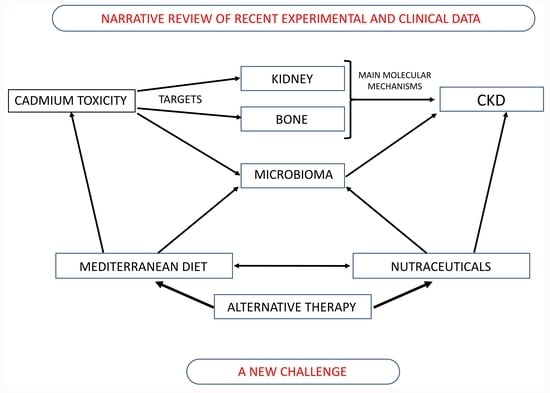
Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review
Abstract
1. Cadmium: Who, Where and How
2. Nutraceuticals: Generalities
3. Cadmium-Induced Pathophysiological Mechanisms and Kidney Dysfunctionality
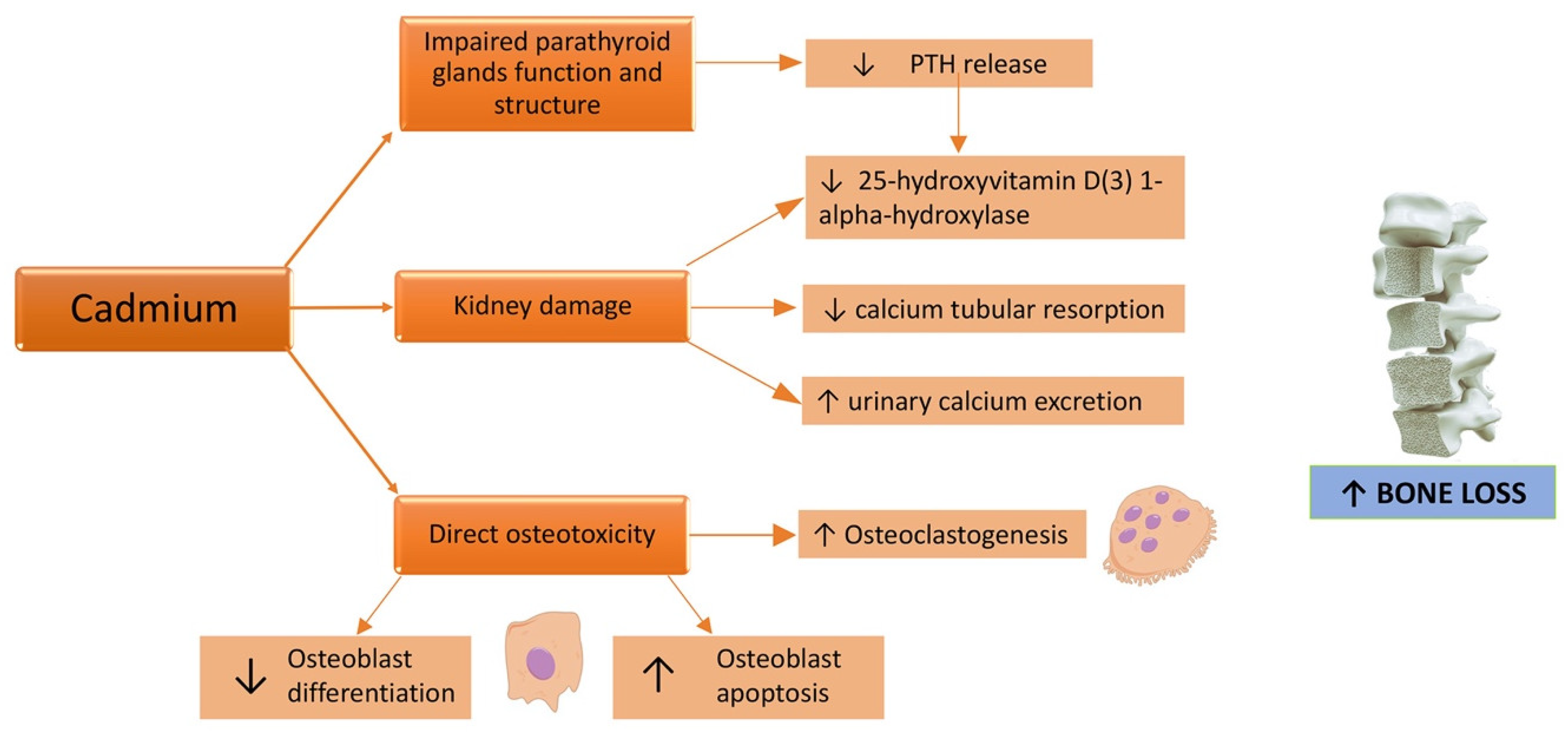
4. Cadmium and Bone Damage in CKD
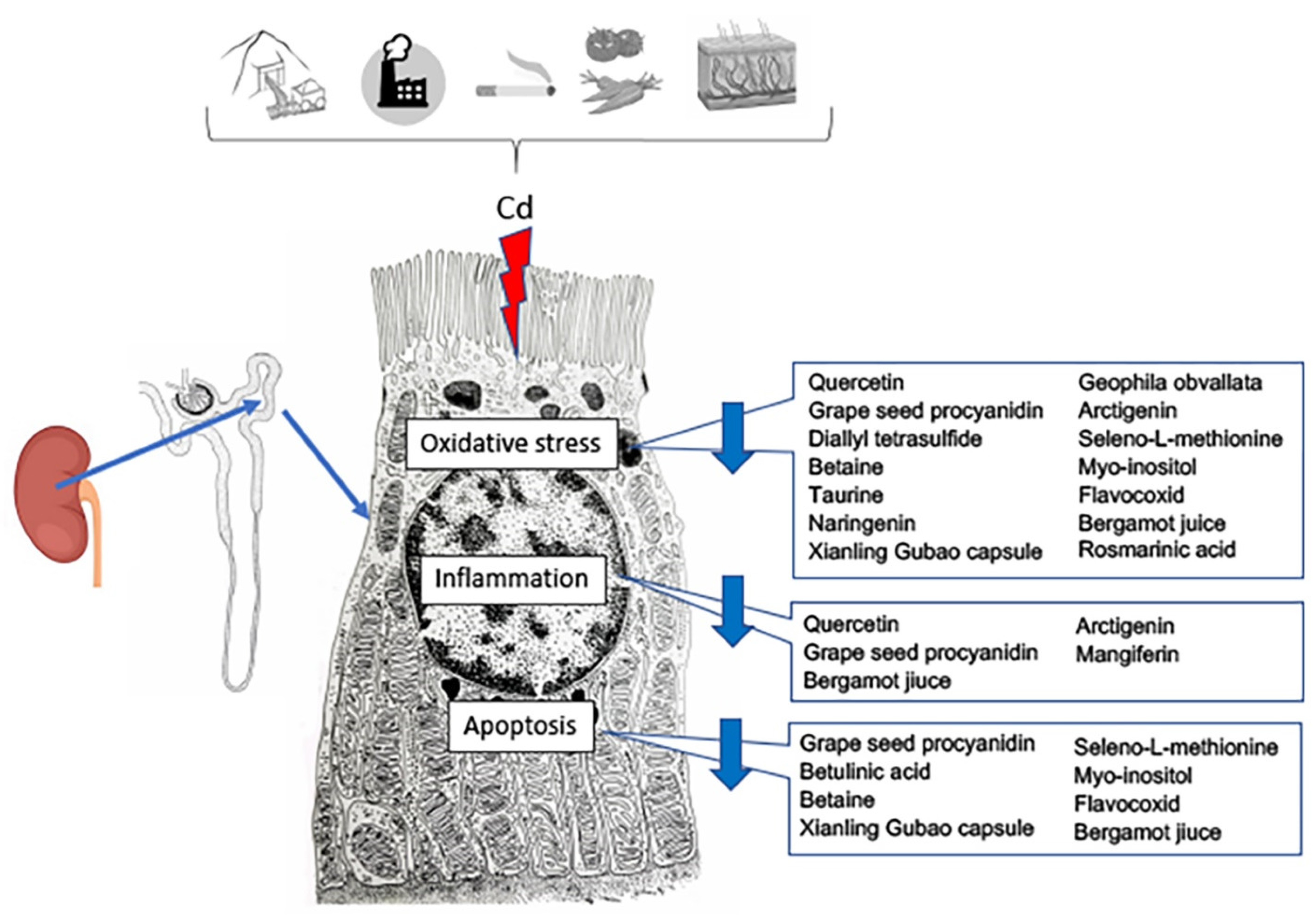
5. Therapeutic Effects of Functional Foods and Nutraceuticals in Cadmium-Induced Kidney Dysfunctionality: The Latest Preclinical Updates
6. Nutraceuticals and Microbioma: Putative Role in Cadmium-Induced Kidney Damage
7. Nutraceuticals and CKD: Chances and Limits in Routine Clinical Setting
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duffus, J.H. Heavy Metal—A Meaningless Term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals" with "Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Liu, X.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P. The global research trend on cadmium in freshwater: A bibliometric review. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.B.; Yanar, A.; Alkan, E.N. Review of heavy metal accumulation on aquatic environment in Northern East Mediterrenean Sea part I: Some essential metals. Rev. Environ. Health 2017, 32, 119–163. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Pelucelli, A.; Medici, S.; Cappai, R.; Nurchi, V.M.; Zoroddu, M.A. Metal Toxicity and Speciation: A Review. Curr. Med. Chem. 2021, 28, 7190–7208. [Google Scholar] [CrossRef]
- Borsari, M. Encyclopedia of Inorganic and Bioinorganic Chemistry; Online © 2011–2014; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Heinrichs, H.; Schulz-Dobrick, B.; Wedepohl, K.H. Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim. Cosmochim. Acta 1980, 44, 1519–1533. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, F.; Xue, S.; Ma, T.; Yu, L. Heavy Metal Pollution and Source Contributions in Agricultural Soils Developed from Karst Landform in the Southwestern Region of China. Toxics 2022, 10, 568. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Rosén, K.; Eriksson, J.; Vinichuk, M. Uptake and translocation of 109Cd and stable Cd within tobacco plants (Nicotiana sylvestris). J. Environ. Radioact. 2012, 113, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, Q.; Lin, Q.; Zeng, C.; Zhong, C. Cadmium source identification in soils and high-risk regions predicted by geographical detector method. Environ. Pollut. 2020, 263, 114338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Piadé, J.J.; Jaccard, G.; Dolka, C.; Belushkin, M.; Wajrock, S. Differences in cadmium transfer from tobacco to cigarette smoke, compared to arsenic or lead. Toxicol. Rep. 2014, 2, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Jumarie, C. Cadmium transport through type II alveolar cell monolayers: Contribution of transcellular and paracellular pathways in the rat ATII and the human A549 cells. Biochim. Biophys. Acta 2002, 1564, 487–499. [Google Scholar] [CrossRef]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public Health 2017, 14, 1154. [Google Scholar] [CrossRef]
