Abstract
Cadmium (Cd) represents a public health risk due to its non-biodegradability and long biological half-life. The main target of Cd is the kidney, where it accumulates. In the present narrative review, we assessed experimental and clinical data dealing with the mechanisms of kidney morphological and functional damage caused by Cd and the state of the art about possible therapeutic managements. Intriguingly, skeleton fragility related to Cd exposure has been demonstrated to be induced both by a direct Cd toxic effect on bone mineralization and by renal failure. Our team and other research groups studied the possible pathophysiological molecular pathways induced by Cd, such as lipid peroxidation, inflammation, programmed cell death, and hormonal kidney discrepancy, that, through further molecular crosstalk, trigger serious glomerular and tubular injury, leading to chronic kidney disease (CKD). Moreover, CKD is associated with the presence of dysbiosis, and the results of recent studies have confirmed the altered composition and functions of the gut microbial communities in CKD. Therefore, as recent knowledge demonstrates a strong connection between diet, food components, and CKD management, and also taking into account that gut microbiota are very sensitive to these biological factors and environmental pollutants, nutraceuticals, mainly present in foods typical of the Mediterranean diet, can be considered a safe therapeutic strategy in Cd-induced kidney damage and, accordingly, could help in the prevention and treatment of CKD.
Keywords:
cadmium; PTE; kidney; CKD; bone; oxidative stress; inflammation; apoptosis; microbiota; nutraceuticals; Mediterranean diet 1. Cadmium: Who, Where and How
Potentially toxic elements (PTEs) are defined as elements that can be found in water, soils, and sediments and are able to progressively accumulate and above certain limits to cause severe damage to humans, animals, and the environment [1,2]. They are classified as essential or nonessential elements [3]. The former are manganese, iron, nickel, and zinc, necessary for the processes of growth, development, and other physiological activities of the organism [4]. Nonessential elements, such as cadmium (Cd), arsenic, mercury, lead, etc., cause trouble in the biological activities of organisms [5], as they can accumulate in the body and are used as substitutes for essential elements. As an example, Cd is able to replace calcium, so that the normal bone structure is altered, inducing bone diseases (osteomalacia, decalcification, and osteoporosis) [6].
Cd is a PTE with atomic number 48, discovered in 1817 by Friedrich Stromeyer in some samples of zinc carbonate; its name, in fact, comes from the Latin word “cadmia” meaning calamine, a mix of minerals rich in zinc carbonate, or from the Greek word “kadmeia” with the same meaning [7].
Cd is particularly rare in the Earth’s crust, with a lithosphere concentration of about 0.1–0.2 mg/kg [8], but it can be mobilized into the atmosphere owing to the action of volcanoes and the weathering of rocks by wind and rain [9], causing pollution of environmental air.
From the atmosphere, Cd is released to agricultural soils in a quantity calculated at 2500–15,000 tons annually [10]. Once in the soil, Cd concentration can increase owing to the use of unprocessed drain waters, phosphate-based fertilizers, particularly those obtained from seabed sediments with high Cd content [10], or different anthropogenic activities. Cd is easily absorbed by plants owing to its high mobility within the soil-plant system [11], even if its uptake is regulated by many factors related to soil characteristics, for example particle size, pH, temperature, and plant activity, such as root size and rate of root exudation and transpiration [12]. Cd absorption from plants may result in serious health problems. In fact, evidence was provided that in people living in Cd-uncontaminated locations, Cd-containing foods, such as vegetables, cereals, and legumes, are the main source of Cd either in animals or in humans [10]. It must be kept in mind that Cd is toxic to humans at lower concentrations than plants; therefore, apparently healthy plants are not safe for human feeding [10].
Anthropogenic activities are considered to contribute almost 80–90% of Cd pollution in the environment [13]. In fact, in addition to the use of phosphate fertilizers because of incorrect waste management, a higher concentration of Cd in the soil was observed around mining areas and industries where Cd is used for many purposes [3]. The extraction of minerals, even if crucial for human progress, causes serious PTE pollution in the environment [14]. Owing to its peculiar characteristics, including great electrical conductivity, resistance to corrosion, and low melting point, Cd has many industrial usaes, including anticorrosive materials production, electronic constituents, plastic stabilizers, nickel-Cd batteries, paints, and pigments [6].
Once in the environment, Cd is available for absorption by the organism. In humans, different ways of penetration have been described: the respiratory apparatus, the digestive apparatus, and the skin.
The main route of Cd exposure is considered cigarette smoke; in fact, Cd is accumulated by tobacco plants in a high concentration (650 to 3630 ng/g tobacco) [15], particularly when they are grown in contaminated soils. Another important environmental respiratory entry is found in workers of mines, and in factories producing paints and batteries, owing to the noteworthy quantities of Cd contained in dust and fumes [16]. It was demonstrated that particles containing Cd are able to induce a direct noxious effect on both cell types (type I and type II pneumocytes) of the alveolar epithelium with cellular injury, inflammation, and fibrosis, increasing the possibility of respiratory diseases [17,18,19]. A large quantity of Cd (50–100% of the inhaled particles with diameters smaller than 2–3 μm) [17] is entrapped in the epithelium, crosses the pulmonary interstice and enters the circulation.
In nonsmokers, food is the main cause of Cd intake, and its absorption is related to the type of toxicant, the amount, and the rate of exposure [20]. Cell death after chronic Cd exposure may cause structural changes of the intestinal epithelium, resulting in larger amounts of Cd permeation. Similarly, Cd-induced lesions of epithelial tight junctions may allow further penetration of Cd through the intestinal barrier [21]. However, it was recently demonstrated that gut bacteria can decrease the intestinal permeability of Cd, thus providing direct protection of the barrier [22]. From the epithelium, Cd is absorbed into the connective tissue and then into the submucosal capillaries.
