Patients: What Do We Know so Far? Acta Gastroenterol. Belg. 2022, 85, 346–357. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Neeland, I.J.; Marso, S.P.; Ayers, C.R.; Lewis, B.; Oslica, R.; Francis, W.; Rodder, S.; Pandey, A.; Joshi, P.H. Effects of Liraglutide on Visceral and Ectopic Fat in Adults with Overweight and Obesity at High Cardiovascular Risk: A Randomised, Double-Blind, Placebo-Controlled, Clinical Trial. Lancet Diabetes Endocrinol. 2021, 9, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Marsico, F.; Paolillo, S.; Gargiulo, P.; Bruzzese, D.; Dell’Aversana, S.; Esposito, I.; Renga, F.; Esposito, L.; Marciano, C.; Dellegrottaglie, S.; et al. Effects of Glucagon-like Peptide-1 Receptor Agonists on Major Cardiovascular Events in Patients with Type 2 Diabetes Mellitus with or without Established Cardiovascular Disease: A Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2020, 41, 3346–3358. [Google Scholar] [CrossRef]
- Patel Chavez, C.; Cusi, K.; Kadiyala, S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022, 107, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, B.A.; Wakefield, A.N.; Triboletti, J.; Gonzalvo, J.D.; Meredith, A.H. GLP-101: A Diabetes Educator’s Guide to Glucagon-Like-Peptide-1 Receptor Agonists. AADE Pract. 2019, 7, 32–41. [Google Scholar] [CrossRef]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 Inhibitors and GLP-1 Receptor Agonists: Established and Emerging Indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Kim, D.D.; Shen, L.; Ruggles, J.A.; Nielsen, L.L.; Fineman, M.S.; Baron, A.D. Pharmacokinetics, Pharmacodynamics, and Safety of Exenatide in Patients with Type 2 Diabetes Mellitus. Am. J. Health Syst. Pharm. 2005, 62, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.H.A.; Stechl, J.; Steinstraesser, A.; Golor, G.; Pellissier, F. Lixisenatide Reduces Postprandial Hyperglycaemia via Gastrostatic and Insulinotropic Effects. Diabetes Metab. Res. Rev. 2015, 31, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.E.; Stewart, M.W.; De Boever, E.H.; Dobbins, R.L.; Hodge, R.J.; Walker, S.E.; Holland, M.C.; Bush, M.A. Albiglutide Study Group Pharmacodynamics, Pharmacokinetics, Safety, and Tolerability of Albiglutide, a Long-Acting Glucagon-like Peptide-1 Mimetic, in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Damholt, B.; Golor, G.; Wierich, W.; Pedersen, P.; Ekblom, M.; Zdravkovic, M. An Open-Label, Parallel Group Study Investigating the Effects of Age and Gender on the Pharmacokinetics of the Once-Daily Glucagon-like Peptide-1 Analogue Liraglutide. J. Clin. Pharmacol. 2006, 46, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Geiser, J.S.; Heathman, M.A.; Cui, X.; Martin, J.; Loghin, C.; Chien, J.Y.; de la Peña, A. Clinical Pharmacokinetics of Dulaglutide in Patients with Type 2 Diabetes: Analyses of Data from Clinical Trials. Clin. Pharmacokinet. 2016, 55, 625–634. [Google Scholar] [CrossRef]
- Marbury, T.C.; Flint, A.; Jacobsen, J.B.; Derving Karsbøl, J.; Lasseter, K. Pharmacokinetics and Tolerability of a Single Dose of Semaglutide, a Human Glucagon-Like Peptide-1 Analog, in Subjects With and Without Renal Impairment. Clin. Pharmacokinet. 2017, 56, 1381–1390. [Google Scholar] [CrossRef]
- Granhall, C.; Donsmark, M.; Blicher, T.M.; Golor, G.; Søndergaard, F.L.; Thomsen, M.; Bækdal, T.A. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clin. Pharmacokinet. 2019, 58, 781–791. [Google Scholar] [CrossRef]
- Napoli, R.; Avogaro, A.; Formoso, G.; Piro, S.; Purrello, F.; Targher, G.; Consoli, A. Beneficial Effects of Glucagon-like Peptide 1 Receptor Agonists on Glucose Control, Cardiovascular Risk Profile, and Non-Alcoholic Fatty Liver Disease. An Expert Opinion of the Italian Diabetes Society. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3257–3270. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver Clinical Practice Guidelines for the Diagnosis and Management of Metabolic Associated Fatty Liver Disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Recommendations|Non-Alcoholic Fatty Liver Disease (NAFLD): Assessment and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng49/chapter/Recommendations (accessed on 20 March 2023).
