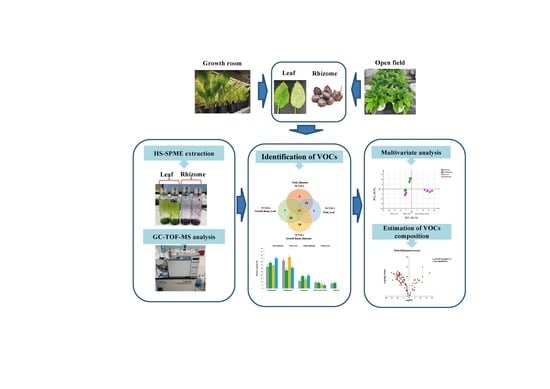
Exploring Volatile Organic Compounds in Rhizomes and Leaves of Kaempferia parviflora Wall. Ex Baker Using HS-SPME and GC–TOF/MS Combined with Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.1.1. Plant Selection and Preparation
2.1.2. Tissue Sample Collection and Preparation
2.2. Sample Processing
2.2.1. Homogenization
2.2.2. Chemicals
2.2.3. VOCs Extraction
2.3. GC-TOF-MS Analysis
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. The VOC Profile in the Headspace of K. parviflora Rhizomes and Leaves
3.2. Multivariate Data Analysis (MVDA) of Omics Data
Principal Component Analysis (PCA)
3.3. Estimated Quantitative VOC Composition of K. parviflora Rhizomes and Leaves
3.4. Effect of Growth Conditions on the Composition of Common VOCs of K. parviflora Tissues
3.5. Identification of VOCs as Basis to Substitute Rhizomes with Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCS) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant. Sci. 2019, 10, 7–37. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional Interaction between Phyllospheric Microbiotas and Plant Volatile Emissions. Trends Plant. Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant. Biol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Hansen, U.; Seufert, G. Temperature and light dependence of β-caryophyllene emission rates. J. Geophys. Res. 2003, 108, 4801. [Google Scholar] [CrossRef]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikova, V. Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Environ. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Munda, S.; Saikia, P.; Lal, M. Chemical composition and biological activity of essential oil of Kaempferia galanga: A review. J. Essent. Oil Res. 2018, 30, 303–308. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-Elgawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Zouaoui, N.; Chenchouni, H.; Bouguerra, A.; Massouras, T.; Barkat, M. Characterization of volatile organic compounds from six aromatic and medicinal plant species growing wild in North African drylands. NFS J. 2020, 18, 19–28. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Abbas, F. Aroma Components in Horticultural Crops: Chemical Diversity and Usage of Metabolic Engineering for Industrial Applications. Plants. 2023, 12, 1748. [Google Scholar] [CrossRef]
- Wangchuk, P. Therapeutic Applications of Natural Products in Herbal Medicines, Biodiscovery Programs, and Biomedicine. J. Biol. Act. Prod. Nat. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Gyawali, R.; Kim, K.S. Bioactive Volatile Compounds of Three Medicinal Plants From Nepal. J. Eng. Sci. Technol. 2012, 8, 51–62. [Google Scholar] [CrossRef]
- Begum, T.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Direct sunlight and partial shading alter the quality, quantity, biochemical activities of Kaempferia parviflora Wall., ex Baker rhizome essential oil: A high industrially important species. Ind. Crops Prod. 2022, 180, 114765. [Google Scholar] [CrossRef]
- Cuervo, L.; Méndez, C.; Salas, J.A.; Olano, C.; Malmierca, G. Volatile Compounds in Actinomycete Communities: A New Tool for Biosynthetic Gene Cluster Activation, Cooperative Growth Promotion, and Drug Discovery. Cells 2022, 11, 3510. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and its methoxyflavones: Chemistry and biological activities. Evid. Based Complement. Altern. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef]
- Labrooy, C. Morphological description for Kunyit Hitam (Kaempferia parviflora) and breaking bud dormancy with BAP and Ethephon treatment. In Proceedings of the 24th Malaysian Society of Plant Physiology Conference, Terengganu, Malaysia, 26 August 2013; pp. 139–141. Available online: https://www.researchgate.net/publication/263772758 (accessed on 26 August 2013).