As to skin absorption, previous experimental papers demonstrated the accumulation of Cd in the shaved skin of mice and rats, causing hyperkeratosis, acanthosis, and ulcerative changes in a dose-related manner [23]. The role of Cd in the prevention of skin pathologies was recently shown in psoriatic subjects evaluated in the NHANES study, which demonstrated a correlation between blood Cd and psoriasis severity [24].
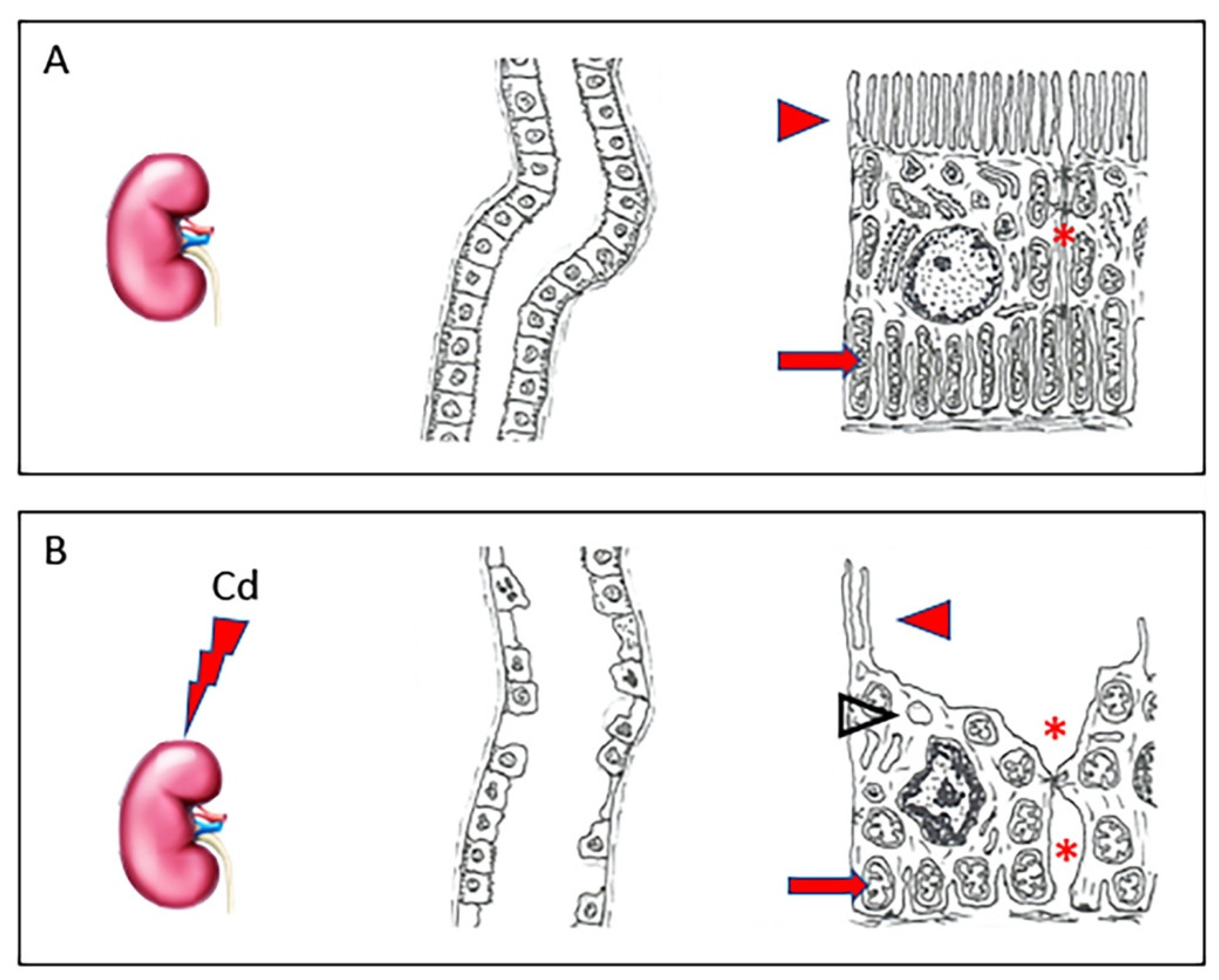
In the circulation, about 90% of Cd binds to α2-macroglobulin and albumin in the serum [25] and reaches the liver, where the complex is destroyed and small cysteine-rich proteins, metallothioneins (MT), are produced [26]. Of the four main isoforms (MT-1, -2, -3, and -4), Cd, likewise to other PTEs, induces the synthesis of MT-1 and MT-2, able to stimulate specific transcriptional factors, while MT-3 and MT-4 seem to have no role in the detoxification of PTEs [27]. The complex Cd-MT is taken from hepatocytes to shield the cells from toxic Cd ions. The excess part of these complexes not stored in the liver is discharged into the blood, reaching the kidney [16], where they are filtered from the glomerulus and then reabsorbed by the proximal tubular epithelial cells [28]. Here, the complex Cd-MT is degraded by lysosomes into amino acids and free Cd ions. In this way, free Cd can accumulate and cause nephrotoxicity, primarily in the proximal tubular region [29]. In fact, as demonstrated by our group in a recent paper [30], in healthy kidneys, tubules have an epithelium with well-evident apical microvilli, elongated mitochondria, and tight intercellular junctions. On the contrary, in kidneys challenged with Cd, tubules show evident morphological changes, as epithelial cells have short, few, or even absent apical microvilli, round or swollen mitochondria, and cytoplasmic vacuoles. Intercellular spaces are wide (Figure 1).
