Untargeted Plant Metabolomics: Evaluation of Lyophilization as a Sample Preparation Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reference Standards and Plant Material
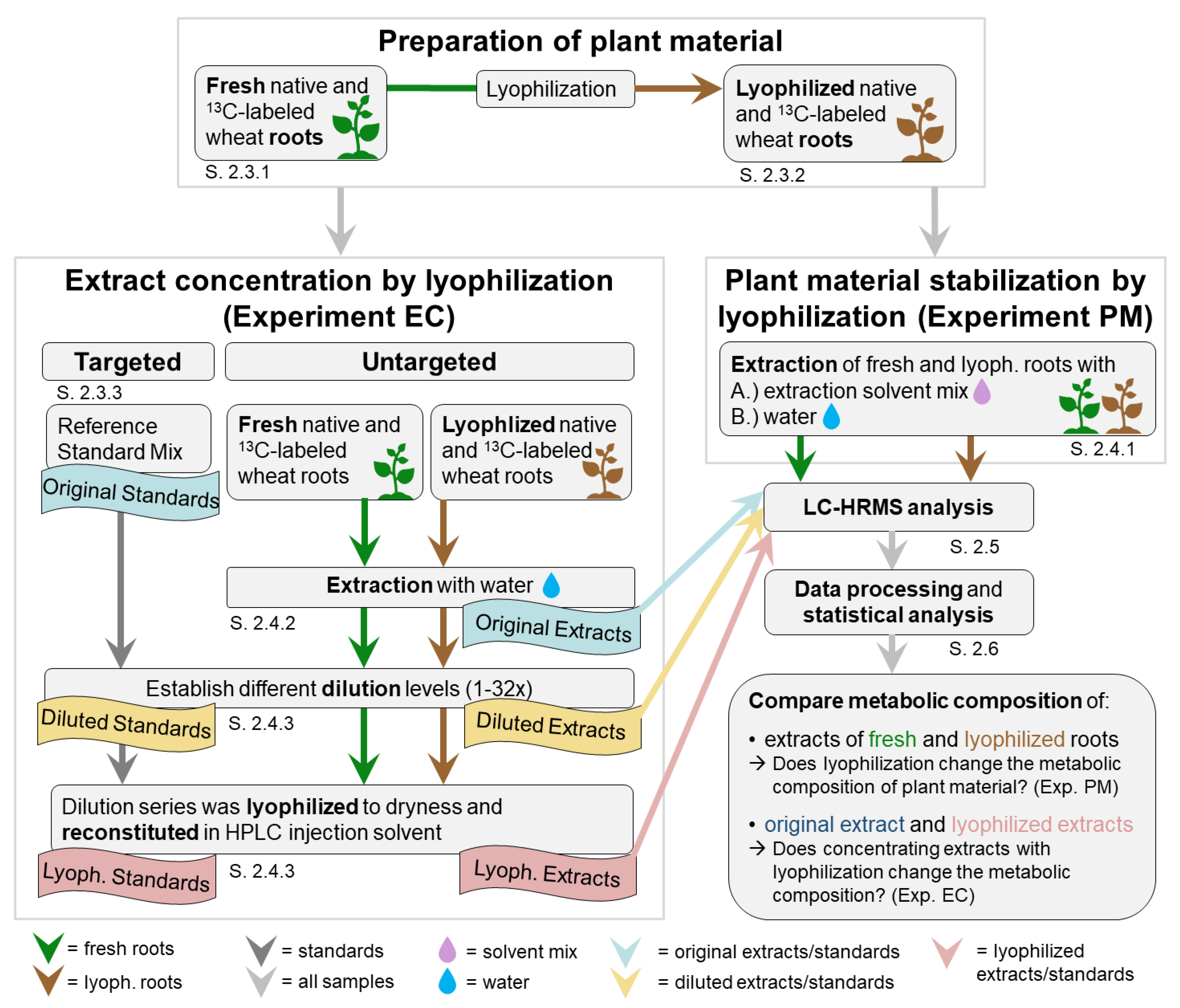
2.2. Overview Experimental Setup
2.2.1. Experimental Setup
2.2.2. Stable Isotopically Labelled Plant Material for Global Internal Standardization
2.3. Sample Preparation
2.3.1. Fresh Root Samples
2.3.2. Lyophilization of Root Samples
2.3.3. Reference Standard Mix
2.4. Extraction and Lyophilization
2.4.1. Extraction for Testing the Effect of Lyophilization on the Metabolome of Plant Material (Experiment PM)
2.4.2. Extraction for Testing the Effect of Lyophilization When Used for Extract Concentration (Experiment EC)
2.4.3. Lyophilization of Extracts (Experiment EC)
2.5. LC-HRMS Analysis
2.6. Data Processing, Statistical Analysis, and Metabolite Annotation
3. Results
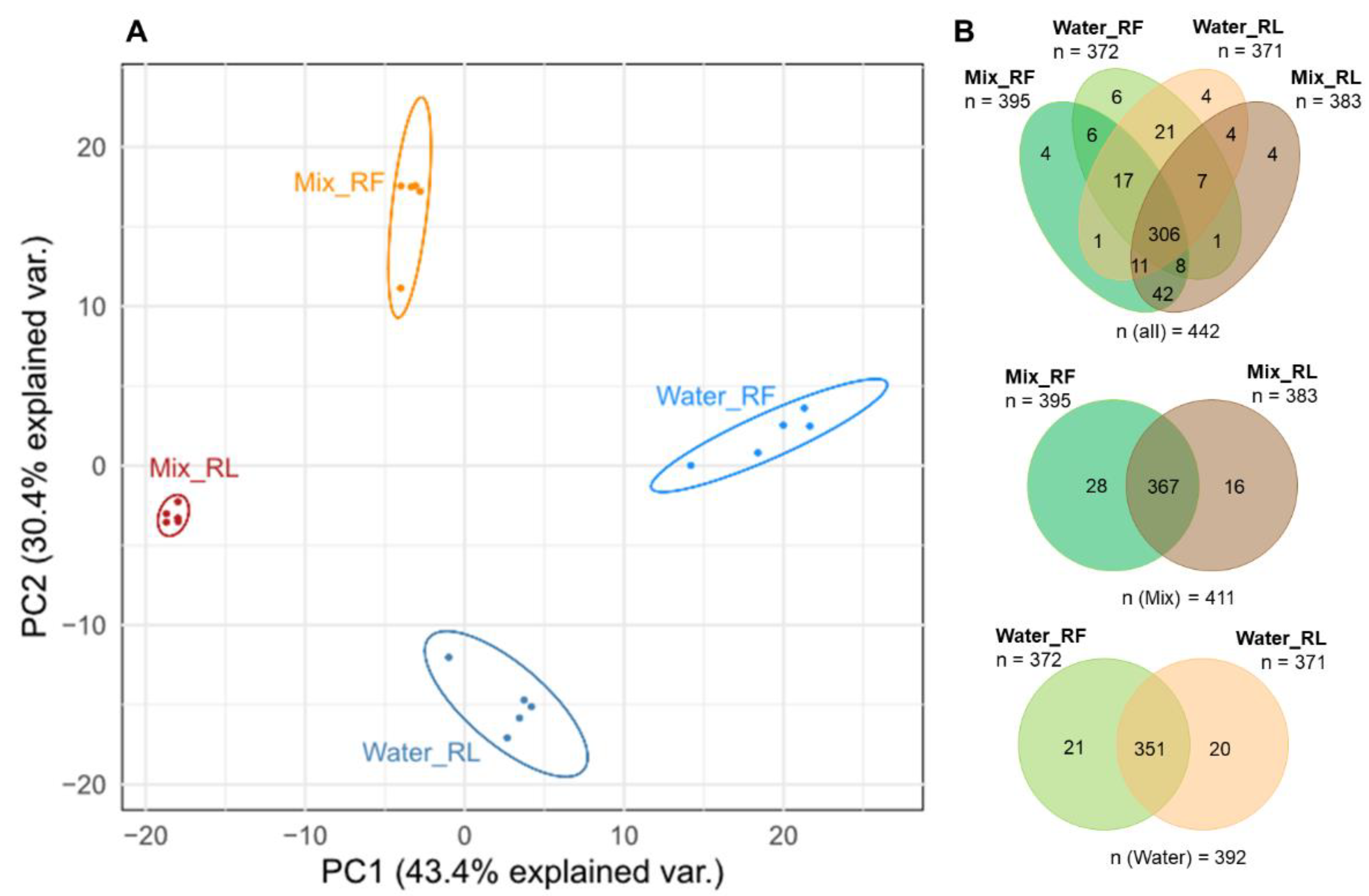
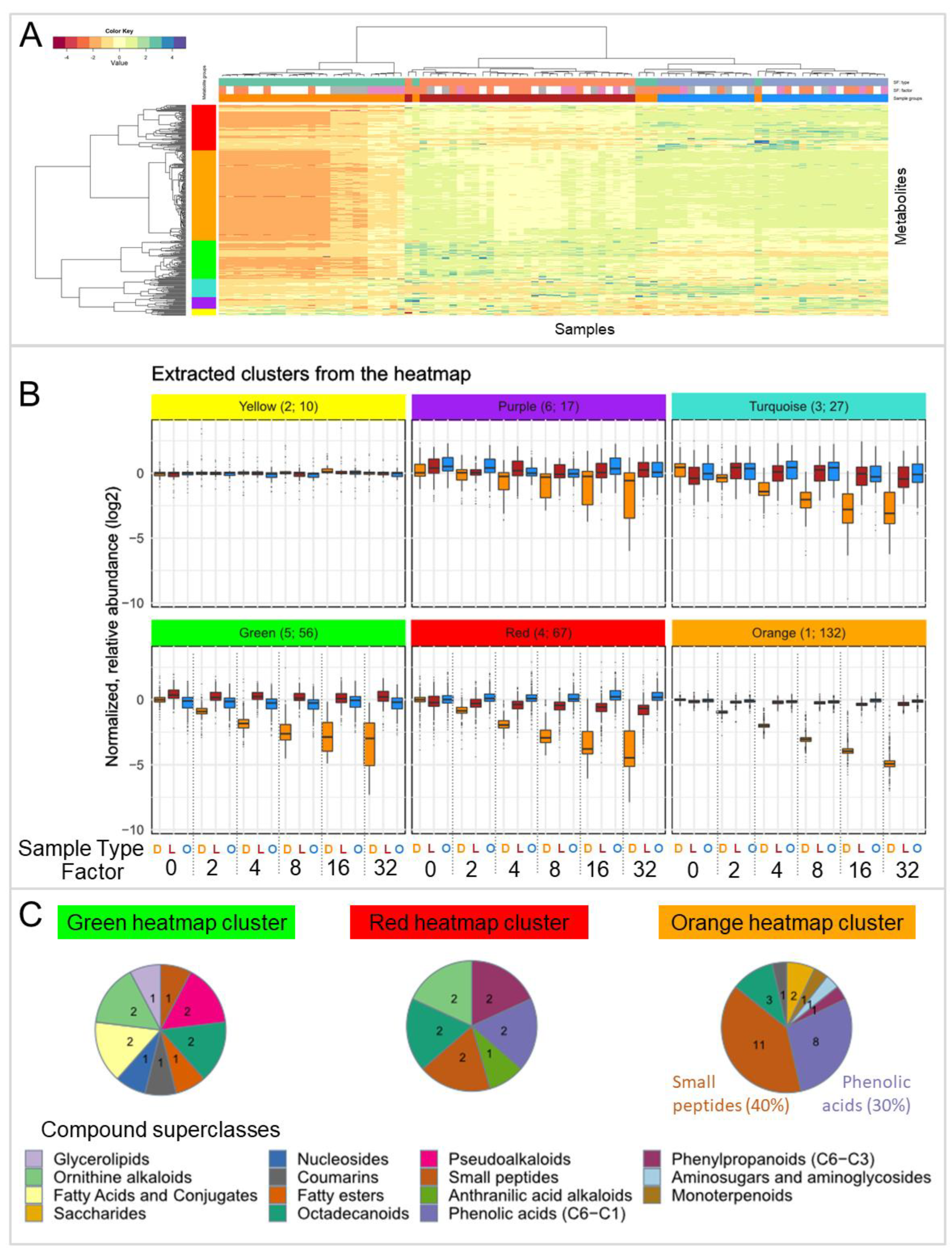
3.1. Experiment for Testing the Effect of Lyophilization on the Metabolic Composition of Plant Material (Experiment PM)
3.1.1. Comparison of Extraction Solvents of Fresh and Dried (Lyophilized) Roots
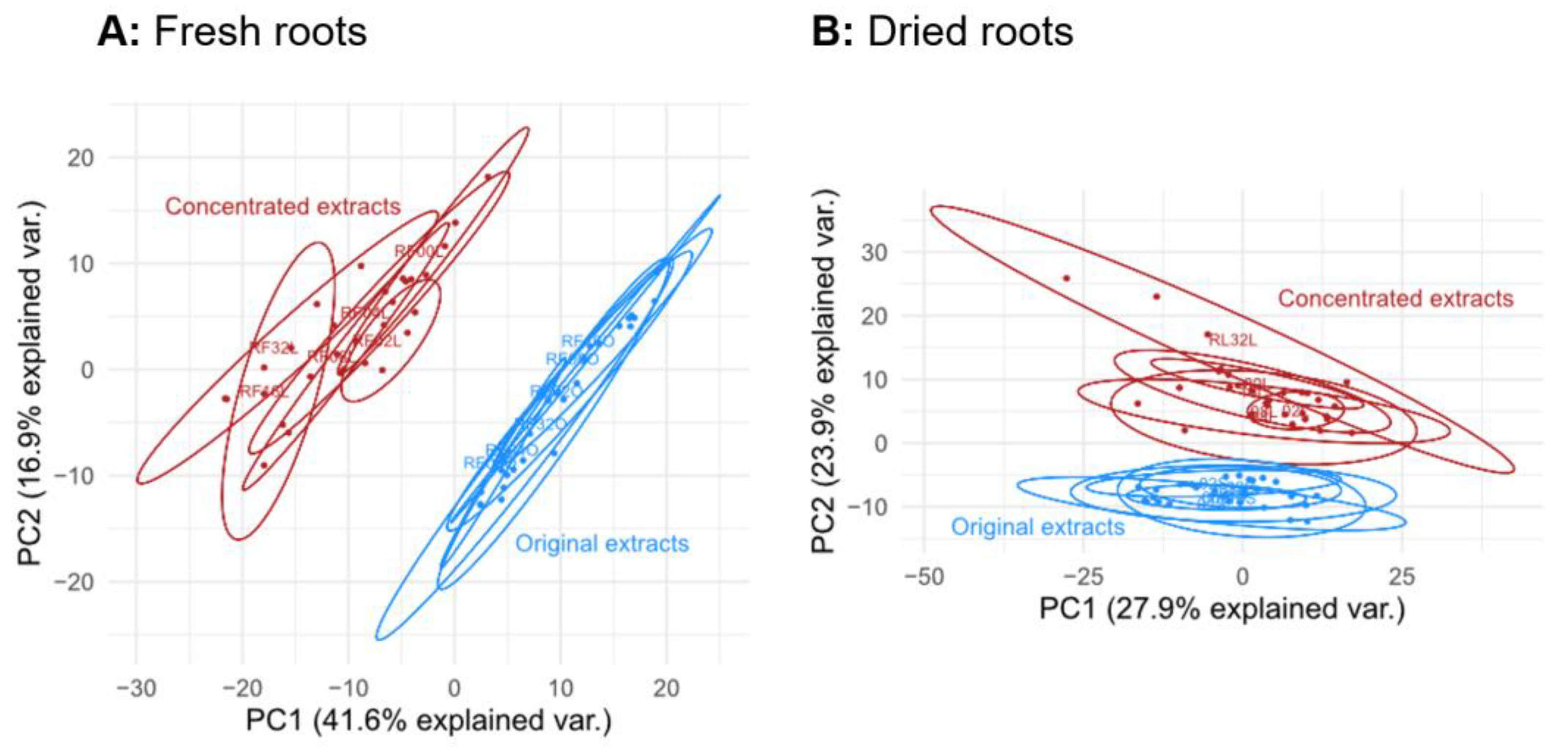
3.1.2. Comparison of Extracts of Fresh and Dried Roots
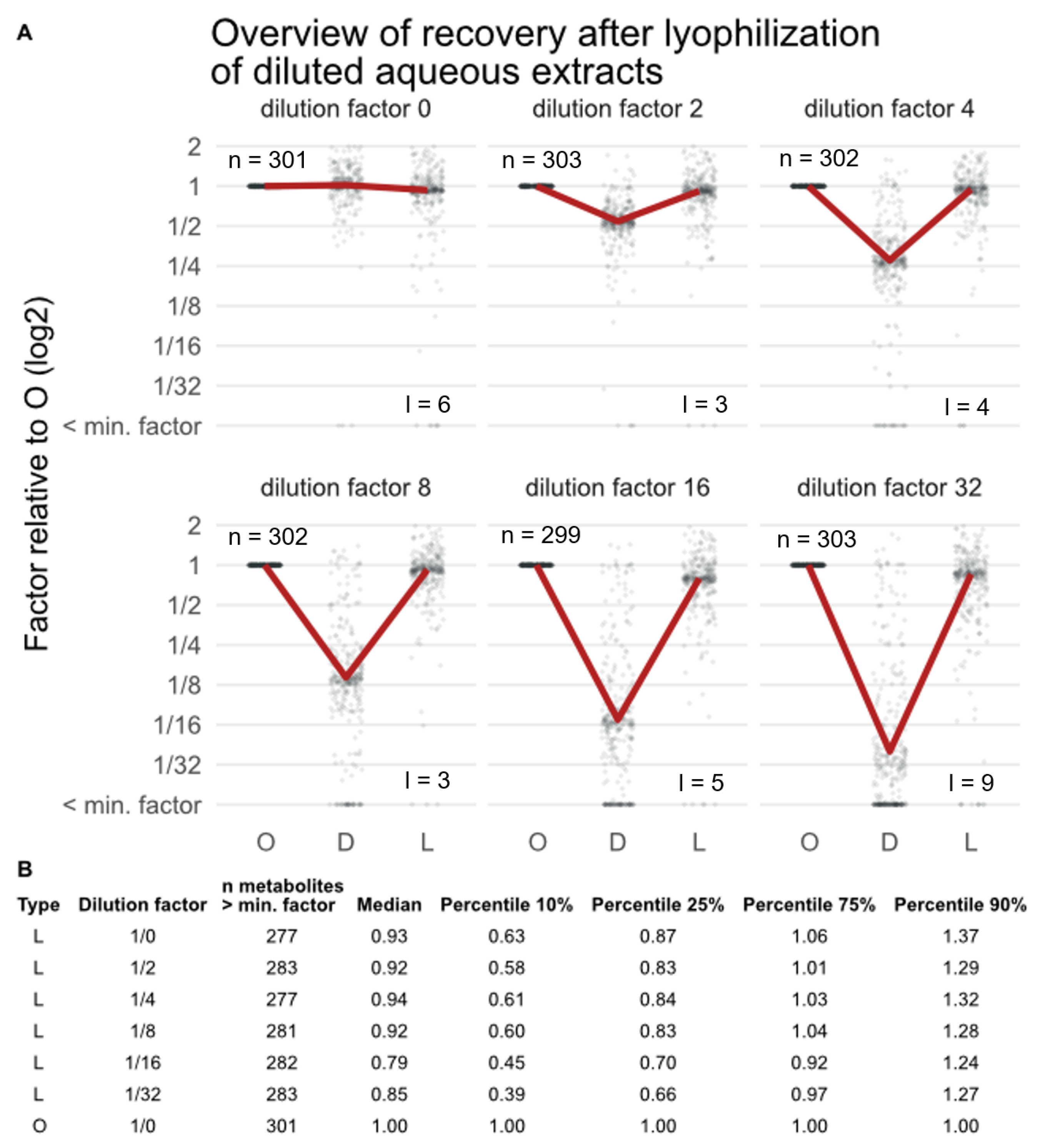
3.2. Effect of Lyophilization on the Metabolic Composition of Extracts after Concentration (Experiment EC)
4. Discussion
4.1. Discussion of General Results
4.2. Experiment for Testing the Effect of Lyophilization on the Metabolic Composition of Plant Material (Experiment PM)
