Repeated Low-Level Blast Exposure Alters Urinary and Serum Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Materials
2.3. Sample Collection
2.4. Sample Preparation
2.5. HPLC-MS/MS Analysis
2.6. Urine Specific Gravity Measurements
2.7. Quality Assurance and Quality Control
2.8. Data Processing and Statistical Analyses
3. Results
3.1. Method Performance
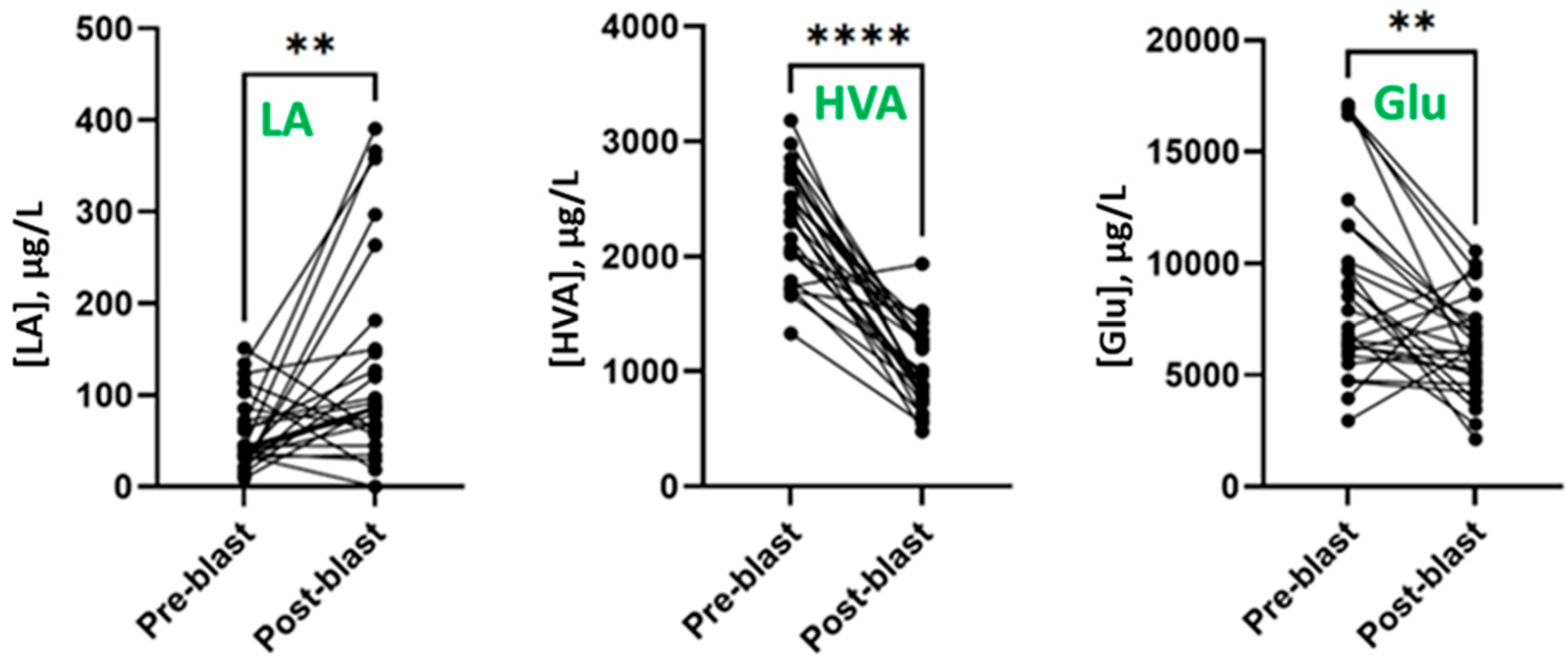
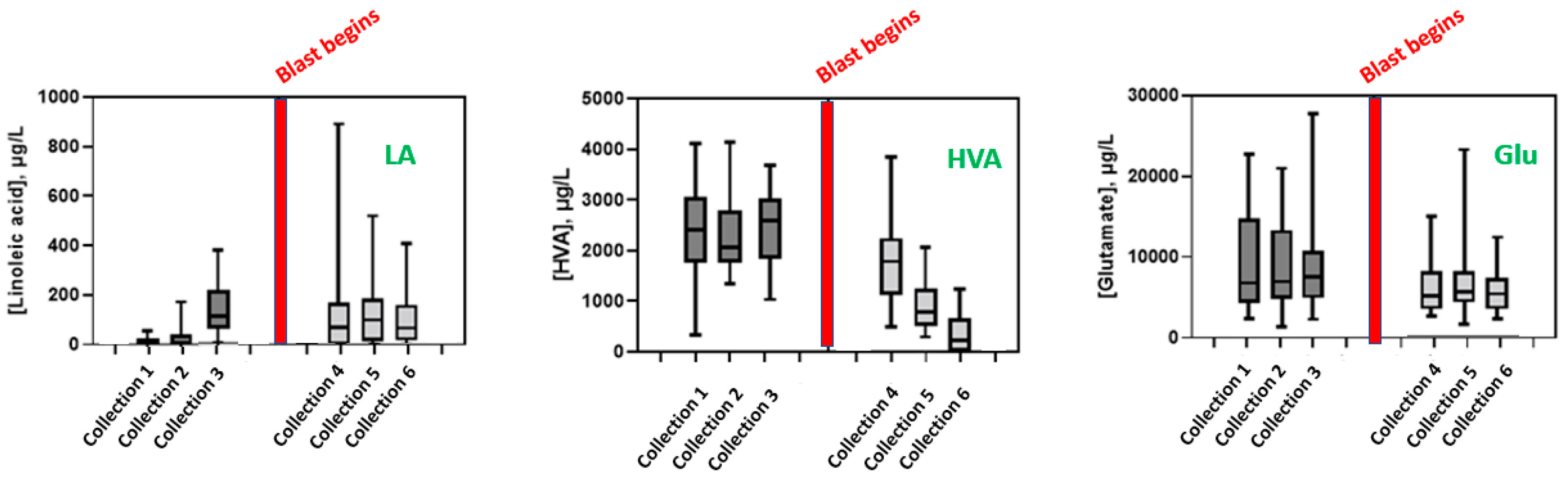
3.2. Effect of Blast Exposure on Urinary Metabolites
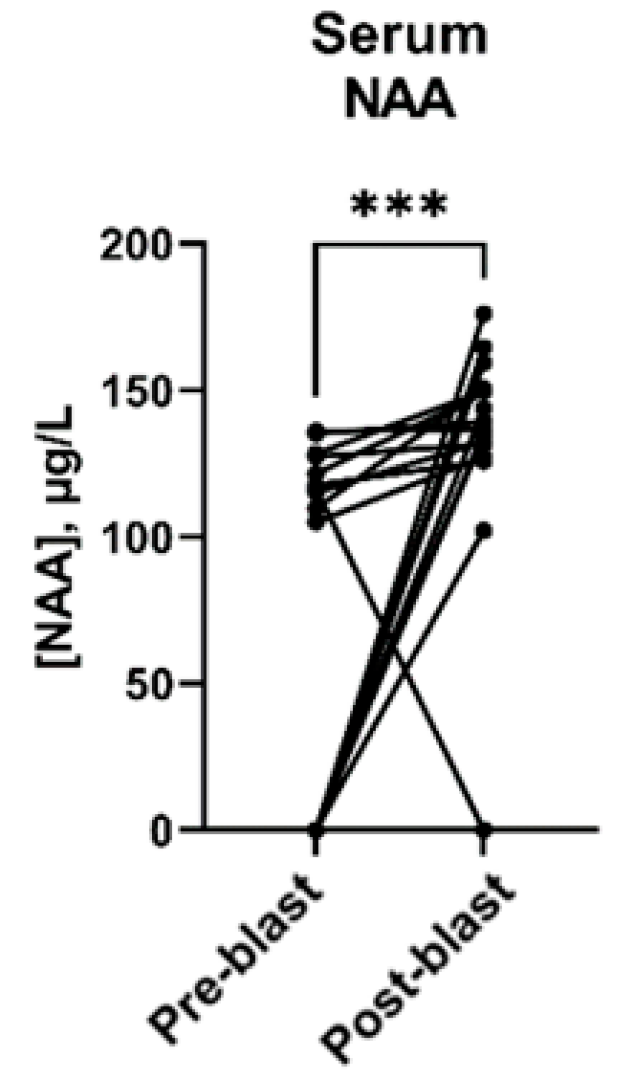
3.3. Effect of Blast Exposure on Serum Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindberg, M.; Sloley, S.; Ivins, B.; Marion, D.; Martin, E.M. Military TBI—What civilian primary care providers should know. J. Fam. Med. Prim. Care 2021, 10, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.; Stone, J.R.; Walilko, T.; Young, L.A.; Snook, T.L.; Paggi, M.E.; Tsao, J.W.; Jankosky, C.J.; Parish, R.V.; Ahlers, S.T. Repeated low-level blast exposure: A descriptive human subjects study. Mil. Med. 2016, 181, 28–39. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.; McCrea, M. Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J. Athl. Train. 2017, 5, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.L.; Gasperi, R.D.; Sosa, M.A.; Perez-Garcia, G.; Short, J.A.; Sosa, H.; Perez, G.; Knesaurek, A.E.; Knutsen, A.; Pham, D.L.; et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol. Psychiatry 2021, 26, 5940–5954. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cowan, M.; Beraldo, F.; Schranz, A.; McCunn, P.; Geremia, N.; Brown, Z.; Patel, M.; Nygard, K.L.; Khazaee, R.; et al. Repetitive mild traumatic brain injury in mice triggers a slowly developing cascade of long-term and persistent behavioral deficits and pathological changes. Acta. Neuropathol. Commun. 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.M.; Walter, A.; Breiter, H.C.; Zhu, D.C.; Bai, X.; Bream, T.; Seidenberg, P.; Mao, X.; Johnson, B.; Talavage, T.M. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: A multi-modal neuroimaging study. Neuroimage Clin. 2017, 14, 708–718. [Google Scholar] [CrossRef]
- Elder, G.A.; Ehrlich, M.E.; Gandy, S. Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci. Lett. 2019, 707. [Google Scholar] [CrossRef]
- Loignon, A.; Ouellet, M.C.; Belleville, G. A systematic review and meta-analysis on PTSD following TBI among military/veteran and vivilian populations. J. Head Trauma Rehabil. 2020, 35, E21–E35. [Google Scholar] [CrossRef]