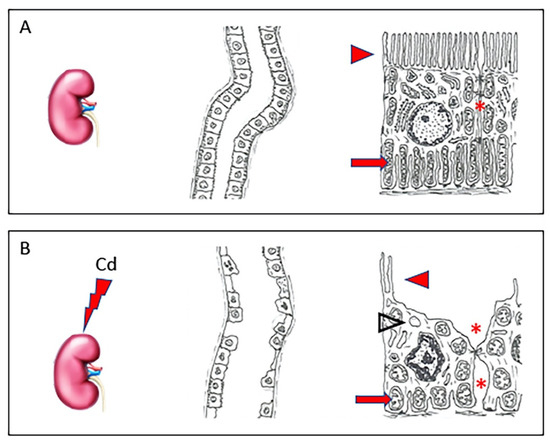
Figure 1.
In a healthy kidney (A), tubules have normal architecture; their epithelium shows well-evident apical microvilli (arrowhead), elongated mitochondria (arrow), and tight intercellular junctions (asterisk). In kidneys challenged with Cd (B), tubules have evident morphological changes, as epithelial cells show short, few, or even absent apical microvilli (arrowhead), round or swollen mitochondria (arrow), and cytoplasmic vacuoles (empty arrowhead). Intercellular spaces are wide (asterisk).
In the last few years, it has been focused on the relative roles of apoptotic, necrotic, and autophagic mechanisms in Cd-induced proximal tubular cell death. So far, these studies have implicated, from a pathophysiological point of view, three possible early response mechanisms in the proximal tubule. These are: (i) disruption of cadherin-mediated cell-cell adhesion; (ii) modulation of intracellular signaling cascades; and (iii) induction of oxidative stress [31]. This discovery has crucial implications for biomonitoring Cd-exposed populations and for the potential treatment of Cd nephrotoxicity. In this context, one novel marker that has shown exceptional promise in preclinical studies is Kidney Injury Molecule-1 (KIM-1). KIM-1 is a transmembrane protein that is not detectable in normal kidney but is expressed at high levels in the proximal tubule after ischemic or toxic injury [32]. Overall, these findings, along with early detection with novel biomarkers such as KIM-1, suggest that it may be possible to use specific agents to modulate or even halt these pathophysiological processes before they become irreversible [31].
The toxic action of Cd was also demonstrated in glomeruli, which showed elongated and fewer podocytes with reduced or lacking contact with the capillaries [30].
Recent data suggest that up to 50% of the deposits of Cd are accumulated in the kidney [28], where it induces renal toxicity owing to its mean half-life. Even if it were stated that the mean half-life of Cd in the kidney is 14 years [33], many variations ranging from 9 to 45 years and correlated with individual variations of MT expression were described [34]. Cd-induced renal toxicity is, therefore, a major risk for human health, particularly in countries where environmental controls are lacking. In fact, in patients exposed to Cd, a significant proximal tubular dysfunction was described, clinically expressed as increased urinary excretion of low-molecular-weight proteins, glucose, amino acids, and electrolytes such as sodium, potassium, and calcium [34]. Several studies have shown that Cd exposure may be related to chronic kidney disease (CKD) [35,36,37]. However, it is not easy to find a reliable exposure biomarker since several studies showed differences in study design (i.e., cross-sectional design) and/or exposure levels. To date, dietary urinary Cd (UCd) or blood Cd (BCd) have been commonly adopted as exposure biomarkers, as have urinary N-acetyl-β-d-glucosaminidase and beta-2-microglobulin. Generally, BCd mainly reflects recent exposure [38], while UCd may be related to long-term exposure [39,40]. Dietary Cd intake is also used as a surrogate indicator of Cd exposure [41]. Finally, Kawata [42] indicated that renal tubular function should be controlled during analysis. Overall, longitudinal studies are needed to better clarify the link between CKD and biomarkers.
2. Nutraceuticals: Generalities
The word “nutraceutical” (a combination of the terms “nutrient” and “pharmaceutical”) refers to “foods (or part of a food) that provide medical or health benefits, including prevention and/or treatment of disease” [43]. Nutraceuticals and pharmaceuticals exhibit high similarities and overlaps among their properties and functionalities [44]. To date, three groups of “healthy foods (or part of a food)” are considered: (i) “dietary supplements”, (ii) “functional foods”, and (iii) “nutraceuticals”. These latter may range from isolated nutrients, herbal products, dietary supplements, novel foods, and processed food ingredients. Indeed, in the global marketplace, nutraceuticals have become a multibillion-dollar industry as consumers in different countries appreciate these substances owing to their plant origin [45]. The popularity of nutraceuticals is also associated with their easy availability, low cost, and their intake in low doses. For this reason, the use of nutraceuticals in the prevention of renal dysfunction and CKD is a very intriguing option [46]. However, for many products, there is no clear data on their safety and effectiveness, possible side effects, interactions with prescribed medicines, or impact on preexisting medical conditions. Moreover, some nutraceuticals may present toxicity and cause adverse interactions with drugs commonly prescribed for CKD [47]. Overall, the topic is debated, and current research would help understand if it will be possible to employ “nutraceuticals” as an alternative approach against Cd-induced kidney damage.
3. Cadmium-Induced Pathophysiological Mechanisms and Kidney Dysfunctionality
Kidney damage induced by Cd has been shown either in vitro or in vivo [48,49,50]. In Japan, the Itai-itai disease, which is able to cause typical signs of CKD such as proteinuria, glicosuria, and aminoaciduria progressively irreversible, was shown to be related to chronic Cd toxication [51,52,53]. Generally, the above-mentioned features are typical of either occupational or environmental Cd poisoning, as experimentally observed. Cd exposure can also impair calcium metabolism, causing hypercalciuria and the formation of kidney stones [54]. The negative molecular cascade is amplified by the generation of reactive oxygen species (ROS), which are able to cause programmed cell death [55]. ROS in turn cause lipid peroxidation and damage to proteins, including Na+/K+ ATPase [56].