4.3. Effect of Lyophilization on the Metabolic Composition of Extracts after Concentration (Experiment EC)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ElNaker, N.A.; Daou, M.; Ochsenkühn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. A metabolomics approach to evaluate the effect of lyophilization versus oven drying on the chemical composition of plant extracts. Sci. Rep. 2021, 11, 22679. [Google Scholar] [CrossRef] [PubMed]
- Tavaria, F.K.; Sousa, M.J.; Malcata, F.X. Storage and lyophilization effects of extracts of Cynara cardunculus on the degradation of ovine and caprine caseins. Food Chem. 2001, 72, 79–88. [Google Scholar] [CrossRef]
- Abla, K.K.; Mehanna, M.M. Freeze-drying: A flourishing strategy to fabricate stable pharmaceutical and biological products. Int. J. Pharm. 2022, 628, 122233. [Google Scholar] [CrossRef]
- Nitika, N.; Chhabra, H.; Rathore, A.S. Raman spectroscopy for in situ, real time monitoring of protein aggregation in lyophilized biotherapeutic products. Int. J. Biol. Macromol. 2021, 179, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Preston, K.B.; Randolph, T.W. Stability of lyophilized and spray dried vaccine formulations. Adv. Drug. Deliv. Rev. 2021, 171, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jalowiecki, L.; Krzyminska, I.; Gorska, M.; Plaza, G.; Ratman-Klosinska, I. Effect of the freeze-drying process on the phenotypic diversity of Pseudomonas putida strains isolated from the interior of healthy roots of Sida hermaphrodita: Phenotype microarrays (PMs). Cryobiology 2020, 96, 145–151. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.S.; Calderon-Santiago, M.; Priego-Capote, F. Lyophilization as pre-processing for sample storage in the determination of vitamin D(3) and metabolites in serum and plasma. Talanta 2021, 222, 121692. [Google Scholar] [CrossRef]