- Carr, W.; Polejaeva, E.; Grome, A.; Crandall, B.; LaValle, C.; Eonta, S.E.; Young, L.A. Relation of repeated low-level blast exposure with symptomology similar to concussion. J. Head Trauma Rehabil. 2015, 30, 47–55. [Google Scholar] [CrossRef]
- Ling, G.; Ecklund, J.M.; Bandak, F.A. Brain injury from explosive blast: Description and clinical management. In Handbook of Clinical Neurology; Vinken, P., Bruyn, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–180. [Google Scholar]
- Edalatfar, M.; Piri, S.; Mehrabinejad, M.; Mousave, M.; Meknakhah, S.; Fattahi, M.; Kavyani, Z.; Hajighadery, A.; Kaveh, M.; Aryannejad, A.; et al. Biofluid biomarkers in traumatic brain injury: A systematic scoping review. Neurocrit. Care 2021, 35, 559–572. [Google Scholar] [CrossRef]
- Agoston, D.V.; Shutes-David, A.; Peskind, E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017, 31, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Schiff, L.; Hadker, N.; Weiser, S.; Rausch, C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol. Diagn. Ther. 2012, 16, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; León-Justel, A.; Gordillo, E.; Revuelto-Rey, J.; Vilches-Arenas, A.; Carrillo-Vico, A.; Dominguez, J.M.; Cabezas, F.M.; Guerrero, J.M. Role of S100B protein in urine and serum as an early predictor of mortality after severe traumatic brain injury in adults. Clin. Chim. Acta 2012, 414, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kulbe, J.R.; Geddes, J.W. Current status of fluid biomarkers in mild traumatic brain injury. Exp. Neurol. 2016, 275, 334–352. [Google Scholar] [CrossRef]
- Jeter, C.B.; Hergenroeder, G.W.; Hylin, M.J.; Redell, J.B.; Moore, A.N.; Dash, P.K. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma 2013, 30, 657–670. [Google Scholar] [CrossRef]
- Vorn, R.; Naunheim, R.; Lai, C.; Wagner, C.; Gill, J.M. Elevated axonal protein markers following repetitive blast exposure in military personnel. Front. Neurosci. 2022, 16, 853616. [Google Scholar] [CrossRef]
- An, M.; Gao, Y. Urinary biomarkers of brain diseases. Genom. Proteom. Bioinform. 2015, 13, 345–354. [Google Scholar] [CrossRef]
- Sigler, A.; He, X.; Bose, M.; Cristea, A.; Liu, W.; Nam, P.K.-S.; James, D.; Burton, C.; Shi, H. Simultaneous determination of eight urinary metabolites by HPLC-MS/MS for noninvasive assessment of traumatic brain injury. J. Am. Soc. Mass Spectrom. 2020, 31, 1910–1917. [Google Scholar] [CrossRef]