- Gastaldelli, A.; Repetto, E.; Guja, C.; Hardy, E.; Han, J.; Jabbour, S.A.; Ferrannini, E. Exenatide and Dapagliflozin Combination Improves Markers of Liver Steatosis and Fibrosis in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2020, 22, 393–403. [Google Scholar] [CrossRef]
- Liu, L.; Yan, H.; Xia, M.; Zhao, L.; Lv, M.; Zhao, N.; Rao, S.; Yao, X.; Wu, W.; Pan, B.; et al. Efficacy of Exenatide and Insulin Glargine on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3292. [Google Scholar] [CrossRef] [PubMed]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide Decreases Liver Fat Content and Epicardial Adipose Tissue in Patients with Obesity and Type 2 Diabetes: A Prospective Randomized Clinical Trial Using Magnetic Resonance Imaging and Spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Shao, N.; Kuang, H.Y.; Hao, M.; Gao, X.Y.; Lin, W.J.; Zou, W. Benefits of Exenatide on Obesity and Non-Alcoholic Fatty Liver Disease with Elevated Liver Enzymes in Patients with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2014, 30, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Pan, Q.; Xu, Y.; Yang, X. Exenatide Improves Type 2 Diabetes Concomitant with Non-Alcoholic Fatty Liver Disease. Arq. Bras. Endocrinol. Metabol. 2013, 57, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Irwin, A.; Gardner, C.J.; Daousi, C.; Purewal, T.; Furlong, N.; Goenka, N.; Thomas, E.L.; Adams, V.L.; Pushpakom, S.P.; et al. Improved Glycaemia Correlates with Liver Fat Reduction in Obese, Type 2 Diabetes, Patients given Glucagon-like Peptide-1 (GLP-1) Receptor Agonists. PLoS ONE 2012, 7, e50117. [Google Scholar] [CrossRef]
- Sathyanarayana, P.; Jogi, M.; Muthupillai, R.; Krishnamurthy, R.; Samson, S.L.; Bajaj, M. Effects of Combined Exenatide and Pioglitazone Therapy on Hepatic Fat Content in Type 2 Diabetes. Obesity 2011, 19, 2310–2315. [Google Scholar] [CrossRef]
- Buse, J.B.; Klonoff, D.C.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Maggs, D.G.; Wintle, M.E. Metabolic Effects of Two Years of Exenatide Treatment on Diabetes, Obesity, and Hepatic Biomarkers in Patients with Type 2 Diabetes: An Interim Analysis of Data from the Open-Label, Uncontrolled Extension of Three Double-Blind, Placebo-Controlled Trials. Clin. Ther. 2007, 29, 139–153. [Google Scholar] [CrossRef]
- Tan, Y.; Zhen, Q.; Ding, X.; Shen, T.; Liu, F.; Wang, Y.; Zhang, Q.; Lin, R.; Chen, L.; Peng, Y.; et al. Association between Use of Liraglutide and Liver Fibrosis in Patients with Type 2 Diabetes. Front. Endocrinol. 2022, 13, 935180. [Google Scholar] [CrossRef]
- Bizino, M.B.; Jazet, I.M.; de Heer, P.; van Eyk, H.J.; Dekkers, I.A.; Rensen, P.C.N.; Paiman, E.H.M.; Lamb, H.J.; Smit, J.W. Placebo-Controlled Randomised Trial with Liraglutide on Magnetic Resonance Endpoints in Individuals with Type 2 Diabetes: A Pre-Specified Secondary Study on Ectopic Fat Accumulation. Diabetologia 2020, 63, 65–74. [Google Scholar] [CrossRef]
- Gameil, M.A.; Rozaik, S.E.; Elsebaie, A.; Marzouk, R. Influence of Liraglutide, Dulaglutide versus Conventional Treatment on Fatty Liver Index and Fibrosis-4 Score in Egyptian Patients with Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Med. J. Viral Hepat. 2020, 5, 25–32. [Google Scholar] [CrossRef]
- Guo, W.; Tian, W.; Lin, L.; Xu, X. Liraglutide or Insulin Glargine Treatments Improves Hepatic Fat in Obese Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease in Twenty-Six Weeks: A Randomized Placebo-Controlled Trial. Diabetes Res. Clin. Pract. 2020, 170, 108487. [Google Scholar] [CrossRef]
- Simeone, P.G.; Costantino, S.; Tripaldi, R.; Liani, R.; Ciotti, S.; Tartaro, A.; Guagnano, M.T.; Cosentino, F.; Consoli, A.; Paneni, F.; et al. Baseline Interleukin1beta Expression in Peripheral Blood Monocytes Predicts the Extent of Weight Loss and Nonalcoholic Fatty Liver Improvement in Obese Subjects with Prediabetes or Type 2 Diabetes. Eur. Heart J. 2020, 41, ehaa946.3033. [Google Scholar] [CrossRef]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zheng, Z.; Zhang, D.; He, S.; Shen, J. Efficacy of Liraglutide in Treating Type 2 Diabetes Mellitus Complicated with Non-Alcoholic Fatty Liver Disease. Biosci. Rep. 2018, 38, BSR20181304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, Y.; Kong, W.; Jin, Q.; Wang, X.; Dong, Y.; Wang, Y.; Li, H. Efficacy and Clinical Value of Liraglutide for Treatment of Diabetes Mellitus Complicated by Non-Alcoholic Fatty Liver Disease. Med. Sci. Monit. 2018, 24, 7399–7404. [Google Scholar] [CrossRef]
- Bouchi, R.; Nakano, Y.; Fukuda, T.; Takeuchi, T.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Reduction of Visceral Fat by Liraglutide Is Associated with Ameliorations of Hepatic Steatosis, Albuminuria, and Micro-Inflammation in Type 2 Diabetic Patients with Insulin Treatment: A Randomized Control Trial. Endocr. J. 2017, 64, 269–281. [Google Scholar] [CrossRef]