- Koopsamy Naidoo, S.V.; Bester, M.J.; Arbi, S.; Venter, C.; Dhanraj, P.; Oberholzer, H.M. Oral exposure to cadmium and mercury alone and in combination causes damage to the lung tissue of Sprague-Dawley rats. Environ. Toxicol. Pharmacol. 2019, 69, 86–94. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef]
- Bolan, S.; Seshadri, B.; Keely, S.; Kunhikrishnan, A.; Bruce, J.; Grainge, I.; Talley, N.J.; Naidu, R. Bioavailability of arsenic, cadmium, lead and mercury as measured by intestinal permeability. Sci. Rep. 2021, 11, 14675. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Sampson, B. Dermal toxicity and percutaneous absorption of cadmium in rats and mice. Lab. Anim. Sci. 1996, 46, 549–554. [Google Scholar]
- Liaw, F.Y.; Chen, W.L.; Kao, T.W.; Chang, Y.W.; Huang, C.F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci. Rep. 2017, 7, 1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant-cadmium (II) with human serum albumin. Environ. Sci. Pollut. Res. Int. 2014, 21, 6994–7005. [Google Scholar] [CrossRef]
- Sabolić, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Fan, R.F.; Tang, K.K.; Wang, Z.Y.; Wang, L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology 2021, 464, 152999. [Google Scholar] [CrossRef]
- Micali, A.; Pallio, G.; Irrera, N.; Marini, H.; Trichilo, V.; Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Laurà, R.; et al. Flavocoxid, a Natural Antioxidant, Protects Mouse Kidney from Cadmium-Induced Toxicity. Oxid. Med. Cell Longev. 2018, 2018, 9162946. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Arab, H.H.; Ashour, A.M.; Eid, A.H.; Arafa, E.A.; Al Khabbaz, H.J.; Abd El-Aal, S.A. Targeting oxidative stress, apoptosis, and autophagy by galangin mitigates cadmium-induced renal damage: Role of SIRT1/Nrf2 and AMPK/mTOR pathways. Life Sci. 2022, 291, 120300. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Weaver, V.; Tellez-Plaza, M.; Guallar, E.; Lee, B.; Navas-Acien, A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ. Health Perspect. 2011, 119, 1800–1805. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012 Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef]
- Gibb, H.J.; Barchowsky, A.; Bellinger, D.; Bolger, P.M.; Carrington, C.; Havelaar, A.H.; Oberoi, S.; Zang, Y.; O’Leary, K.; Devleesschauwer, B. Estimates of the 2015 global and regional disease burden from four foodborn-arsenic, cadmium, lead and methylmercury. Environ. Res. 2019, 174, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Adams, S.V.; Newcomb, P.A. Cadmium blood and urine concentrations as measures of exposure, NHANES 1999–2010. J. Exp. Sci. Environ. Epidemiol. 2014, 24, 163–170. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Vacchi-Suzzi, C.; Eriksen, K.T.; Levine, K.; McElroy, J.; Tjønneland, A.; Raaschou-Nielsen, O.; Harrington, J.M.; Meliker, J.R. Dietary intake estimates and urinary cadmium levels in danish postmenopausal women. PLoS ONE 2015, 10, e0138784. [Google Scholar] [CrossRef]