- Maraldi, M.; Ferrari, R.; Auriemma, R.; Sponchioni, M.; Moscatelli, D. Concentration of Polymer Nanoparticles Through Dialysis: Efficacy and Comparison With Lyophilization for PEGylated and Zwitterionic Systems. J. Pharm. Sci. 2020, 109, 2607–2614. [Google Scholar] [CrossRef]
- Doppler, M.; Kluger, B.; Bueschl, C.; Schneider, C.; Krska, R.; Delcambre, S.; Hiller, K.; Lemmens, M.; Schuhmacher, R. Stable Isotope-Assisted Evaluation of Different Extraction Solvents for Untargeted Metabolomics of Plants. Int. J. Mol. Sci. 2016, 17, 1017. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Ruttanakorn, M.; Sukadeetad, K.; Sakkayawong, N.; Damrianant, S. Effects of drying and extraction methods on phenolic compounds and in vitro assays of Eclipta prostrata Linn leaf extracts. ScienceAsia 2019, 45, 127–137. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.Y. Physicochemical property analysis of lyophilized fresh, wet-, and dry-aged beef powders: Application of dry-aged beef crust as a food additive. Meat Sci. 2023, 195, 109014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kebede, B.; Chen, G.; McComb, K.; Frew, R. Impact of freeze-drying and subsequent storage on milk metabolites based on 1H NMR and UHPLC-QToF/MS. Food Control 2020, 116, 107017. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Villate, A.; San Nicolas, M.; Gallastegi, M.; Aulas, P.A.; Olivares, M.; Usobiaga, A.; Etxebarria, N.; Aizpurua-Olaizola, O. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2021, 303, 110789. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Verpoorte, R. Sample preparation for plant metabolomics. Phytochem. Anal. 2010, 21, 4–13. [Google Scholar] [CrossRef]