- Feng, W.; Gao, C.; Bi, Y.; Wu, M.; Li, P.; Shen, S.; Chen, W.; Yin, T.; Zhu, D. Randomized Trial Comparing the Effects of Gliclazide, Liraglutide, and Metformin on Diabetes with Non-Alcoholic Fatty Liver Disease. J. Diabetes 2017, 9, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.-M.; Cercueil, J.-P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients with Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.A.; Kramer, M.H.H.; Pouwels, P.J.W.; Pieters-van den Bos, I.C.; Hoekstra, T.; Diamant, M.; van Raalte, D.H.; Cahen, D.L. Twelve Week Liraglutide or Sitagliptin Does Not Affect Hepatic Fat in Type 2 Diabetes: A Randomised Placebo-Controlled Trial. Diabetologia 2016, 59, 2588–2593. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Harrison, L.B.; Warshauer, J.T.; Adams-Huet, B.; Li, X.; Yuan, Q.; Hulsey, K.; Dimitrov, I.; Yokoo, T.; Jaster, A.W.; et al. Mechanisms of Action of Liraglutide in Patients with Type 2 Diabetes Treated With High-Dose Insulin. J. Clin. Endocrinol. Metab. 2016, 101, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Rabasa-Lhoret, R.; Castel, H.; Wartelle-Bladou, C.; Gilbert, G.; Massicotte-Tisluck, K.; Chartrand, G.; Olivié, D.; Julien, A.-S.; de Guise, J.; et al. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients With Type 2 Diabetes: A Randomized Trial. Diabetes Care 2015, 38, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Isogawa, A.; Iwamoto, M.; Ohsugi, M.; Yoshida, H.; Toda, N.; Tagawa, K.; Omata, M.; Koike, K. The Effectiveness of Liraglutide in Nonalcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Compared to Sitagliptin and Pioglitazone. Sci. World J. 2012, 2012, e496453. [Google Scholar] [CrossRef]
- Carretero-Gómez, J.; Carrasco-Sánchez, F.J.; Fernández-Rodríguez, J.M.; Casado-Escribano, P.; Miramontes-González, J.P.; Seguí-Ripoll, J.M.; Ena, J.; Arévalo-Lorido, J.C. Effect of Semaglutide on Fatty Liver Disease Biomarkers in Patients with Diabetes and Obesity. Rev. Clín. Española (Engl. Ed.) 2023, 223, 134–143. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Ono, H.; Kawano, T.; Yoshida, Y.; Okubo, T.; Hayama, K.; Nakagawa-Iwashita, A.; Itokawa, N.; et al. Efficacy and Safety of Oral Semaglutide in Patients with Non-Alcoholic Fatty Liver Disease Complicated by Type 2 Diabetes Mellitus: A Pilot Study. JGH Open 2022, 6, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.P.; Liu, S.K. The Effect of Semaglutide on Glycolipid Metabolism in Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Indian J. Pharm. Sci. 2022, 84, 171–175. [Google Scholar] [CrossRef]
- Volpe, S.; Lisco, G.; Fanelli, M.; Racaniello, D.; Colaianni, V.; Triggiani, D.; Donghia, R.; Crudele, L.; Rinaldi, R.; Sabbà, C.; et al. Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study. Nutrients 2022, 14, 4673. [Google Scholar] [CrossRef]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Sundby Palle, M.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised Clinical Trial: Semaglutide versus Placebo Reduced Liver Steatosis but Not Liver Stiffness in Subjects with Non-Alcoholic Fatty Liver Disease Assessed by Magnetic Resonance Imaging. Aliment. Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of Dulaglutide on Liver Fat in Patients with Type 2 Diabetes and NAFLD: Randomised Controlled Trial (D-LIFT Trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef]
- Bogomolov, P.O.; Bueverov, A.O.; Dreval, A.V.; Nechaeva, O.A.; Mayorov, A.Y.; Mishina, E.E.; Fedosina, E.A.; Koblov, S.V.; Sumtsova, O.V.; Jallow, A.; et al. Preliminary results of dulaglutide treatment in patients with non-alcoholic fatty liver disease (DEMOS study). Exp. Clin. Gastroenterol. 2019, 9, 4–10. [Google Scholar] [CrossRef]
- Giugliano, D.; Scappaticcio, L.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Ceriello, A.; Chiodini, P.; Esposito, K. GLP-1 Receptor Agonists and Cardiorenal Outcomes in Type 2 Diabetes: An Updated Meta-Analysis of Eight CVOTs. Cardiovasc. Diabetol. 2021, 20, 189. [Google Scholar] [CrossRef]
- Boyle, J.G.; Livingstone, R.; Petrie, J.R. Cardiovascular Benefits of GLP-1 Agonists in Type 2 Diabetes: A Comparative Review. Clin. Sci. 2018, 132, 1699–1709. [Google Scholar] [CrossRef]
- Cusi, K. Incretin-Based Therapies for the Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes. Hepatology 2019, 69, 2318–2322. [Google Scholar] [CrossRef]