- Yee, T.T.; War, K.; Lwin, Y. Study of Phytochemical Composition on Kaempferia parviflora Wall. ex Baker *. IEEE Pers. Commun. 2019, 7, 128–136. [Google Scholar]
- Singh, A.; Singh, N.; Singh, S.; Srivastava, R.P.; Singh, L.; Verma, P.C.; Saxena, G. The industrially important genus Kaempferia: An ethnopharmacological review. Front. Pharmacol. 2023, 14, 1099523. [Google Scholar] [CrossRef]
- Thairat, S.; Srichan, R.; Mala, S. Cytotoxic effect of Kaempferia parviflora extract on normal oral keratinocytes and a human squamous carcinoma cell line. Mahidol Dent. J. 2022, 42, 33–38. Available online: https://he02.tci-thaijo.org/index.php/mdentjournal/article/view/244781 (accessed on 23 March 2022).
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 143, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Aburada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Wasuntarawat, C.; Pengnet, S.; Walaikavinan, N.; Kamkaew, N.; Bualoang, T.; Toskulkao, C.; Mcconell, G. No effect of acute ingestion of Thai ginseng (Kaempferia parviflora) on sprint and endurance exercise performance in humans. J. Sport. Sci. 2010, 28, 1243–1250. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Vichitphan, S.; Vichitphan, K.; Sirikhansaeng, P. Flavonoid content and antioxidant activity of krachai-dum (Kaempferia parviflora) wine. Curr. Appl. Sci. Technol. 2007, 7, 97–105. [Google Scholar]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and Antioxidant Activities by Rhizomes of Kaempferia parviflora Wall. ex Baker. Nat. Prod. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods. 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Ahmed, F.R.S.; Amin, R.; Hasan, I.; Asaduzzaman, A.K.M.; Kabir, S.R. Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol. 2017, 102, 952–959. [Google Scholar] [CrossRef]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med. 2018, 18, 178. [Google Scholar] [CrossRef]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Yoshino, S.; Awa, R.; Miyake, Y.; Fukuhara, I.; Sato, H.; Ashino, T.; Tomita, S.; Kuwahara, H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018, 11, 447–458. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Tong-Un, T.; Muchimapura, S.; Wannanon, P.; Sripanidkulchai, B.; Phachonpai, W. Anti-Stress Effects of Kaempferia parviflora in Immobilization Subjected Rats. Am. J. Pharmacol. Toxicol. 2013, 8, 31–38. [Google Scholar] [CrossRef]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef]
- Youn, K.; Lee, J.; Ho, C.T.; Jun, M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods. 2016, 20, 567–574. [Google Scholar] [CrossRef]
- Plaingam, W.; Sangsuthum, S.; Angkhasirisap, W.; Tencomnao, T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complement. Med. 2017, 7, 538–552. [Google Scholar] [CrossRef]
- Chaturapanich, G.; Chaiyakul, S.; Verawatnapakul, V.; Pholpramool, C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction 2008, 136, 515–522. [Google Scholar] [CrossRef]
- Sitthichai, P.; Chanpirom, S.; Maneerat, T.; Charoensup, R.; Tree-Udom, T.; Pintathong, P.; Laphookhieo, S.; Sripisut, T. Kaempferia parviflora Rhizome Extract as Potential Anti-Acne Ingredient. Molecules 2022, 27, 4401. [Google Scholar] [CrossRef]
- Azuma, T.; Kayano, S.I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, K.; Deguchi, T.; Itoh, K.; Fujita, T.; Higashino, M.; Yoshioka, Y.; Matsumura, S.; Tanaka, R.; Shinada, T.; et al. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011, 34, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Horigome, S.; Maeda, M.; Ho, H.J.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Foods 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Ochiai, W.; Kobayashi, H.; Kitaoka, S.; Kashiwada, M.; Koyama, Y.; Nakaishi, S.; Nagai, T.; Aburada, M.; Sugiyama, K. Effect of the active ingredient of Kaempferia parviflora, 5,7-dimethoxyflavone, on the pharmacokinetics of midazolam. J. Nat. Med. 2018, 72, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef]