- Baker, A.J.; Moulton, R.J.; MacMillan, V.H.; Shedden, P.M. Excitatory amino acids in cerebrospinal fluid following traumatic brain injury in humans. J. Neurosurg. 1993, 79, 369–372. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Graham, S.; Han, B.; Turkoglu, O.; Ziaeh, J.; Mandal, R.; Er, A.; Wishart, D.S.; Stahel, P. Serum metabolomic markers for traumatic brain injury: A mouse model. Metabolomics 2016, 12, 100. [Google Scholar] [CrossRef]
- Jalloh, I.; Helmy, A.; Howe, D.J.; Shannon, R.J.; Grice, P.; Mason, A.; Gallagher, C.N.; Murphy, M.P.; Pickard, J.D.; Menon, D.K.; et al. Comparison of oxidative lactate metabolism in traumatically injured brain and control brain. J. Neurotrauma 2018, 35, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Anthonymuthu, T.S.; Kenny, E.M.; Lamade, A.M.; Kagan, V.E.; Bayır, H. Oxidized phospholipid signaling in traumatic brain injury. Free Radic. Biol. Med. 2018, 124, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cui, J.; Simonyi, A.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z. Linking blast physics to biological outcomes in mild traumatic brain injury: Narrative review and preliminary report of an open-field blast model. Behav. Brain Res. 2018, 340, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Corte, A.D.; Chitarrini, G.; Di Gangi, I.M.; Masuero, D.; Soini, E.; Mattivi, F.; Vrhovsek, U. A rapid LC–MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta 2015, 40, 52–61. [Google Scholar] [CrossRef]
- Burton, C.; Shi, H.; Ma, Y. Normalization of urinary pteridines by urine specific gravity for early cancer detection. Clin. Chim. Acta 2014, 435, 42–47. [Google Scholar] [CrossRef]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid transport and metabolism at the blood-brain interface: Implications in health and disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef]
- Ozga, J.; Povroznik, J.; Engler-Chiurazzi, E.; Haar, C. Executive (dys)function after traumatic brain injury: Special considerations for behavioral pharmacology. Behav. Pharmacol. 2018, 7, 617–637. [Google Scholar] [CrossRef]
- Wagner, A.; Kline, A.; Sokoloski, J.; Zafonte, R.; Capulong, E.; Dixon, C. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 2002, 334, 165–168. [Google Scholar] [CrossRef]
- Vecht, C.J.; van Woerkom, T.C.A.M.; Teelken, A.W.; Minderhoud, J.M. Homovanillic acid and 5-hydroxyindoleacetic acid cerebrospinal fluid level, a study with and without probenecid administration of their relationship to the state of consciousness after heard injury. Arch. Neurol. 1975, 32, 792–797. [Google Scholar] [CrossRef]