- Lian, J.; Fu, J. Efficacy of Various Hypoglycemic Agents in the Treatment of Patients With Nonalcoholic Liver Disease With or Without Diabetes: A Network Meta-Analysis. Front. Endocrinol. 2021, 12, 649018. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A.; Clausen, W.H.O.; Elbrønd, B.; Gough, S.C.L.; Tomlinson, J.W.; Newsome, P.N. Safety and Efficacy of Liraglutide in Patients with Type 2 Diabetes and Elevated Liver Enzymes: Individual Patient Data Meta-Analysis of the LEAD Program. Aliment. Pharmacol. Ther. 2013, 37, 234–242. [Google Scholar] [CrossRef]
- Cusi, K.; Sattar, N.; García-Pérez, L.-E.; Pavo, I.; Yu, M.; Robertson, K.E.; Karanikas, C.A.; Haupt, A. Dulaglutide Decreases Plasma Aminotransferases in People with Type 2 Diabetes in a Pattern Consistent with Liver Fat Reduction: A Post Hoc Analysis of the Award Programme. Diabet. Med. 2018, 35, 1434–1439. [Google Scholar] [CrossRef]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of Semaglutide on Liver Enzymes and Markers of Inflammation in Subjects with Type 2 Diabetes and/or Obesity. Aliment. Pharmacol. Ther. 2019, 50, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.R.; Kulkarni, V.V.; Pokhrel, S.; Akram, H.; Abdelgadir, A.; Chatterjee, A.; Khan, S.; Ahmed, N.R.; Kulkarni, V.V.; Pokhrel, S.; et al. Comparing the Efficacy and Safety of Obeticholic Acid and Semaglutide in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e24829. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Knop, F.K.; Vilsbøll, T. Effects of Lixisenatide on Elevated Liver Transaminases: Systematic Review with Individual Patient Data Meta-Analysis of Randomised Controlled Trials on Patients with Type 2 Diabetes. BMJ Open 2014, 4, e005325. [Google Scholar] [CrossRef]
- Ghosal, S.; Datta, D.; Sinha, B. A Meta-Analysis of the Effects of Glucagon-like-Peptide 1 Receptor Agonist (GLP1-RA) in Nonalcoholic Fatty Liver Disease (NAFLD) with Type 2 Diabetes (T2D). Sci. Rep. 2021, 11, 22063. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of 12-Week Dulaglutide Therapy in Japanese Patients with Biopsy-Proven Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Hepatol. Res. 2017, 47, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Hilliard, M.E.; Isaacs, D.; et al. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S49–S67. [Google Scholar] [CrossRef]
- Barritt, A.S.; Marshman, E.; Noureddin, M. Review Article: Role of Glucagon-like Peptide-1 Receptor Agonists in Non-Alcoholic Steatohepatitis, Obesity and Diabetes-What Hepatologists Need to Know. Aliment. Pharmacol. Ther. 2022, 55, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Novo Nordisk A/S. The Effect of Semaglutide in Subjects with Non-Cirrhotic Non-Alcoholic Steatohepatitis; ClinicalTrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
- Kumar, J.; Memon, R.S.; Shahid, I.; Rizwan, T.; Zaman, M.; Menezes, R.G.; Kumar, S.; Siddiqi, T.J.; Usman, M.S. Antidiabetic Drugs and Non-Alcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis and Evidence Map. Dig. Liver Dis. 2021, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Beatrice, G.; Petracca, G.; Pampagnin, F.; Sandri, D.; Targher, G. GLP-1 Receptor Agonists for NAFLD Treatment in Patients with and without Type 2 Diabetes: An Updated Meta-Analysis. Explor. Med. 2020, 1, 108–123. [Google Scholar] [CrossRef]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.-M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging Therapeutic Approaches for the Treatment of NAFLD and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef]
- Hamaguchi, E.; Takamura, T.; Sakurai, M.; Mizukoshi, E.; Zen, Y.; Takeshita, Y.; Kurita, S.; Arai, K.; Yamashita, T.; Sasaki, M.; et al. Histological Course of Nonalcoholic Fatty Liver Disease in Japanese Patients: Tight Glycemic Control, Rather than Weight Reduction, Ameliorates Liver Fibrosis. Diabetes Care 2010, 33, 284–286. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like Peptide-1 Receptor Agonists Improve Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Long, X.; Ni, Y.; Qian, L.; Nychas, E.; Siliceo, S.L.; Pohl, D.; Hanhineva, K.; Liu, Y.; Xu, A.; et al. Risk Assessment with Gut Microbiome and Metabolite Markers in NAFLD Development. Sci. Transl. Med. 2022, 14, eabk0855. [Google Scholar] [CrossRef] [PubMed]