- Shen, S.; Zhan, C.; Yang, C.; Fernie, A.R.; Luo, J. Metabolomics-centered mining of plant metabolic diversity and function: Past decade and future perspectives. Mol. Plant 2023, 16, 43–63. [Google Scholar] [CrossRef]
- Da Silva Oliveira, J.P.; de Oliveira, R.T.; Guedes, A.L.; da Costa Oliveira, M.; Macedo, A.F. Metabolomic studies of anthocyanins in fruits by means of a liquid chromatography coupled to mass spectrometry workflow. Curr. Plant Biol. 2022, 32, 100260. [Google Scholar] [CrossRef]
- Aggio, R.B.; Mayor, A.; Coyle, S.; Reade, S.; Khalid, T.; Ratcliffe, N.M.; Probert, C.S. Freeze-drying: An alternative method for the analysis of volatile organic compounds in the headspace of urine samples using solid phase micro-extraction coupled to gas chromatography - mass spectrometry. Chem. Cent. J. 2016, 10, 9. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barber, D.; Barbas, C.; Perez-Gordo, M.; Rojo, D. Sample pre-treatment procedures for the omics analysis of human gut microbiota: Turning points, tips and tricks for gene sequencing and metabolomics. J. Pharm. Biomed. Anal. 2020, 191, 113592. [Google Scholar] [CrossRef]
- Rakusanova, S.; Fiehn, O.; Cajka, T. Toward building mass spectrometry-based metabolomics and lipidomics atlases for biological and clinical research. TrAC Trends Anal. Chem. 2023, 158, 116825. [Google Scholar] [CrossRef]