- Kawata, T. Cadmium intake and chronic kidney disease. Clin. Nutr. 2018, 37, 1779. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Singh, J.; Sinha, S. Classification, Regulatory Acts and Applications of Nutraceuticals for Health. Int. J. Pharma. Biosci. 2012, 2, 177–187. [Google Scholar]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Rysz, J.; Franczyk, B.; Kujawski, K.; Sacewicz-Hofman, I.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Are Nutraceuticals Beneficial in Chronic Kidney Disease? Pharmaceutics 2021, 13, 231. [Google Scholar] [CrossRef]
- Salis, S. Role of nutraceuticals and probiotics in chronic kidney disease. J. Renal Nutr. Metab. 2018, 4, 47. [Google Scholar] [CrossRef]
- Pari, L.; Murugavel, P.; Sitasawad, S.L.; Kumar, K.S. Cytoprotective and antioxidant role of diallyl tetrasulfide on cadmium induced renal injury: An in vivo and in vitro study. Life Sci. 2007, 80, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Lee, J.Y.; Tokumoto, M.; Satoh, M. Cadmium renal toxicity via apoptotic pathways. Biol. Pharm. Bull. 2012, 35, 1892–1897. [Google Scholar] [CrossRef]
- Lauwers, R.; De Wals, P. Environmental pollution by cadmium and mortality from renal diseases. Lancet 1981, 317, 383. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nishijo, M.; Morikawa, Y.; Tabata, M.; Miura, K.; Takahara, H.; Okumura, Y.; Yoshita, K.; Kawano, S.; Nishi, M.; et al. Increased urinary β2-microglobulin and mortality rate by cause of death in a Cadmium-polluted area. Environ. Health Prev. Med. 1996, 1, 144–148. [Google Scholar] [CrossRef]
- Nishijo, M.; Morikawa, Y.; Nakagawa, H.; Tawara, K.; Miura, K.; Kido, T.; Ikawa, A.; Kobayashi, E.; Nogawa, K. Causes of death and renal tubular dysfunction in residents exposed to cadmium in the environment. Occup. Environ. Med. 2006, 63, 545–550. [Google Scholar] [CrossRef]
- Kazantzis, G. Cadmium, osteoporosis and calcium metabolism. Biometals 2004, 17, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Khandelwal, S. Influence of cadmium on murine thymocytes: Potentiation of apoptosis and oxidative stress. Toxicol. Lett. 2006, 165, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free. Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Kayama, F.; Yoshida, T.; Elwell, M.R.; Luster, M.I. Cadmium- induced renal damage and proinflammatory cytokines: Possible role of IL-6 in tubular epithelial cell regeneration. Toxicol. Appl. Pharmacol. 1995, 134, 26–34. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Vu, T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium hepatotoxicity and nephrotoxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Thevenod, F. Nephrotoxicity and the proximal tubules: Insights from cadmium. Nephron Physiol. 2003, 93, 87–93. [Google Scholar] [CrossRef]
- Fouad, A.A.; Jresat, I. Protective effect of telmisartan against cadmium-induced nephrotoxicity in mice. Life Sci. 2011, 89, 29–35. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Mohamed, W.R.; Ahmed, O.S.; Abdel-Daim, M.M.; Sayed, A.M. The role of inflammation in cadmium nephrotoxicity: NF-κB comes into view. Life Sci. 2022, 308, 120971. [Google Scholar] [CrossRef] [PubMed]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Grape seedproanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem. Cell Biol. 2015, 93, 210–226. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Sandoval, J.M.; Egido, J.; Mayoral, P.; Arevalo, M.A.; Fernández-Tagarro, M.; Lopez-Novoa, J.M.; Pérez-Barriocanal, F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Kidney Cadmium Toxicity, Diabetes and High Blood Pressure: The Perfect Storm. Tohoku, J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef]