- Pezzino, S.; Sofia, M.; Faletra, G.; Mazzone, C.; Litrico, G.; La Greca, G.; Latteri, S. Gut-Liver Axis and Non-Alcoholic Fatty Liver Disease: A Vicious Circle of Dysfunctions Orchestrated by the Gut Microbiome. Biology 2022, 11, 1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cai, B.-Y.; Zhu, L.-X.; Xin, X.; Wang, X.; An, Z.-M.; Li, S.; Hu, Y.-Y.; Feng, Q. Liraglutide Modulates Gut Microbiome and Attenuates Nonalcoholic Fatty Liver in Db/Db Mice. Life Sci. 2020, 261, 118457. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Rongjiong, Z.; Kahaer, M.; Chunhui, J.; Wulasihan, M. Therapeutic Efficacy of Liraglutide versus Metformin in Modulating the Gut Microbiota for Treating Type 2 Diabetes Mellitus Complicated with Nonalcoholic Fatty Liver Disease. Front. Microbiol. 2023, 14, 1088187. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Du, J.; Xi, L.; Zhang, Z.; Ge, X.; Li, W.; Peng, W.; Jiang, X.; Liu, W.; Zhao, N.; Wang, X.; et al. Metabolic Remodeling of Glycerophospholipids Acts as a Signature of Dulaglutide and Liraglutide Treatment in Recent-Onset Type 2 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 1097612. [Google Scholar] [CrossRef]
- Ye, J.; Xu, J.; Wen, W.; Huang, B. Effect of Liraglutide on Serum TSH Levels in Patients with NAFLD and Its Underlying Mechanisms. Int. J. Clin. Pract. 2022, 2022, 1786559. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef]
- Clayton-Chubb, D.; Kemp, W.; Majeed, A.; Lubel, J.S.; Hodge, A.; Roberts, S.K. Understanding NAFLD: From Case Identification to Interventions, Outcomes, and Future Perspectives. Nutrients 2023, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.E.R.; Robertson, D.; Wang, T.; Hornigold, D.C.; Petrone, M.; Cooper, A.T.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Schlichthaar, H.; et al. Efficacy, Safety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J. Clin. Endocrinol. Metab. 2020, 105, 803–820. [Google Scholar] [CrossRef]
- Boland, M.L.; Laker, R.C.; Mather, K.; Nawrocki, A.; Oldham, S.; Boland, B.B.; Lewis, H.; Conway, J.; Naylor, J.; Guionaud, S.; et al. Resolution of NASH and Hepatic Fibrosis by the GLP-1R and GCGR Dual-Agonist Cotadutide via Modulating Mitochondrial Function and Lipogenesis. Nat. Metab. 2020, 2, 413–431. [Google Scholar] [CrossRef]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults with Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Vedtofte, L.; Bahne, E.; Foghsgaard, S.; Bagger, J.I.; Andreasen, C.; Strandberg, C.; Gørtz, P.M.; Holst, J.J.; Grønbæk, H.; Svare, J.A.; et al. One Year’s Treatment with the Glucagon-Like Peptide 1 Receptor Agonist Liraglutide Decreases Hepatic Fat Content in Women with Nonalcoholic Fatty Liver Disease and Prior Gestational Diabetes Mellitus in a Randomized, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 3213. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study Comparing the Efficacy and Safety of Tirzepatide versus Placebo in Patients with Nonalcoholic Steatohepatitis (NASH); ClinicalTrials.gov: Bethesda, MD, USA, 2023. [Google Scholar]
- Central Hospital, Nancy, France. A Multicentre Controlled and Randomized Study Assessing the Effect of Dulaglutide Add-On to Dietary Reinforcement versus Dietary Reinforcement Alone in Patients with Type 2 Diabetes and Carriers of a Non-Alcoholic Steatohepatitis; ClinicalTrials.gov: Bethesda, MD, USA, 2019. [Google Scholar]
- The Deutsche Diabetes Forschungsgesellschaft e.V. Combined Active Treatment in Type 2 Diabetes with NASH; ClinicalTrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
- AstraZeneca. A Phase IIb/III Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Cotadutide in Participants with Non-Cirrhotic Non-Alcoholic Steatohepatitis with Fibrosis; ClinicalTrials.gov: Bethesda, MD, USA, 2023. [Google Scholar]
- Agha, D.A. Effect on Non-Alcoholic Fatty Liver Disease with Advanced Fibrosis in Patients with Type 2 Diabetes Mellitus on Treatment with Gastric Inhibitory Polypeptide/Glucagon Like Peptide-1 Analogue (Tirzpatide); ClinicalTrials.gov: Bethesda, MD, USA, 2023. [Google Scholar]
- Caprio, S. Semaglutide, 2.4 mg, Once Weekly: Effects on Beta-Cell Preservation and Reduction of Intrahepatic Triglyceride Content in Obese Youth with Prediabetes (IGT)/Early Type 2 Diabetes (T2D) and Non-Alcoholic Fatty Liver Disease (NAFLD); ClinicalTrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
| GLP-1RA Generic Name, Ref. | Trade Name Manufacturer | First Approved | Dose and Route of Administration | Administration Schedule | Side Effects |
|---|---|---|---|---|---|
| Short-acting |
| ||||
| Exenatide [32] | Byetta® AstraZeneca, Cambridge, England | 2005 (US) 2006 (EU) | 5–10 mcg sc | Twice daily prior to meals | |
| Lixisenatide [33] | Adlyxin®, Lyxumia® Sanofi, Paris, France | 2013 (EU) 2016 (US) | 10–20 mg sc | Once daily prior to the first meal | |
| Long-acting | |||||
| Exenatide ER [32] | Bydureon® AstraZeneca, Cambridge, England | 2012 (US) 2011 (EU) | 2 mg sc | Once weekly, unrelated to meals | |
| Albiglutide [34] | Eperzan® Tanzeum® GlaxoSmithKline, London, England | 2014 (EU) 2014 (US) | 30–50 mg sc | Once weekly, unrelated to meals | |
| Liraglutide [35] | Victoza® Novo Nordisk, Bagsvaerd, Denmark | 2010 (US) 2009 (EU) | 0.6–1.8 mg sc | Once daily, unrelated to meals | |