- Luque de Castro, M.D. Lyophilization a useful approach to the automation of analytical processes. J. Autom. Chem. 1990, 12, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Escolà Casas, M.; Matamoros, V. Analytical challenges and solutions for performing metabolomic analysis of root exudates. Trends Environ. Anal. Chem. 2021, 31, e00130. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Paterna, A.; Biondi, D.M.; Patti, A. Lyophilized extracts from vegetable flours as valuable alternatives to purified oxygenases for the synthesis of oxylipins. Bioorg. Chem. 2019, 93, 103325. [Google Scholar] [CrossRef] [PubMed]
- Sauerschnig, C.; Doppler, M.; Bueschl, C.; Schuhmacher, R. Methanol Generates Numerous Artifacts during Sample Extraction and Storage of Extracts in Metabolomics Research. Metabolites 2017, 8, 1. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Patel, S.M.; Suryanarayanan, R.; Rinella, J.V., Jr.; Bogner, R.H. Key factors governing the reconstitution time of high concentration lyophilized protein formulations. Eur. J. Pharm. Biopharm. 2021, 165, 361–373. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; Segura-Carretero, A.; Guerra-Hernandez, E.; Garcia-Villanova, B.; Fernandez-Gutierrez, A. Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 2013, 141, 869–878. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Effects of different drying methods on the bioactive compounds and antioxidant properties of edible Centaurea (Centaurea cyanus) petals. Braz. J. Food Technol. 2018, 21, e2017211. [Google Scholar] [CrossRef]
- Pham, H.; Nguyen, V.; Vuong, Q.; Bowyer, M.; Scarlett, C. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta Lour. Leaves. Technologies 2015, 3, 285–301. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef]