Oxidative stress can also lead to inflammation with increased production of proinflammatory cytokines, various chemokines, cellular adhesion molecules, and inducible enzymes that in turn can contribute to CKD [57,58,59,60,61]. In fact, the ROS increase induced by Cd challenge activates nuclear factor kappa B (NF-κB), which is a transcription factor able to control inflammation and regulate some components of the immune system. Once induced, it moves into the nucleus, regulating the synthesis of different mediators, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-12, cycloxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and macrophage migration inhibitory factor [62,63]. Therefore, inflammatory and immune disorders could be the consequence of NF-κB dysregulation; moreover, as reported in their intriguing review, Satarug and coworkers [64] highlighted the role of inflammation and oxidative stress as mechanistic pathways altered by Cd exposure (“the perfect storm”) in the physiopathology of diabetes and hypertension, that, in turn, cause CKD.
Additionally, Cd-induced renal inflammation through the NF-κB signaling pathway is able to activate the NLR family Pyrin Domain Containing 3 (NLRP3) inflammasome, a component of the innate immune system, whose role is still to be fully elucidated [65].
Lipid peroxidation, in turn, induces apoptosis by the following mechanisms: (i) the endoplasmic reticulum (ER)-mediated pathway through ER stress and calcium release; (ii) the mitochondria-mediated molecular signals; and (iii) the p53-dependent apoptotic pathway [50].
Specifically, it has been shown that the Cd-induced oxidative stress/inflammatory cascade activates apoptosis through the Fas/FasL pathway [66], and this molecular signal appears crucial in CKD induced by different nephrotoxic agents [67,68,69]. In the kidney, as also observed by our research group, a crucial role for mitochondria-dependent apoptosis is played by the B-cell lymphoma-2 (Bcl-2)/Bcl-2-associated X protein (Bax) system [70]. Moreover, it has been demonstrated in kidney tubules that, after Cd challenge, autophagia followed by apoptosis involves the upregulation of KIM-1 expression and changes in the localization and function of typical transmembrane adhesion molecules such as N-cadherin and claudin-2 [71,72]. The reduction of N-cadherin and claudin-2, a which are able to modify tubular epithelial polarization and junctional complexes, can be related to the presence of KIM-1 [31]. Finally, it was demonstrated that, experimentally, Cd suppressed renal erythropoietin (EPO) production through a direct effect and destruction of EPO-producing cells, driving anemia in Cd toxicity [73]. Moreover, it has been suggested that inhibition of EPO gene expression by Cd depends on the suppression of Hypoxia-Inducible Factor (HIF)-1 binding activity [74].
4. Cadmium and Bone Damage in CKD
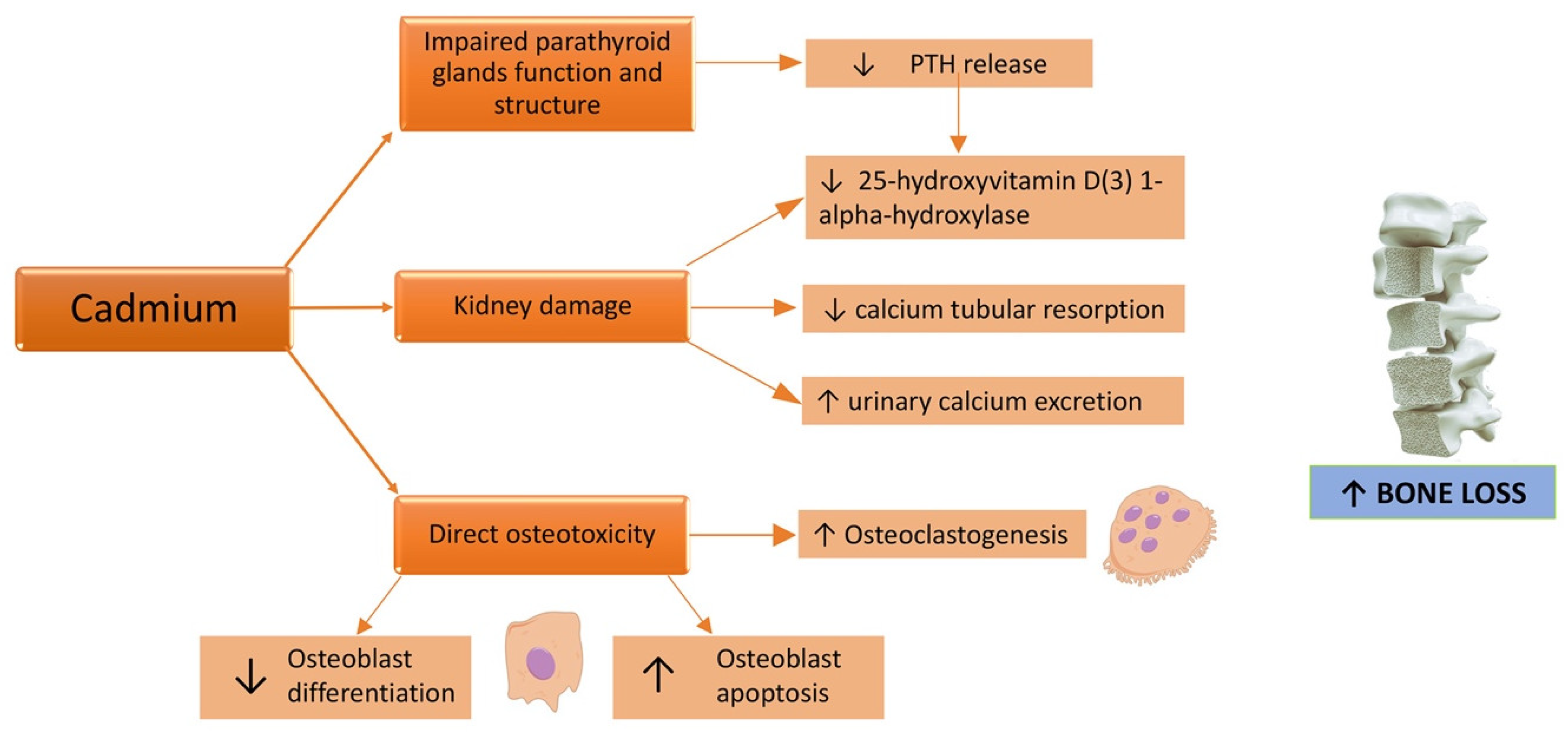
Osteotoxicity is a known effect of Cd [75]. In fact, the Itai-Itai disease, caused by a chronic exposure to Cd due to the use of Cd-polluted water to irrigate the rice fields [38], in addition to kidney failure, caused osteomalacia and osteoporosis. Skeleton fragility related to Cd exposure can be induced both by a direct Cd toxic effect on bone mineralization and by renal failure (Figure 2), even if the critical exposure levels and exact underlying mechanisms remain unclear [76].