| Dulaglutide [36] | Trulicity® Eli Lilly and Company, Indianapolis, Indiana, USA | 2014 (US, EU) | 0.75–1.5 mg sc | Once weekly, unrelated to meals | |
| Semaglutide [37,38] | Ozempic® Novo Nordisk, Bagsvaerd, Denmark | 2017 (US) 2018 (EU) | 0.25–1 mg sc | Once weekly, unrelated to meals | |
| Rybelsus® Novo Nordisk, Bagsvaerd, Denmark | 2019 (US) 2020 (EU) | 3–14 mg po | Once daily, one hour prior to the first meal | ||
| Guidelines/Guidance | Recommendations |
|---|---|
| AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease, 2023 [15] | Semaglutide can be considered for T2DM or obesity in patients with NASH, adding cardiovascular benefit and improving NASH. |
| AACE Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings, 2022 [12] | Recommend pioglitazone and GLP-1RAs for people with T2DM and biopsy-proved NASH. Consider treating T2DM with GLP-1RAs or pioglitazone in the situation of possible NASH with modified noninvasive tests and elevated levels of hepatic enzymes, also offering cardiometabolic benefits, even in pediatric obesity and T2DM. |
| APASL clinical practice guidelines for the diagnosis and management of metabolic-associated fatty liver disease, 2020 [40] | Insufficient evidence in the Asian population. |
| EASL–EASD–EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease, 2016 [17] | Pharmacotherapy reserved for patients with NASH and significant fibrosis (≥stage F2) or NASH with a high risk for disease progression (elevated ALT, T2DM, MS). No firm recommendation for the use of pioglitazone or vitamin E in NASH. Insufficient evidence for GLP-1RAs. |
| NICE Guideline on Liver Disease (NAFLD), 2016 [41] | GLP-1RAs not mentioned. Insufficient evidence for pioglitazone and vitamin E. |
| GLP-1RA | Author, Year, Ref. | Country | Total Patients (GLP-1RA/Control) | Control Arm | GLP-1RA Arm | Follow-Up Duration | Diagnosis Method | Results of GLP-1RAs |
|---|---|---|---|---|---|---|---|---|
| Exenatide | Gastaldelli et al., 2020 [42] | Italy | 228/227/230 | Dapagliflozin + placebo Exenatide + placebo | Exenatide 2 mg OW and Dapagliflozin 10 mg daily | 104 weeks | Steatosis and fibrosis scores | Improved biomarkers of steatosis and fibrosis in the exenatide and dapagliflozin group vs. exenatide or dapagliflozin alone. |
| Liu et al., 2020 [43] | China | 35/36 | Glargine insulin | Exenatide 5 μg BID/4 weeks, then 10 μg BID/20 weeks | 24 weeks | 1H-MRS | Greater reductions in body weight, visceral fat area, liver enzymes, FIB-4, postprandial plasma glucose, and LDL-C. | |
| Dutour et al., 2016 [44] | France | 22/22 | Standard of care without GLP-1RA | Exenatide 5 μg BID/4 weeks, then 10 μg BID/22 weeks | 26 weeks | 1H-MRS | Reduced liver fat content and epicardial fat dependent on weight loss. | |
| Shao et al., 2014 [45] | China | 30/30 | Insulin-based therapy | Exenatide 5 μg BID/4 weeks, then 10 μg BID/8 weeks | 12 weeks | US | Lower AST, ALT, and GGT (p < 0.001) correlated with body weight change. | |
| Fan et al., 2013 [46] | China | 49/68 | Metformin | Exenatide 5 μg BID/4 weeks, then 10 μg BID/8 weeks | 12 weeks | US | Controlled blood glucose, reduced body weight, and improved hepatic enzymes. | |
| Cuthbertson et al., 2012 [47] | United Kingdom | 25 19/6 | - | Exenatide 5 μg BID/4 weeks, then 10 μg BID/20 weeks Or Liraglutide 0.6–1.2 mg daily | 24 weeks | 1H-MRS MRI | Correlation between intrahepatic lipid and AST, ALT, and GGT levels (p < 0.05). Significant body weight relative reduction of 4.3%. Reduction in VAT and SAT volume. | |
| Sathyanarayana et al., 2011 [48] | USA | 11/10 | Pioglitazone | Exenatide 5 μg BID/2 weeks, then 10 μg BID/50 weeks | 52 weeks | MRS, plasma adiponectin | Significantly greater reduction in hepatic fat (Δ = 61% vs. 41%, p < 0.05), and greater increase in circulating adiponectin in combination therapy vs. pioglitazone alone. | |
| Buse, 2007 [49] | USA | 283 | Placebo | Exenatide 5 μg or 10 μg BID | 2 years | Liver enzymes | HbA1c and ALT reduction, insulin resistance improvement, weight loss, and blood pressure. | |
| Liraglutide | Tan et al., 2022 [50] | China | 262/1503 | Conventional drug therapy | Liraglutide daily | 12 months | Fibrosis scores LSM | Decrease of NFS (p = 0.043), FIB-4 (p = 0.044), and LSM (p = 0.007). Reduced prevalence of advanced fibrosis (3.1% vs. 6.1%, p = 0.218) in the liraglutide group vs. control group. |
| Bizino et al., 2020 [51] | Netherlands | 23/26 | Placebo | Liraglutide 1.8 mg daily | 26 weeks | MRI | Significantly reduced SAT No significant change in VAT, hepatic, epicardial, and myocardial fat content. | |