- Sookkongwaree, K.; Geitmann, M.; Roengsumran, S.; Petsom, A.; Danielson, U.H.; Danielson, U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie 2006, 61, 717–721. [Google Scholar]
- Pitakpawasutthi, Y.; Palanuvej, C.; Ruangrungsi, N. Quality evaluation of Kaempferia parviflora rhizome with reference to 5,7-dimethoxyflavone. J. Adv. Pharm. Technol. Res. 2018, 9, 26–31. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef]
- Wungsintaweekul, J.; Sitthithaworn, W.; Putalun, W.; Pfeifhoffer, H.W.; Brantner, A. Antimicrobial, antioxidant activities and chemical composition of selected Thai spices. Songklanakarin J. Sci. Technol. 2010, 32, 589–598. [Google Scholar]
- Wongpia, A.; Samphunphuang, C.; Pipithsangchan, K.; Somprasong, W.; Boonpradub, S.; Luangsuphabool, T.; Thammasiri, K. Chemical composition analysis of essential oil from black gingers (Kaempferia parviflora) by gas chromatography-mass spectrometry (GC-MS). Acta Hortic. 2021, 1339, 323–330. [Google Scholar] [CrossRef]
- Labrooy, C.D.; Abdullah, T.L.; Abdullah, N.A.P.; Stanslas, J. Optimum shade enhances growth and 5,7-Dimethoxyflavone accumulation in Kaempferia parviflora Wall. ex Baker cultivars. Sci. Hortic. 2016, 213, 346–353. [Google Scholar] [CrossRef]
- Techaprasan, J.; Klinbunga, S.; Ngamriabsakul, C.; Jenjittikul, T. Genetic variation of Kaempferia (Zingiberaceae) in Thailand based on chloroplast DNA (psbA-trnH and petA-psbJ) sequences. Genet. Mol. Res. 2010, 9, 1957–1973. [Google Scholar] [CrossRef]
- Labrooy, D.C.; Abdullah, L.T.; Stanslas, J. Pre-soak Technique Using Bap or Ethephon to Break Dormancy in Black Galingale (Kaempferia parviflora). Am.-Eurasian J. Agric. Environ. Sci. 2016, 16, 1577–1582. [Google Scholar]
- Labrooy, D.C.; Abdullah, L.T.; Stanslas, J. Influence of N6-Benzyladenine and Sucrose on In Vitro Direct Regeneration and Microrhizome Induction of Kaempferia parviflora Wall. Ex Baker, An Important Ethnomedicinal Herb of Asia. Trop. Life Sci. Res. 2020, 31, 123–139. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B. The leaf flavonoids of the Zingiberales. Biochem. Syst. Ecol. 1977, 5, 221–229. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Begum, J.; Anwar, M. Essential oils of leaves and rhizomes of Kaempferia galanga Linn. Chittagong Univ. J. Biol. Sci. 2008, 3, 65–76. [Google Scholar] [CrossRef]
- Ali, M.S.; Dash, P.R.; Nasrin, M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complement. Altern. Med. 2015, 15, 158. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Zakaria, Z.A.; Daud, I.A.; Ng, F.N.; Ng, Y.C.; Hidayat, M.T. Antinociceptive and anti-inflammatory activities of the aqueous extract of Kaempferia galanga leaves in animal models. J. Nat. Med. 2008, 62, 221–227. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Preetha, T.S.; Hemanthakumar, A.S.; Krishnan, P.N. A comprehensive review of Kaempferia galanga L. (Zingiberaceae): A high sought medicinal plant in Tropical Asia. J. Med. Plants Stud. 2016, 4, 270–276. [Google Scholar]