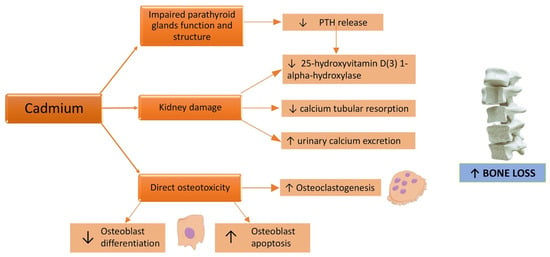
Figure 2.
Effects of a cadmium challenge on the bone. ↑: increased; ↓: decreased.
Cd exerts its direct toxicity either by a reduction in bone formation or an increase in bone resorption [77]. Indeed, this PTE affects mostly osteoblastic cells via the inhibition of osteoblast differentiation, synthesis activity, and the mineralization process of the extracellular matrix [78]. Scimeca et al. analyzed bone head biopsies demonstrating that Cd accumulation was associated with lower bone quality parameters and reduction and/or absence of osteoblasts; curiously, through an immunohistochemistry method, high levels of sclerostin, a glycoprotein belonging to the family of bone morphogenetic protein antagonists, were found in bone tissue of osteoporotic patients with Cd accumulation [79]. Cd exposure also determines an increase of tartrate resistant acid phosphatase (TRAP) activity and the formation of TRAP positive activated osteoclasts in the presence of receptor-activated nuclear factor κ B ligand (RANKL), inducing the differentiation of osteoclast precursors into osteoclasts and consequently leading to increased bone resorption [80].
Furthermore, Cd could induce proximal tubular dysfunction [77] with impaired calcium tubular resorption and consequently augmented urinary calcium excretion [81]; this, in turn, leads to bone demineralization and an increased risk of kidney stones [82]. Interference with parathyroid hormone (PTH) release has also been demonstrated: in the setting of Cd exposed workers, a significant negative correlation between the Cd-exposure index and plasma PTH levels was shown [83]. In other terms, Cd exposure leads to a decrease in PTH levels [84]. A structural and functional damage of the parathyroid glands, with a dose-dependent behavior and intensity related to Cd exposure duration has been demonstrated in murine models [85]. This implies a reduced activation of 25(OH)D3 vitamin D, because of impaired 1-alpha-hydroxylation in the kidney from its inactive form to the active one, 1,25(OH)2D3, followed by reduced intestinal calcium absorption and decreased reabsorption of bone mineral matrix [54]. Many cross-sectional and prospective population-based studies showed a negative correlation between Cd exposure and bone mineral density (BMD) [86,87,88,89,90,91,92] (Table 1).

Table 1.
Main clinical studies about cadmium exposure and fracture risk.
In detail, U-Cd concentrations have been contrariwise associated with BMD at the total body, lumbar spine, hip, femoral neck, and volumetric femoral neck [93]. Nevertheless, Kim et al. recently demonstrated no direct dose-response relationship at the highest Cd levels; this was related to a greater awareness of the disease by participants with osteoporosis and to higher Cd levels, resulting in improved therapeutic adherence, resulting in better BMD. Another reason for their results could simply be selection bias [92].
To date, little evidence exists on the protective role of some nutraceuticals against the damage to bone integrity induced by Cd exposure [94]. The supplementation of a natural polyphenol, resveratrol (RES), was shown to prevent Cd-induced apoptosis in osteoblastic MC3T3-E1 cells and to mitigate the inhibition of osteogenic differentiation induced by Cd chloride (CdCl2) by modulating ERK1/2 and JNK signaling [95]. Zinc supplementation has been demonstrated to prevent an increased risk of femoral neck fractures in rats with chronic exposure to Cd [96]. In Cd-exposed rats with a vitamin D-deficient diet, the toxic effect of Cd on kidney, bone, and hematopoietic systems was significantly higher than in Cd-exposed rats with a normal diet, suggesting a potential protective role of vitamin D administration against Cd-induced bone and kidney damage [54]. Through its antioxidant activity, spirulina, a filamentous cyanobacterium (also called blue-green algae), showed a significantly reduced frequency of fetal anencephaly, micro maxillary deformity, and skeletal deformities in pregnant mice orally administered with a high dose of Cd [97]. Essential elements, such as calcium, zinc, and vitamins, with which Cd shares a very similar way of metabolism and absorption, can attenuate Cd toxicity, particularly in bone tissue [94].
Notwithstanding, further research is needed to better define dietary strategies for preventing Cd-induced bone loss.