- Oh, S.H.; Choi, J.E.; Lim, S.C. Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 2006, 220, 1–12. [Google Scholar] [CrossRef]
- Yang, C.W.; Faulkner, G.R.; Wahba, I.M.; Christianson, T.A.; Bagby, G.C.; Jin, D.C.; Abboud, H.E.; Andoh, T.F.; Bennett, W.M. Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am. J. Transplantat. 2002, 2, 391–399. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Choi, D.E.; Jeong, J.Y.; Lim, B.J.; Lee, K.W.; Shin, Y.T.; Na, K.R. Pretreatment with darbepoetin attenuates renal injury in a rat model of cisplatin-induced nephrotoxicity. Korean J. Intern. Med. 2009, 24, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Lynch, S.M. Cadmium alters the localization of N-cadherin, E-cadherin, and b-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003, 189, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Kayama, F. Cadmium and cisplatin damage erythropoietin-producing proximal renal tubular cells. Arch. Toxicol. 2006, 80, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Kayama, F.; Oguma, E.; Willmore, W.G.; Hradecky, P.; Bunn, H.F. Cadmium and platinum suppression of erythropoietin production in cell culture: Clinical implications. Blood 2000, 96, 3743–3747. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, J.; Chen, Y.; Shi, C.; Zhang, Q.; Ning, Z.; Yu, Y.; Li, Y. Urinary cadmium in relation to bone damage: Cadmium exposure threshold dose and health-based guidance value estimation. Ecotoxicol. Environ. Saf. 2021, 226, 112824. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicol. Appl. Pharmacol. 2005, 202, 68–83. [Google Scholar] [CrossRef]
- Scimeca, M.; Feola, M.; Romano, L.; Rao, C.; Gasbarra, E.; Bonanno, E.; Brandi, M.L.; Tarantino, U. Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environ. Toxicol. 2017, 32, 1333–1342. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Jin, T.; Gu, S.; Xiao, H.; Qiu, J. Cadmium induces differentiation of RAW264.7 cells into osteoclasts in the presence of RANKL. Food Chem. Toxicol. 2011, 49, 2392–2397. [Google Scholar] [CrossRef]
- Nambunmee, K.; Honda, R.; Nishijo, M.; Swaddiwudhipong, W.; Nakagawa, H.; Ruangyuttikarn, W. Bone resorption acceleration and calcium reabsorption impairment in a Thai population with high cadmium exposure. Toxicol. Mech. Methods. 2010, 20, 7–13. [Google Scholar] [CrossRef]
- Wallin, M.; Sallsten, G.; Fabricius-Lagging, E.; Öhrn, C.; Lundh, T.; Barregard, L. Kidney cadmium levels and associations with urinary calcium and bone mineral density: A cross-sectional study in Sweden. Environ. Health. 2013, 12, 22. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Beshir, S.; Shahy, E.M.; Shaheen, W. Effect of Occupational Cadmium Exposure on Parathyroid Gland. Open Access Maced. J. Med. Sci. 2016, 4, 302–306. [Google Scholar] [CrossRef]
- Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. Int. J. Mol. Sci. 2021, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Pilat-Marcinkiewicz, B.; Brzóska, M.; Moniuszko-Jakoniuk, J. Thyroid and parathyroid function and structure in male rats chronically exposed to cadmium. Pol. J. Environ. Stud. 2008, 17, 113–120. [Google Scholar]
- Alfvén, T.; Elinder, C.G.; Carlsson, M.D.; Grubb, A.; Hellström, L.; Persson, B.; Pettersson, C.; Spång, G.; Schütz, A.; Järup, L. Low-level cadmium exposure and osteoporosis. J. Bone Miner. Res. 2000, 15, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Åkesson, A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef]
- Chen, X.; Wang, K.; Wang, Z.; Gan, C.; He, P.; Liang, Y.; Jin, T.; Zhu, G. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone 2014, 63, 76–80. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B.R. Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J. Bone Metab. 2016, 23, 223–231. [Google Scholar] [CrossRef]
- Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Karlsson, M.K.; Lorentzon, M.; Ohlsson, C.; Mellström, D. Low-Level Cadmium Exposure Is Associated with Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study. J. Bone Miner. Res. 2016, 31, 732–741. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, P.; Huang, R.; Liang, X.; Wang, P.; Tan, J.; Chen, Z.; Dun, Z.; Wang, J.; Jiang, Q.; et al. Cadmium Exposure and Osteoporosis: A Population-Based Study and Benchmark Dose Estimation in Southern China. J. Bone Miner. Res. 2017, 32, 1990–2000. [Google Scholar] [CrossRef]
- Kim, E.S.; Shin, S.; Lee, Y.J.; Ha, I.H. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch. Osteoporos. 2021, 16, 22. [Google Scholar] [CrossRef]
- Elonheimo, H.; Lange, R.; Tolonen, H.; Kolossa-Gehring, M. Environmental Substances Associated with Osteoporosis-A Scoping Review. Int. J. Environ. Res. Public Health. 2021, 18, 738. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Song, D.; Wu, Z.; Yang, L.; Wang, P.; Zhang, R.; Zhu, X. Resveratrol protects MC3T3-E1 cells against cadmium-induced suppression of osteogenic differentiation by modulating the ERK1/2 and JNK pathways. Ecotoxicol. Environ. Saf. 2021, 214, 112080. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Roszczenko, A.; Galażyn-Sidorczuk, M.; Majewska, K. Zinc supplementation can protect from enhanced risk of femoral neck fracture in male rats chronically exposed to cadmium. Exp. Toxicol. Pathol. 2011, 63, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Hernández-Navarro, D.; Pérez-Pastén, R.; Chamorro-Cevallos, G. Spirulina (Arthrospira) protects against cadmium-induced teratogenic damage in mice. J. Med. Food. 2011, 14, 398–404. [Google Scholar] [CrossRef]
- Rajak, C.; Singh, N.; Parashar, P. Metal toxicity and natural antidotes: Prevention is better than cure. Environ. Sci. Pollut. Res. Int. 2020, 27, 43582–43598. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Jerkic, M.; Santiago, J.M.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N.Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, W.M.; Niuc, Y.J.; Guoc, H.C.; Liuc, X.H.; Houc, Y.C.; Zhaoc, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Env. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.C.; Wang, Y.; Lin, H.Y.; Su, K.; Feng, X.S.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef]