- Eric Chan, W.C.; Lim, Y.Y.; Wong, S.K. Antioxidant properties of ginger leaves: An overview. Free. Radic. Antioxid. 2011, 1, 6–16. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, K.S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.-H.; Sivanesan, L. Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Saini, R.K.; Keum, Y.S.; Sivanesan, I. Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC-MRM-MS and GC-FID/MS. Antioxidants 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Subramaniam, P. Phytochemical Profiling of Leaf, Stem, and Tuber Parts of Solena amplexicaulis (Lam.) Gandhi Using GC-MS. Int. Sch. Res. Not. 2014, 2014, 567409. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kuś, P.; Marijanović, Z.; Tuberoso, C.I.G.; Fecka, I.; Jerković, I. Headspace Solid-Phase Micro-Extraction Versus Hydrodistillation of Volatile Compounds from Leaves of Cultivated Mentha Taxa: Markers of Safe Chemotypes. Molecules 2022, 27, 6561. [Google Scholar] [CrossRef]
- Canuti, V.; Conversano, M.; Calzi, M.L.; Heymann, H.; Matthews, M.A.; Ebeler, S.E. Headspace solid-phase microextraction–gas chromatography–mass spectrometry for profiling free volatile compounds in Cabernet Sauvignon grapes and wines. J. Chromatogr. A 2009, 1216, 3012–3022. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Appl. Sci. 2018, 2, 10–14. [Google Scholar]
- Qi, S.; Zha, L.; Peng, Y.; Luo, W.; Chen, K.; Li, X.; Yin, D. Quality and metabolomics analysis of Houttuynia cordata based on HS-SPME/GC-MS. Molecules 2022, 27, 3921. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, S.; Niu, Y.; Yu, H.; Zhu, J.; Tian, H.; Gu, Y. Discrimination of Chinese vinegars based on headspace solid-phase microextraction-gas chromatography mass spectrometry of volatile compounds and multivariate analysis. J. Food Sci. 2011, 76, 1125–1135. [Google Scholar] [CrossRef]
- Gonzalez, A.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.X.; Molinié, R.; Meffre, P. Extraction and Identification of Volatile Organic Compounds in Scentless Flowers of 14 Tillandsia Species Using HS-SPME/GC-MS. Metabolites 2022, 12, 628. [Google Scholar] [CrossRef]
- Burzynski-Chang, E.A.; Ryona, I.; Reisch, B.I.; Gonda, I.; Foolad, M.R.; Giovannoni, J.J.; Sacks, G.L. HS-SPME-GC-MS analyses of volatiles in plant populations—Quantitating compound × individual matrix effects. Molecules 2018, 23, 2436. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; de Villiers, A.; McNeish, C.; Seethapathy, S.; Mostafa, A.; Górecki, T.; Crouch, A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC× GC–TOFMS). Food Chem. 2011, 129, 188–199. [Google Scholar] [CrossRef]
- Guilhaus, M. Mass Spectrometry|Time-of-Flight. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 412–423. [Google Scholar]
- Haglund, P.S.; Löfstrand, K.; Siek, K.; Asplund, L. Powerful GC-TOF-MS Techniques for Screening, Identification and Quantification of Halogenated Natural Products. Mass Spectrom. 2013, 2, S0018. [Google Scholar] [CrossRef]
- Chen, Y.; Shukurova, M.K.; Asikin, Y.; Kusano, M.; Watanabe, K.N. Characterization of volatile organic compounds in mango ginger (Curcuma amada Roxb.) from Myanmar. Metabolites 2020, 11, 21. [Google Scholar] [CrossRef]
- Shukurova, M.K.; Asikin, Y.; Chen, Y.; Kusano, M.; Watanabe, K.N. Profiling of volatile organic compounds in wild indigenous medicinal ginger (Zingiber barbatum wall.) from Myanmar. Metabolites 2020, 10, 248. [Google Scholar] [CrossRef]
- Joothamongkhon, J.; Susantikarn, P.; Kongkachana, W.; Ketngamkum, Y.; Batthong, S.; Jomchai, N.; Pongpamorn, P. Quantitative analysis of methoxyflavones discriminates between the two types of Kaempferia parviflora. Phytochem. Anal. 2022, 33, 670–677. [Google Scholar] [CrossRef]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils; Routledge Handbooks Online; Hüsnü Can Başer, K., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 43–86. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- ChemSpider Search and Share Chemistry. Available online: https://www.chemspider.com/ (accessed on 20 December 2019).