5. Therapeutic Effects of Functional Foods and Nutraceuticals in Cadmium-Induced Kidney Dysfunctionality: The Latest Preclinical Updates
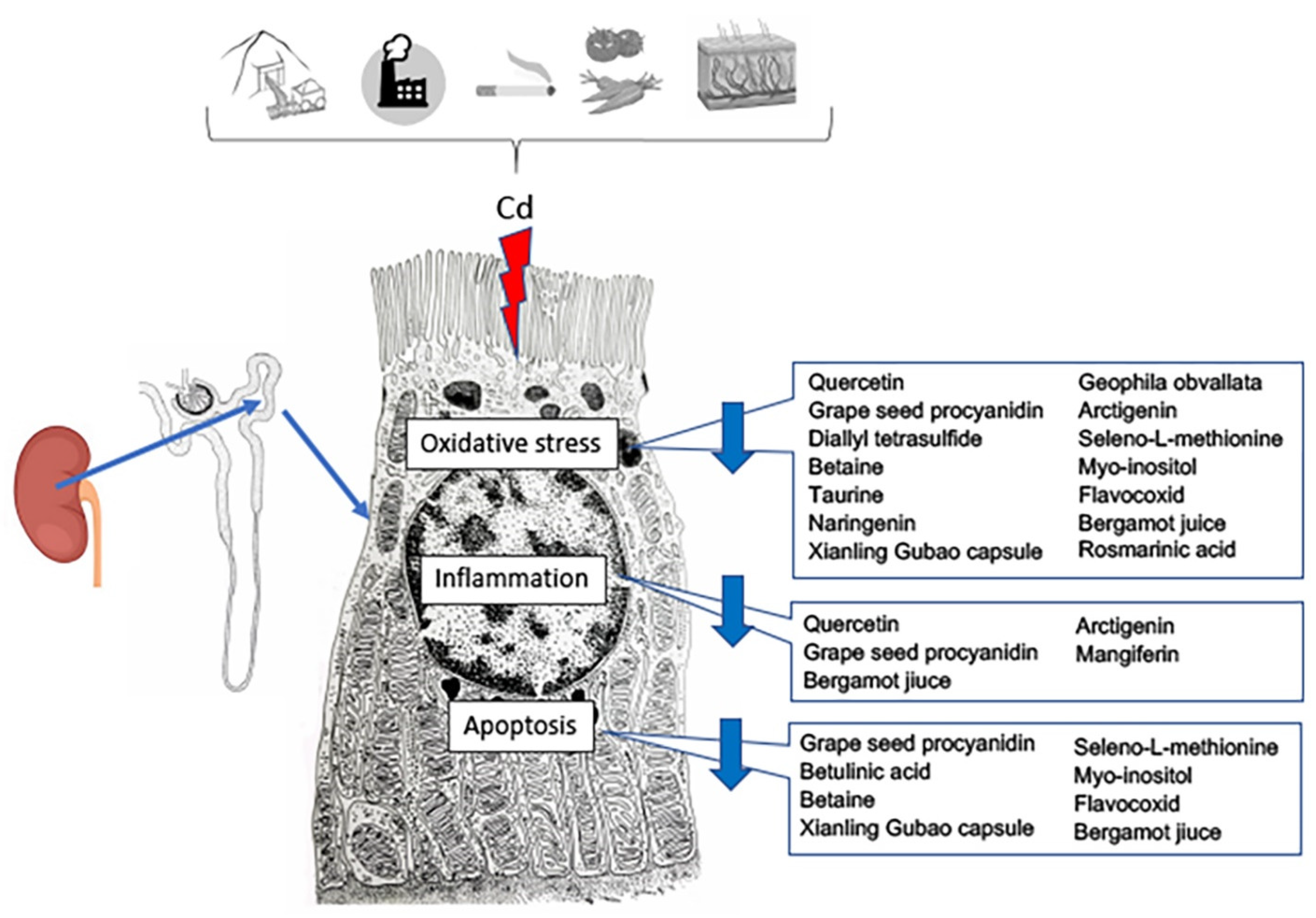
In the last few years, the protective role of antioxidants in food against PTEs has been evaluated [98] (Table 2; Figure 3).

Table 2.
Data obtained from in vivo and in vitro studies on nutraceuticals utilized as a possible approach against Cd-induced kidney toxicity.
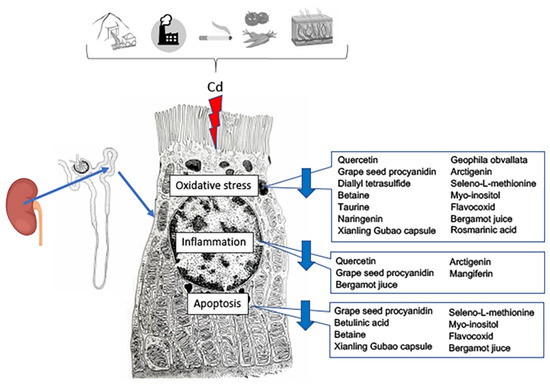
Figure 3.
Effects of nutraceutical treatment on the different pathways triggered by cadmium challenge in the proximal tubule cells of the kidney.
In fruits, vegetables, and wine, the polyphenolic compounds, flavonoids, are broadly distributed. Specifically, quercetin is the most abundant (60–75% of the polyphenols ingested). Quercetin has antioxidant, anti-inflammatory, and chelating activities, so it is protected from nephrotoxicity even after Cd intoxication [63,99]. It acts mainly as an antioxidant by contrasting the action of superoxide anion and lowering xanthine, NADPH oxidase, and superoxide dismutase (SOD). Moreover, an indirect action through increased MT-1 and MT-2 activity has been revealed [63,99]. In fact, the administration of quercetin plus Cd has increased MT-1 and MT-2 expression, thus lowering acute renal Cd toxicity, probably owing to its antioxidant activity. Quercetin also shows a crucial anti-inflammatory action through an augmented activity of both MT and endothelial nitric oxide synthase (eNOS) expression, together with an inhibition of both COX-2 and iNOS expression. Finally, a potent chelating capacity of quercetin, through the reduction of Cd uptake and accumulation in the kidney, has been demonstrated to further protect against Cd tubular damage [63,99].