| Gameil et al., 2020 [52] | Egypt | 79/80/65 | Conventional treatment | Liraglutide 1.8 mg daily Or Dulaglutide 1.5 mg OW | 24 weeks | FLI FIB-4 score | Significant reduction of median FLI and FIB-4 score in the liraglutide and dulaglutide groups vs. the conventional treatment group (p < 0.001). Greater change in the liraglutide group vs. dulaglutide group (p = 0.027). | |
| Guo et al., 2020 [53] | China | 32/32/32 | Insulin glargine Placebo | Liraglutide 1.8 mg daily | 26 weeks | 1H-MRS | Significantly decreased IHCL (26.4% ± 3.2% to 20.6% ± 3.9%, p < 0.05). Significantly decreased of SAT and VATgroup (SAT, 331.7 ± 79.0 cm2 to 295.3 ± 80.3 cm2, p < 0.05; and VAT, 235.6 ± 30.8 cm2 to 188.2 ± 26.6 cm2, p < 0.05). | |
| Simeone et al., 2020 [54] | Italy | 16 people with T2DM, 16 with prediabetes | Lifestyle counseling | Liraglutide 1.8 mg daily | Not mentioned | MRI | Improvement in glycemic control, CRP, IL-1β level, BMI, and NAFLD degree. | |
| Yan et al., 2019 [55] | China | 24/27 | Sitagliptin Glargine | Liraglutide 1.8 mg daily | 26 weeks | MRI-PDFF | Reduced body weight, intrahepatic lipid, and VAT in addition to improving glycemic control. | |
| Tian et al., 2018 [56] | China | 52/75 | Metformin 1–1.5 g/day | Liraglutide 0.6–1.8 mg daily | 12 weeks | B-mode Ultrasonic Scanning | Significant decrease of 2 h plasma glucose, AST, ALT, and adiponectin levels. | |
| Zhang et al., 2018 [57] | China | 424/411 | Conventional drug therapy | Liraglutide 1.2 mg daily | 24 weeks | Biochemical analyzer | Significant improvement in blood glucose level, HbA1c, lipid profile, and liver function. | |
| Bouchi et al., 2017 [58] | Japan | 8/9 | Insulin | Liraglutide 0.9 mg daily | 36 weeks | CT | Significantly reduced VFA, LAI, ACR, and CRP levels. | |
| Feng et al., 2017 [59] | China | 29/29 | Metformin Gliclazide | Liraglutide daily | 24 weeks | US | Lower HbA1c, improved liver enzymes, weight loss on liraglutide and metformin. Decreased liver fat content in all groups, greater in liraglutide compared with others. | |
| Petit et al., 2017 [60] | France | 68 | Conventional drug therapy | Liraglutide 1.2 mg daily | 24 weeks | MRS | Significantly reduced LFC (−31%, p = 0.0001) and body weight. | |
| Armstrong et al., 2016 [61] | United Kingdom | 26/26 | Placebo | Liraglutide 1.8 mg daily | 48 weeks (extension to 72 weeks) | Liver biopsy | Significantly higher NASH resolution (39% liraglutide vs. 9% placebo). No worsening of fibrosis. | |
| Smits et al., 2016 [62] | Netherlands | 17/17 | Sitagliptin 100 mg Placebo | Liraglutide 1.8 mg daily | 12 weeks | 1H-MRS Fibrosis scores | Reduced glycemia, HbA1c, insulin levels. Reduced steatosis by 10% (p = 0.98). No influence on hepatic fibrosis. Reduced plasma albumin levels (p = 0.03). | |
| Vanderheiden et al., 2016 [63] | USA | 35/36 | Placebo | Liraglutide 1.8 mg daily | 24 weeks | MRI/MRS | Significant reduction in abdominal SAT, no change in VAT, and the ratio of visceral to total fat. | |
| Tang et al., 2015 [64] | Canada | 18/17 | Glargine | Liraglutide 1.8 mg daily | 12 weeks | MRI-PDFF | Similar improvement in the liver fat fraction in both groups (p = 0.34). No weight gain from insulin therapy. | |
| Ohki et al., 2012 [65] | Japan | 26/36 26/20 | Sitagliptin Pioglitazone | Liraglutide daily 0.3 mg/first week, 0.6 mg/next week, and finally up to the limit dose of 0.9 mg if necessary | 48 weeks for liraglutide | US | Body weight decreased from 81.1 to 78 kg. BMI from 30.1 to 28.6 kg/m2. Fasting glycemia from 207 to 168 mg/dL. HbA1c from 8.4% to 7.6%. AST from 50 IU/L to 35 IU/L. ALT from 65 IU/L to 48 IU/L. APRI index from 0.73 to 0.49. | |
| Semaglutide | Carretero-Gomez et al., 2023 [66] | Spain | 213 | - | Semaglutide sc OW | 24 weeks | HSI FIB-4 | Significant reduction in HSI (−2.36, p < 0.00001) and FIB-4 (−0.075, p < 0.016), related to the decrease of body weight, triglyceride levels, insulin resistance, and liver enzymes. |
| Arai et al., 2022 [67] | Japan | 16 | - | Oral semaglutide daily 3 mg/4 weeks, then 7 mg/4 weeks, then 14 mg/16 weeks | 24 weeks | CAP, LSM | Reduced HbA1c, HOMA-IR, ferritin normalized hepatic enzymes. Decreased FIB-4 index from 1.42 to 1.1. Significantly decreased CAP values from 344 to 279 dB/m. | |
| Ding et al., 2022 [68] | China | 75/75 | Metformin | Semaglutide sc OW 0.6 mg/1.2 mg/1.8 mg | 12 weeks | US | Decreased AST, ALT, and GGT levels (p < 0.05). Significantly reduced moderate to severe NAFLD patients. | |
| Volpe et al., 2022 [69] | Italy | 48 | - | Semaglutide sc 0.25 mg OW for 4 weeks, then 0.5 mg OW for 20 weeks | 52 weeks | US US-Liver Steatosis Score | Reduced body weight, HbA1c, HOMA-IR, serum lipid, AST, ALT, GGT, FLI. Significant improvement in liver steatosis severity, body composition. | |