- Stévant, P.; Indergård, E.; Ólafsdóttir, A.; Marfaing, H.; Larssen, W.E.; Fleurence, J.; Roleda, M.Y.; Rustad, T.; Slizyte, R.; Nordtvedt, T.S. Effects of drying on the nutrient content and physico-chemical and sensory characteristics of the edible kelp Saccharina latissima. J. Appl. Phycol. 2018, 30, 2587–2599. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Dai, Y.; Cheng, H.Y.; Lam, W.; Cheng, Y.C.; Li, K.; Peng, W.H.; Pao, L.H.; Hsieh, M.T.; Qin, X.M.; et al. Improving the Concentrations of the Active Components in the Herbal Tea Ingredient, Uraria crinita: The Effect of Post-harvest Oven-drying Processing. Sci. Rep. 2017, 7, 38763. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Otsuka, T.; Jikumaru, Y.; Yamaguchi, S.; Matsuda, F.; Nakabayashi, R.; Takashina, T.; Isuzugawa, K.; Saito, K.; Shiratake, K. Effects of freeze-drying of samples on metabolite levels in metabolome analyses. J. Sep. Sci. 2011, 34, 3561–3567. [Google Scholar] [CrossRef] [PubMed]
- Ceranic, A.; Doppler, M.; Buschl, C.; Parich, A.; Xu, K.; Koutnik, A.; Burstmayr, H.; Lemmens, M.; Schuhmacher, R. Preparation of uniformly labelled (13)C- and (15)N-plants using customised growth chambers. Plant Methods 2020, 16, 46. [Google Scholar] [CrossRef]
- Doppler, M.; Bueschl, C.; Kluger, B.; Koutnik, A.; Lemmens, M.; Buerstmayr, H.; Rechthaler, J.; Krska, R.; Adam, G.; Schuhmacher, R. Stable Isotope-Assisted Plant Metabolomics: Combination of Global and Tracer-Based Labeling for Enhanced Untargeted Profiling and Compound Annotation. Front. Plant Sci. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Bueschl, C.; Kluger, B.; Neumann, N.K.N.; Doppler, M.; Maschietto, V.; Thallinger, G.G.; Meng-Reiterer, J.; Krska, R.; Schuhmacher, R. MetExtract II: A Software Suite for Stable Isotope-Assisted Untargeted Metabolomics. Anal. Chem. 2017, 89, 9518–9526. [Google Scholar] [CrossRef]