Another substance with widespread antioxidant and anti-inflammatory action is grape seed procyanidin extract (GSPE), which is typical of tea leaves, fruits, vegetables, and seeds of many plants, such as grapes and apples [100]. When compared to vitamins C, E, and β-carotene, GSPE demonstrated a broad spectrum of antioxidant activity [101]. In Cd-challenged mice, GSPE was able to increase glutathione (GSH)-peroxidase (GPx) and SOD activities and decrease malondialdehyde levels in the kidneys. Moreover, GSPE antagonized renal apoptosis, as indicated by the expression of Bax and Bcl-2 [100].
Betulinic acid, a natural pentacyclic triterpenoid present in the bark of a number of trees, including white birch, bear tree, sycamore, and other members of the Platanus family, has, among other things, antioxidative, anti-inflammatory, and anti-apoptoptic properties. A protective effect of betulinic acid in the course of renal ischemia/reperfusion was demonstrated, as it induced antioxidant responses, improved structural changes, and renal function by modulating apoptosis of leukocytes [116]. Recently, a positive effect of betulinic acid on CdCl2-induced kidney injury was demonstrated by a direct inhibition of apoptosis [102].
Diallyl tetrasulfide (DTS) is a substance with antioxidant effects, found in garlic and, as an essential oil, in other plants [103]. It protects tubular cells, either in vivo or in vitro, after CdCl2 toxicity, owing to its antioxidant and metal chelating activities [48].
Betaine (glycine betaine or trimethylglycine), a natural antioxidant, can be obtained from the diet or from its precursor, choline [117]. As a result, reduced lipid peroxidation, an increased antioxidant status, a blunting of caspase-3 activity, and a reduction of tubular morphological changes were observed in the kidneys of rats challenged with Cd plus betaine [104].
An evident protection against oxidative stress caused by a Cd challenge was also observed after administration of the essential amino acid taurine (2-aminoethanesulfonic acid) [105,106]. When taurine is administered before Cd challenge, a reduction of morphological damages and of antioxidant enzyme levels, such as catalase (CAT), glutathione S-transferase (GST), glutathione reductase (GR), SOD, GPx, and glucose-6-phosphate dehydrogenase, was observed in mice’s kidneys [105].
Another substance with strong nephroprotective activity (antioxidant and metal chelating properties) is the bioflavonoid naringenin (4,5,7-trihydroxy flavonone), particularly abundant in citrus fruits [49,107]. A significant reduction of the structural changes and an increase of antioxidants and glutathione metabolizing enzymes were observed in the kidneys of Cd-exposed rats after oral coadministration of naringenin [49].
Xianling Gubao Capsule, a preparation of a mixture of Chinese herbs [Epimedii Folium (Epimedium brevicomu Maxim), Salvia miltiorrhiza Radix Rhizoma (Salvia miltiorrhiza Bunge), Anemarrhenae Rhizoma (Anemarrhena asphodeloides Bunge), Psoraleae Fructus (Cullen corylifolium (Linnaeus) Medikus), Dipsaci Radix (Dipsacus asper Wallich ex Candolle), and Rehmanniae Radix (Rehmannia glutinosa Libosch. ex Fisch. et Mey)] showed an important protective role in Cd-exposed mice, as it positively regulated oxidative stress, autophagy, and apoptosis, owing to the actions of the single components of the mixture [108].
The methanolic extract of Geophila obvallata (Rubiacea), a medicinal herb used in African ethnomedicine for treating kidney diseases, possesses bioactive principles able to show potent antioxidant action and downregulate KIM-1 and MT-1 in rats, thus providing renal protection against Cd-induced nephrotoxicity [109].
Arctigenin, a lignan naturally present in several plants, showed anti-inflammatory and antioxidant actions and reduced the expression of KIM-1 in the kidneys of Cd-treated rats [110].
Mangiferin (MGN) is a glucosylxanthone particularly abundant in the leaves and edible mango fruits of Mangifera indica. In vitro studies showed that MGN showed a potent antiinflammatory effect against Cd toxicity in human glomerulus renal endothelial cells through the reduction of IL-6 and IL-8, which play a significant role in renal inflammation [111].
Rosmarinic acid (RA), a naturally occurring polyphenolic nutraceutical, is an active constituent of Rosmarinus officinalis. In vitro and in vivo data revealed that RA treatment significantly counteracted the Cd-induced nephrotoxicity by blunting ROS, promoting cellular redox defense, and Cd clearance, thus positively modulating the altered pathological signal transduction [112].
An evident protection from oxidative stress of the kidney both in vivo and in vitro was observed after treatment with selenium (Se), which was related to ROS scavenging [113,114], through the activation of c-Jun N-terminal kinase phosphorylation [115]. Se inhibited the oxidative stress based on a reduction of ROS and blunted apoptosis through mitochondrial dysfunction, then confirmed a cytoprotective role against Cd toxicity in the kidney [113,114].
The treatment with the natural nutraceutical myo-inositol (MI) in Cd-treated mice showed protection against kidney damage. In fact, MI significantly reduced urea nitrogen and creatinine levels, oxidative marker expression, modulated apoptosis, increased GSH content and GPx activity, and preserved kidney morphology, suggesting a strong antioxidant role against Cd with harmful effects on kidney lesions [72].