- Kusano, M.; Kobayashi, M.; Iizuka, Y.; Fukushima, A.; Saito, K. Unbiased profiling of volatile organic compounds in the headspace of Allium plants using an in-tube extraction device. BMC Res. Notes 2016, 9, 133. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Viant, M.R. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Jonsson, P.; Johansson, E.S.; Wuolikainen, A.; Lindberg, J.; Schuppe-Koistinen, I.; Kusano, M.; Antti, H. Predictive metabolite profiling applying hierarchical multivariate curve resolution to GC− MS data a potential tool for multi-parametric diagnosis. J. Proteome Res. 2006, 5, 1407–1414. [Google Scholar] [CrossRef]
- KÖnig, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids Library—MassFinder. Available online: https://massfinder.com/wiki/Terpenoids_Library (accessed on 20 March 2020).
- Stein, S.E.; Ausloos, P.; Clifton, C.L.; Klassen, J.K.; Lias, S.G.; Mikaya, A.I.; Sparkman, O.D.; Tchekhovskoi, D.V.; Zaikin, V.; Zhu, D. Evaluation of the NIST/EPA/NIH Mass Spectral Library. Abstr. Pap. Am. Chem. S. 1999, 218, 368. [Google Scholar]
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured: Carol Stream, IL, USA, 2012. [Google Scholar]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Panyakaew, J.; Sookkhee, S.; Rotarayanont, S.; Kittiwachana, S.; Wangkarn, S.; Mungkornasawakul, P. Chemical Variation and Potential of Kaempferia Oils as Larvicide Against Aedes aegypti. J. Essent. Oil-Bear. Plants 2017, 20, 1044–1056. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives-Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry, and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, Ü. Environmental Impacts on Plant Volatile Emission. In Deciphering Chemical Language of Plant Communication; Signaling and Communication in Plants; Blande, J., Glinwood, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 35–59. [Google Scholar]
- Rinnan, R.; Iversen, L.L.; Tang, J.; Vedel-Petersen, I.; Schollert, M.; Schurgers, G. Separating direct and indirect effects of rising temperatures on biogenic volatile emissions in the Arctic. Proc. Natl. Acad. Sci. USA 2020, 117, 32476–32483. [Google Scholar] [CrossRef]
- Michael, R.; Ranjan, A.; Kumar, R.S.; Pathak, P.K.; Trivedi, P.K. Light-regulated expression of terpene synthase gene, AtTPS03, is controlled by the bZIP transcription factor, HY5, in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 529, 437–443. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thawtar, M.S.; Kusano, M.; Yingtao, L.; Wunna; Thein, M.S.; Tanaka, K.; Rivera, M.; Shi, M.; Watanabe, K.N. Exploring Volatile Organic Compounds in Rhizomes and Leaves of Kaempferia parviflora Wall. Ex Baker Using HS-SPME and GC–TOF/MS Combined with Multivariate Analysis. Metabolites 2023, 13, 651. https://doi.org/10.3390/metabo13050651
Thawtar MS, Kusano M, Yingtao L, Wunna, Thein MS, Tanaka K, Rivera M, Shi M, Watanabe KN. Exploring Volatile Organic Compounds in Rhizomes and Leaves of Kaempferia parviflora Wall. Ex Baker Using HS-SPME and GC–TOF/MS Combined with Multivariate Analysis. Metabolites. 2023; 13(5):651. https://doi.org/10.3390/metabo13050651
Chicago/Turabian StyleThawtar, May San, Miyako Kusano, Li Yingtao, Wunna, Min San Thein, Keisuke Tanaka, Marlon Rivera, Miao Shi, and Kazuo N. Watanabe. 2023. "Exploring Volatile Organic Compounds in Rhizomes and Leaves of Kaempferia parviflora Wall. Ex Baker Using HS-SPME and GC–TOF/MS Combined with Multivariate Analysis" Metabolites 13, no. 5: 651. https://doi.org/10.3390/metabo13050651
APA StyleThawtar, M. S., Kusano, M., Yingtao, L., Wunna, Thein, M. S., Tanaka, K., Rivera, M., Shi, M., & Watanabe, K. N. (2023). Exploring Volatile Organic Compounds in Rhizomes and Leaves of Kaempferia parviflora Wall. Ex Baker Using HS-SPME and GC–TOF/MS Combined with Multivariate Analysis. Metabolites, 13(5), 651. https://doi.org/10.3390/metabo13050651