- Bareggi, S.R.; Porta, M.; Selenati, A.; Assael, B.; Calderini, G.; Collice, M.; Rossanda, M.; Morselli, P.L. Homovanillic acid and 5-hydroxyindole-acetic acid in the CSF of patients after a severe head injury I. Lumbar CSF concentrations in chronic brain post-traumatic syndromes. Eur. Neurol. 1975, 13, 528–544. [Google Scholar] [CrossRef]
- Bykowski, E.A.; Petersson, J.N.; Dukelow, S.; Ho, C.; Delbert, C.T.; Montina, T.; Metz, G. Urinary metabolomic signatures as indicators of injury severity following traumatic brain injury: A pilot study. IBRO Rep. 2021, 11, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Schmid, K.; Berger, R.; Kobeissy, F.; Italiano, D.; Jeromin, A.; Hayes, R.; Tortella, F.; Buki, A. The challenge of mild traumatic brain injury: Role of biochemical markers in diagnosis of brain damage. Med. Res. Rev. 2014, 34, 503–531. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, M.; Zhang, Z.; Metherel, A.; Kitson, A.; Otoki, Y.; Richardson, C.; Yang, J.; Lee, K.; Hammock, B.; Zhang, L.; et al. Linoleic acid participates in the response to ischemic brain injury through oxidized metabolites that regulate neurotransmission. Sci. Rep. 2017, 7, 4342. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.; Rapoport, S.I.; Purdon, A.D. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem. Res. 1997, 22, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Strokin, M.; Sergeeva, M.; Reiser, G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br. J. Pharmacol. 2003, 139, 1014–1022. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Hajiaghamemar, M.; Kilbaugh, T.; Arbogast, K.B.; Master, C.L.; Margulies, S.S. Using serum amino acids to predict traumatic brain injury: A systematic approach to utilize multiple biomarkers. Int. J. Mol. Sci. 2020, 21, 1786. [Google Scholar] [CrossRef]
- Simone, I.L.; Ruggieri, M.; Tortelli, R.; Ceci, E.; D’Errico, E.; Leo, A.; Zoccolella, S.; Mastrapasqua, M.; Capozzo, R.; Livrea, P.; et al. Serum N-acetylaspartate level in amyotrophic lateral sclerosis. Arch. Neurol. 2011, 68, 1308–1312. [Google Scholar] [CrossRef]
- Bernarroch, E.E. N-acetylaspartate and N-acetylaspartylglutamate: Neurobiology and clinical significance. Neurology 2008, 70, 1353–1357. [Google Scholar] [CrossRef]
| Compound | Ion Pairs | Declustering Potential (DP, V) | Collision Energy (CE, V) | Collision Cell Exit Potential (CXP, V) |
|---|---|---|---|---|
| Arachidonic Acid (AA) | 349.4/303.6 | −35 | −18 | −7 |
| 349.4/259.6 | −35 | −24 | −17 | |
| Linoleic Acid (LA) | 325.4/279.6 | −30 | −18 | −7 |
| 325.4/45.2 | −30 | −30 | −1 |