| Flint et al., 2021 [70] | Germany | 34/33 | Placebo | Semaglutide sc 0.4 mg daily | 72 weeks | MRE MRI-PDFF | ≥30% reduction in liver fat content, but no significant change in liver stiffness. Decrease of HbA1c, liver enzymes, body weight. | |
| Newsome et al., 2021 [71] | United Kingdom | 82/80 | Placebo | Semaglutide sc 0.4 mg daily | 72 weeks | Liver biopsy | Resolution of NASH (59% vs. 17% placebo, p < 0.001). Slowed fibrosis progression (4.9% vs. 18.8% placebo), but no significant reduction in fibrosis stages. | |
| Dulaglutide | Kuchay MS, 2020 [72] | India | 32/32 | Standard of care without GLP-1RA | Dulaglutide 0.75 mg OW for 4 weeks, then 1.5 mg OW for 20 weeks | 24 weeks | MRI-PDFF | Significant reduction of LFC (−3.5%, p = 0.025) and GGT levels (−13.1 IU/L, p = 0.025) in patients with NAFLD. No significant reductions in PFC, liver stiffness, serum AST and ALT levels. |
| Bogomolov et al., 2020 [73] | Russia | 65 | - | Dulaglutide 0.75 mg OW for 2 weeks, then 1.5 mg OW for 24 weeks | 26 weeks | FLI | Significant reduction of body weight, BMI, waist circumference, glucose, HbA1c, insulin resistance indexes, transaminases, and GGT. Decreased FLI and liver stiffness. |
| Trial Name | Estimated Enrollment | Start Date | Completion Date | Intervention | Criteria for Diabetes | Primary Outcome | Secondary Outcome |
|---|---|---|---|---|---|---|---|
| A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study Comparing the Efficacy and Safety of Tirzepatide Versus Placebo in Patients with Nonalcoholic Steatohepatitis (SYNERGY-NASH) [112] | 196 participants | 19 November 2019 | 13 February 2024 | Tirzepatide 5/10/15 mg sc OW vs. placebo | Patients with T2DM (HbA1c ≤ 9.5%) or without T2DM |
|
|
| Researching an Effect of GLP-1 Agonist on Liver Steatosis (REALIST) [113] | 93 participants | 1 September 2019 | 30 March 2024 | Dulaglutide 1.5 mg sc OW | Moderately controlled T2DM under OADs stable dose for at least 3 months |
|
|
| Combined Active Treatment in Type 2 Diabetes with NASH (COMBAT_T2_NASH) [114] | 192 participants | 26 March 2021 | December 2023 | Empagliflozin 10 mg/d + semaglutide 1 mg/week vs. empagliflozin 10 mg/d vs. placebo | Patient with T2D (HbA1c ≤ 9.5%) and NASH (F1–F3 fibrosis stage) |
|
|
| The Effect of Semaglutide in Subjects with Noncirrhotic Nonalcoholic Steatohepatitis (ESSENCE) [87] | 1200 participants | 1 April 2021 | 26 May 2028 | Semaglutide sc OW | Patients with T2DM, without any glucose-lowering agents |
|
|
| A Phase IIb/III Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of Cotadutide in Participants with Noncirrhotic Nonalcoholic Steatohepatitis with Fibrosis (PROXYMO-ADV) [115] | 1860 participants | 14 July 2022 | 26 March 2026 | Cotadutide 300 μg/600 μg sc once daily vs. placebo | Patients with or without T2DM |
|
|
| Effect on Nonalcoholic Fatty Liver Disease with Advanced Fibrosis in Patients with Type 2 Diabetes Mellitus on Treatment with Gastric Inhibitory Polypeptide/Glucagon-Like Peptide-1 Analogue NCT05751720 [116] | 30 participants | April 2023 | February 2025 | Tirzepatide sc 0.25 mg OW/4 weeks then 0.5 mg OW, or Oral semaglutide 3 mg daily/first month, then 7 mg daily for 6 months | Patients with T2DM for > 1 year in presence of NAFLD advanced fibrosis |
|
|
| Semaglutide, 2.4 mg, Once Weekly: Effects on Beta-cell Preservation and Reduction of Intrahepatic Triglyceride Content in Obese Youth with Prediabetes (IGT)/Early Type 2 Diabetes (T2D) and Nonalcoholic Fatty Liver Disease (NAFLD) NCT05067621 [117] | 60 participants | January 2023 | January 2027 | Semaglutide sc 0.24 mg/0.5 mg/1.0 mg/1.7 mg/2.4 mg OW | Impaired glucose tolerance or new onset T2DM (<6 months duration) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazac, G.-D.; Lăcătușu, C.-M.; Ștefănescu, G.; Mihai, C.; Grigorescu, E.-D.; Onofriescu, A.; Mihai, B.-M. Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives. Metabolites 2023, 13, 581. https://doi.org/10.3390/metabo13050581
Cazac G-D, Lăcătușu C-M, Ștefănescu G, Mihai C, Grigorescu E-D, Onofriescu A, Mihai B-M. Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives. Metabolites. 2023; 13(5):581. https://doi.org/10.3390/metabo13050581
Chicago/Turabian StyleCazac, Georgiana-Diana, Cristina-Mihaela Lăcătușu, Gabriela Ștefănescu, Cătălina Mihai, Elena-Daniela Grigorescu, Alina Onofriescu, and Bogdan-Mircea Mihai. 2023. "Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives" Metabolites 13, no. 5: 581. https://doi.org/10.3390/metabo13050581
APA StyleCazac, G.-D., Lăcătușu, C.-M., Ștefănescu, G., Mihai, C., Grigorescu, E.-D., Onofriescu, A., & Mihai, B.-M. (2023). Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives. Metabolites, 13(5), 581. https://doi.org/10.3390/metabo13050581