- Bueschl, C.; Kluger, B.; Lemmens, M.; Adam, G.; Wiesenberger, G.; Maschietto, V.; Marocco, A.; Strauss, J.; Bödi, S.; Thallinger, G.G.; et al. A novel stable isotope labelling assisted workflow for improved untargeted LC–HRMS based metabolomics research. Metabolomics 2013, 10, 754–769. [Google Scholar] [CrossRef]
- Ceranic, A.; Bueschl, C.; Doppler, M.; Parich, A.; Xu, K.; Lemmens, M.; Buerstmayr, H.; Schuhmacher, R. Enhanced Metabolome Coverage and Evaluation of Matrix Effects by the Use of Experimental-Condition-Matched (13)C-Labeled Biological Samples in Isotope-Assisted LC-HRMS Metabolomics. Metabolites 2020, 10, 434. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
- Doppler, M.; Kluger, B.; Bueschl, C.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Krska, R.; Adam, G.; Schuhmacher, R. Stable Isotope-Assisted Plant Metabolomics: Investigation of Phenylalanine-Related Metabolic Response in Wheat Upon Treatment With the Fusarium Virulence Factor Deoxynivalenol. Front. Plant Sci. 2019, 10, 1137. [Google Scholar] [CrossRef]
- Blazenovic, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Asami, D.K.; Hong, Y.; Barrett, D.M.; Mitchell, A.E. Comparison of the Total Phenolic and Ascorbic Acid Content of Freeze-Dried and Air-Dried Marionberry, Strawberry, and Corn Grown Using Conventional, Organic, and Sustainable Agricultural Practices. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Leroi, J.-M.; Jeffrey, S.W. Essential polyunsaturated fatty acids from 14 species of diatom (bacillariophyceae). Phytochemistry 1993, 35, 155–161. [Google Scholar] [CrossRef]