| Analyte | Pre-Blast | Post-Blast | p-Value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Linoleic acid (LA) | 42.7 | <LOD–352 | 173.9 | <LOD–857 | 0.0030 |
| Homovanillic acid (HVA) | 2320 | 300–4108 | 864.6 | <LOD–3850 | <0.0001 |
| Lactic acid (Lac) | 933.7 | 65.18–2466 | 897.1 | 120.4–2724 | 0.7140 |
| N-Acetylaspartic acid (NAA) | 5831 | 2784–9879 | 6638 | 3127–12,926 | 0.2687 |
| Arachidonic acid (AA) | 18.82 | 6.045–59.14 | 26.87 | 8.483–81.41 | 0.2792 |
| Pyruvic acid (Pyr) | 1461 | 553.4–3731 | 1397 | 349.8–1959 | 0.2792 |
| Methionine sulfoxide (MetSO) | 105.2 | 11.91–398.6 | 138.6 | 61.54–302.3 | 0.1855 |
| 5-Hydroxyindoleacetic acid (5-HIAA) | 2776 | 1149–5515 | 2397 | 936.5–5157 | 0.9153 |
| Glutamic acid (Glu) | 7906 | 1927–27,132 | 6161 | 2122–24,891 | 0.0027 |
| Analyte | Pre-Blast | Post-Blast | p-Value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Linoleic acid (LA) | <LOD | <LOD–9.154 | <LOD | <LOD–34.69 | >0.999 |
| Homovanillic acid (HVA) | <LOD | <LOD–513.7 | <LOD | <LOD–382.3 | 0.4121 |
| Lactic acid (Lac) | 146,806 | 78,478–419,083 | 128,171 | 9011–373,531 | 0.1964 |
| N-Acetylaspartic acid (NAA) | 107.3 | <LOD–136.0 | 136.5 | <LOD–176.1 | 0.0006 |
| Arachidonic acid (AA) | <LOD | <LOD–7.913 | <LOD | <LOD–29.36 | 0.8203 |
| Pyruvic acid (Pyr) | 9003 | 2858–19,318 | 11,221 | <LOD–26,216 | 0.2288 |
| Methionine sulfoxide (MetSO) | <LOD | <LOD–175.4 | <LOD | <LOD–141.4 | 0.9219 |
| 5-Hydroxyindoleacetic acid (5-HIAA) | <LOD | <LOD–338.2 | <LOD | <LOD | 0.0625 |
| Glutamic acid (Glu) | 2954 | 1118–4330 | 2827 | <LOD–5108 | >0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigler, A.; Wu, J.; Pfaff, A.; Adetunji, O.; Nam, P.; James, D.; Burton, C.; Shi, H. Repeated Low-Level Blast Exposure Alters Urinary and Serum Metabolites. Metabolites 2023, 13, 638. https://doi.org/10.3390/metabo13050638
Sigler A, Wu J, Pfaff A, Adetunji O, Nam P, James D, Burton C, Shi H. Repeated Low-Level Blast Exposure Alters Urinary and Serum Metabolites. Metabolites. 2023; 13(5):638. https://doi.org/10.3390/metabo13050638
Chicago/Turabian StyleSigler, Austin, Jiandong Wu, Annalise Pfaff, Olajide Adetunji, Paul Nam, Donald James, Casey Burton, and Honglan Shi. 2023. "Repeated Low-Level Blast Exposure Alters Urinary and Serum Metabolites" Metabolites 13, no. 5: 638. https://doi.org/10.3390/metabo13050638
APA StyleSigler, A., Wu, J., Pfaff, A., Adetunji, O., Nam, P., James, D., Burton, C., & Shi, H. (2023). Repeated Low-Level Blast Exposure Alters Urinary and Serum Metabolites. Metabolites, 13(5), 638. https://doi.org/10.3390/metabo13050638