- Pari, L.; Murugavel, P. Role of diallyltetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ. Toxicol. Pharmacol. 2005, 20, 493–500. [Google Scholar] [CrossRef]
- Hagar, H.; Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-Environ. Toxicol. Pharmacol. 2014, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Wang, L.C. Effect of taurine on toxicity of cadmium in rats. Toxicology 2001, 167, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Sil, P.C. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 2009, 36, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Batoye, S.; Jindal, R. Protective efficacy of naringenin against cadmium-induced redox imbalance in Labeo rohita: An integrated biomarker approach. Environ. Sci. Pollut. Res. Int. 2021, 29, 25591–25604. [Google Scholar] [CrossRef]
- Huang, J.; Ma, X.T.; Xu, D.D.; Yao, B.J.; Zhao, D.Q.; Leng, X.Y.; Liu, J. Xianling Gubao Capsule Prevents Cadmium-Induced Kidney Injury. Biomed. Res. Int. 2021, 2021, 3931750. [Google Scholar] [CrossRef]
- Iserhienrhien, L.O.; Okolie, N.P. Protective effect of Geophila obvallata (Shumach) Didr leaf extract and its fractions against cadmium-induced nephrotoxicity in male Wistar rats. Toxicol. Rep. 2021, 9, 87–93. [Google Scholar] [CrossRef]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nishigaki, Y.; Palaniswami, R.; Nishigaki, I. In vitro studies on mangiferin protection against cadmium-induced human renal endothelial damage and cell death via the MAP kinase and NF-κB pathways. J. Recept. Signal Transduct. Res. 2016, 36, 57–66. [Google Scholar] [CrossRef]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Luo, K.; Liu, Y.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol. 2013, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhang, S.P.; Liu, C.W.; Cai, Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol. In Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jin, X.; Fan, R.; Xing, M.; Guo, J.; Zhang, Z.; Zhang, J.; Xu, S. Cadmium-mediated miR-30a-GRP78 leads to JNK-dependent autophagy in chicken kidney. Chemosphere 2019, 215, 710–715. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Hamm, R.; Kuete, V. Harmful and Protective Effects of Terpenoids from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 557–576. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Nutraceuticals as modulators of gut microbiota: Role in therapy. Br. J. Pharmacol. 2020, 177, 1351–1362. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Davis, L.M.; Martínez, I.; Walter, J.; Hutkins, R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int. J. Food Microbiol. 2010, 144, 285–292. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Meijers, B.; Farré, R.; Dejongh, S.; Vicario, M.; Evenepoel, P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med. Food. 2017, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, X.; Lei, C.; Huang, Q.; Song, W.; Fang, R.; Li, C.; Li, X.; Mo, H.; Sun, N.; et al. High-Fat Diet Affects Heavy Metal Accumulation and Toxicity to Mice Liver and Kidney Probably via Gut Microbiota. Front. Microbiol. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Pérez-Torres, A.; Caverni-Muñoz, A.; González García, E. Mediterranean Diet and Chronic Kidney Disease (CKD): A Practical Approach. Nutrients 2022, 15, 97. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef] [PubMed]