- Dunstan, G.A. The Effect of Lyophilization on the Solvent Extraction of Lipid Classes Fatty Acids and Sterols from Pyster Crassostrea gigas. LIPIDS 1993, 28, 937–944. [Google Scholar] [CrossRef]
- Çoklar, H.; Akbulut, M. Effect of Sun, Oven and Freeze-Drying on Anthocyanins, Phenolic Compounds and Antioxidant Activity of Black Grape (Ekşikara) (Vitis vinifera L.). S. Afr. J. Enol. Vitic. 2017, 38, 264–272. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M.; Kilinc, S.; Yildirim, A.; Alhassan, I. Effect of Freeze, Oven and Microwave Pretreated Oven Drying on Color, Browning Index, Phenolic Compounds and Antioxidant Activity of Hawthorn (Crataegus orientalis) Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 449–456. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisl, C.; Doppler, M.; Seidl, B.; Bueschl, C.; Schuhmacher, R. Untargeted Plant Metabolomics: Evaluation of Lyophilization as a Sample Preparation Technique. Metabolites 2023, 13, 686. https://doi.org/10.3390/metabo13060686
Maisl C, Doppler M, Seidl B, Bueschl C, Schuhmacher R. Untargeted Plant Metabolomics: Evaluation of Lyophilization as a Sample Preparation Technique. Metabolites. 2023; 13(6):686. https://doi.org/10.3390/metabo13060686
Chicago/Turabian StyleMaisl, Christina, Maria Doppler, Bernhard Seidl, Christoph Bueschl, and Rainer Schuhmacher. 2023. "Untargeted Plant Metabolomics: Evaluation of Lyophilization as a Sample Preparation Technique" Metabolites 13, no. 6: 686. https://doi.org/10.3390/metabo13060686