| Authors | Study Design | Population | Measurements | Main Findings |
|---|---|---|---|---|
| Alfvén et al., 2000 [86] | Retrospective cohort study | 520 men and 544 women, aged 16–81 years, environmentally or occupationally exposed to Cd for at least 5 years | U-Cd, protein HC forearm BMD by DXA | U-Cd was negatively related to BMD, particularly in patients aged more than 60; in men over 60 the ORs for osteoporosis in the highest U-Cd category were 3.5 (95% CI, 0.6–19) in the group without tubular proteinuria, and 4.2 (95% CI, 1.0–20) in the group with tubular proteinuria |
| Engström et al., 2012 [87] | Prospective cohort study | 2676 women aged 56–69 years selected from the Swedish Mammography Cohort | Dietary Cd exposure assessed by a food frequency questionnaire, U-Cd BMD at the total body, femoral neck and lumbar spine by DXA, incidence of fractures | High dietary Cd exposure (≥13 μg/day, median) was associated with an increased risk of osteoporosis (OR = 1.32; 95% CI: 1.02–1.71) and of any first incident fracture (OR = 1.31; 95% CI: 1.02–1.69) |
| Chen et al., 2014 [88] | Cross-sectional, case–control study | 321 Chinese subjects (202 women and 119 men), aged 27 years and older living in control and polluted areas | U-Cd, U-Pb, B-Cd and B-Pb BMD at the proximal radius and ulna by DXA | Cd and Pb levels of people in the polluted area higher than those in the control area (p < 0.05); BMD of women in the polluted area lower than that of women in the control area (p < 0.05) and BMD decreased with increasing of B-Cd (p < 0.05), B-Pb and U-Pb in women. The likelihood of low BMD was associated with higher B-Cd in women (OR = 2.5, 95% CI: 1.11–5.43) and B-Pb in men (OR = 4.49, 95% CI: 1.37–14.6) |
| Lim et al., 2016 [89] | Nationwide cross-sectional study | Data of 2429 subjects from the KNHANES between 2008–2011 | B-Cd, B-Pb and B-Hg BMD at total hip, femoral neck and lumbar spine | In subjects with the highest quartile of B-Cd (≥1.439 μg/L) the risk for osteopenia or osteoporosis increased 2.1 times (95% CI 1.64–2.68) |
| Wallin et al., 2016 [90] | Prospective cohort study | 936 men from the MrOS study aged 70 to 81 years | U-Cd BMD at total body, hip, and lumbar spine, incidence of fractures | Significant negative associations between U-Cd and BMD, with lower BMD (4% to 8%) for all sites in the fourth quartile of U-Cd; positive associations between U-Cd and incident fractures, especially nonvertebral fractures in the fourth quartile of U-Cd |
| Lv et al., 2017 [91] | Cross-sectional study | 1116 subjects (832 and 284 subjects from a Cd-polluted area and a non-Cd-polluted area respectively) | U-Cd BMD at forearm | Significant negative association of U-Cd concentrations with BMD |
| Kim et al., 2021 [92] | Nationwide cross-sectional study | Data of 1031 post-menopausal women ≥50 years of age from the 4th and 5th KNHANES | B-Cd, nutrient intake BMD at total hip, femoral neck, and lumbar spine by DXA | Significant positive association between B-Cd levels and the risk of osteopenia and osteoporosis, but the OR at the 4th level was lower than that at the 3rd level (OR and 95% CI for osteopenia: 2nd quartile: 1.24, 0.88–1.74; 3rd quartile: 3.22, 2.24–4.64; 4th quartile: 1.27, 0.87–1.85; p < 0.001; OR and 95% CI for osteoporosis: 2nd quartile: 1.54, 1.05–2.25; 3rd quartile: 3.63, 2.31–5.69; 4th quartile: 1.70, 1.03–2.81; p < 0.001) |
| Authors | Study Design | Sample | Substance |
|---|---|---|---|
| Morales AI et al., 2006 [63] Morales AI et al., 2006 [99] | In vivo | Rats | Quercetin |
| Bagchi D et al., 2002 [100] Chen Q et al., 2013 [101] | In vitro In vivo | Human cells Mice | Grape seed procyanidin extract (GSPE) |
| Fan R et al., 2018 [102] | In vivo | Mice | Betulinic acid |
| Pari L and Murugavel P, 2005 [103] | In vivo | Rats | Diallyl tetrasulfide (DTS) |
| Hagar H and Al Malki W, 2014 [104] | In vivo | Rats | Betaine |
| Hwang DF and Wang LC, 2001 [105] Manna P et al., 2009 [106] | In vivo | Rats Mice | Taurine |
| Verma S et al., 2021 [107] | In vivo | Fish | Naringenin |
| Huang J et al., 2021 [108] | In vivo | Mice | Xianling Gubao |
| Iserhienrhien LO and Okolie NP, 2021 [109] | In vivo | Rats | Geophila obvallata |
| Salama SA et al., 2021 [110] | In vivo | Rats | Arctigenin |
| Rajendran P et al., 2016 [111] | In vitro | Human cells | Mangiferin |
| Joardar S et al., 2019 [112] | In vitro | Murine kidney cells | Rosmarinic acid |
| Wang Y et al., 2013 [113] Zhou YJ et al., 2009 [114] Shi Q et al., 2019 [115] | In vitro In vivo | LLC-PK1 cells Chicken | Selenium (Se) |
| Pallio G et al., 2019 [72] | In vivo | Mice | Myo-inositol |
| Micali A et al., 2018 [30] | In vivo | Mice | Flavocoxid |
| Cirmi S et al., 2021 [70] | In vivo | Mice | Bergamot juice extract (BJe) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, H.R.; Bellone, F.; Catalano, A.; Squadrito, G.; Micali, A.; Puzzolo, D.; Freni, J.; Pallio, G.; Minutoli, L. Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review. Metabolites 2023, 13, 722. https://doi.org/10.3390/metabo13060722
Marini HR, Bellone F, Catalano A, Squadrito G, Micali A, Puzzolo D, Freni J, Pallio G, Minutoli L. Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review. Metabolites. 2023; 13(6):722. https://doi.org/10.3390/metabo13060722
Chicago/Turabian StyleMarini, Herbert Ryan, Federica Bellone, Antonino Catalano, Giovanni Squadrito, Antonio Micali, Domenico Puzzolo, José Freni, Giovanni Pallio, and Letteria Minutoli. 2023. "Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review" Metabolites 13, no. 6: 722. https://doi.org/10.3390/metabo13060722
APA StyleMarini, H. R., Bellone, F., Catalano, A., Squadrito, G., Micali, A., Puzzolo, D., Freni, J., Pallio, G., & Minutoli, L. (2023). Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review. Metabolites, 13(6), 722. https://doi.org/10.3390/metabo13060722