Flavocoxid, a flavonoid containing both baicalin from Scutellaria baicalensis (Chinese skullcap) and catechin from Acacia catechu (Black catechu), reduced CdCl2-induced oxidative damage secondary to ROS generation in the kidney of C57 BL/6J mice. A significant reduction of iNOS, phosphoextracellular signal-regulated protein kinase 1/2, and matrix metalloproteinase-9 expression and of morphological changes of glomeruli and proximal tubules was in fact observed [30].
Recently, our research group evaluated the effects of a flavonoid-rich extract of bergamot juice (BJe), alone or in association with curcumin and resveratrol, in the kidneys of mice exposed to CdCl2 [70]. BJe, obtained from Citrus bergamia Risso et Poiteau (bergamot) fruits, showed antioxidant, anti-inflammatory, and antiapoptotic properties, as it significantly decreased urea nitrogen and creatinine levels, along with p53, Bax, Nos2, and IL-1ß mRNA, while increasing Bcl2, glutathione content, and glutathione peroxidase activity. Moreover, there was also a reduction of the glomerular and tubular damage, and of nuclear factor erythroid factor 2-related factor 2, NAD(P)H:quinone acceptor oxidoreductase 1 and heme oxygenase 1 gene expression, thus suggesting a new potential strategy in the management of CKD in subjects exposed to environmental toxicants.
6. Nutraceuticals and Microbioma: Putative Role in Cadmium-Induced Kidney Damage
The importance of the complex interactions between the microbiome and the human body is now well recognized, and the contributions of this relationship to host health are increasingly appreciated [118].
Indeed, any discussion of functional foods, nutraceuticals, or dietary supplements in the context of PTE-induced organ damage should address the impact on the microbiome of food and all potential interactions with a preventive and/or therapeutic intervention [119].
Several nutraceuticals act as “prebiotics”, which according to a description by a panel of experts convened by the International Scientific Association for Probiotics and Prebiotics (ISAPP), are defined as “a substrate that is selectively utilized by host microorganisms conferring health benefit” [120]. In this context, the metabolic products of the microbiota, such as short-chain fatty acids (SCFAs) and gases [121,122], appear to play a crucial role in the host.
Therefore, CKD is associated with the presence of dysbiosis, and the results of recent studies have confirmed the altered composition and functions of the gut microbial communities in CKD. In fact, during CKD, protein-bound uremic toxins are progressively accumulated [123]. Moreover, the presence of CKD may be accompanied by the development of intestinal inflammation and epithelial barrier impairment, leading to the translocation of bacterial-derived uremic toxins to the submucosal compartment, where they activate mast cells and lymphocytes, causing the release of proteases, cytokines/chemokines, and other crucial mediators of inflammation. In other words, the loss of kidney function results in structural and functional alterations of the intestinal barrier, contributing to the syndrome of uremia. This finding strongly suggests that a complex bidirectional metabolic and immunological crosstalk involving the kidney and gut is present [124]. Moreover, the aforementioned molecules can activate sensory afferents leading to local reflex responses and/or central transmission, as well as gain access to the portal and systemic circulations via the submucosal vasculature, leading, in turn, to oxidative stress injury, particularly involving the cardiovascular and endocrine systems [125].
Recently, it has been suggested that one of the useful properties of probiotic bacteria is their capacity to bind different targets, thus eliminating them through feces [126]. Specifically, it is supposed that one of these targets could be Cd. As a matter of fact, Djurasevic and coworkers experimentally showed that the rise in lactobacilli number in the feces of rats treated simultaneously with Cd and probiotics resulted in a strong correlation between the increase in Cd concentration in their feces and the decrease in Cd concentration in their blood. These findings suggest that probiotics actively contribute to Cd excretion through feces, probably by binding to the bacterial cell wall, opening the possibility of their therapeutic applications against Cd toxicity [126].
So far, gut microbiota are very sensitive to nutraceuticals, functional foods, probiotics, diet, and even environmental pollutants. Then, it is undeniable that dietary components and supplements interact in one way or another with the gut microbiome. Therefore, the possible effects on the health of environmental pollutants such as antibiotics, PTEs (including Cd), persistent organic pollutants, pesticides, nanomaterials, and food additives on the gut microbiota and their subsequent effects will continue to represent a major focus of future experimental [127] and clinical research [128].
7. Nutraceuticals and CKD: Chances and Limits in Routine Clinical Setting
Currently, there is no effective treatment for Cd poisoning. The principal therapeutic protocol involves the employment of metal chelators, although they cause several undesirable effects, such as redistribution/translocation of PTEs and other serious toxic events. This caught the interest of scientists who have sought an effective remedy from natural sources and/or from foods/healthy eating habits that are less likely to produce toxic effects. In this context, it appears crucial to add more information on the molecular mechanisms of Cd-induced structural damage of the kidney leading to CKD. So far, although in the present narrative review we considered a lot of preclinical studies and new data are currently available, unfortunately, to date, it is very hard to define Cd exposure levels related to the described biological effects on the kidney and the overall human health risk assessment. However, despite this scarcity of information and the limitations related to the type of review, dietary strategies and the use of nutraceuticals, which are present in foods typical of Mediterranean-style eating patterns, appear very useful in the management of non-communicable diseases, particularly CKD [129,130,131]. Pérez-Torres and colleagues, in their recent review [131], suggest a practical approach to Mediterranean diet adaptation as nutritional treatment in CKD patients. Indeed, there are several studies that suggest the use of a Mediterranean-style eating pattern as the dietary approach of choice for patients with CKD, regardless of the CKD stage [132,133]. In this context, it is well-known that the traditional Mediterranean diet is particularly abundant in cereals, legumes, nuts, fruits, vegetables, and herbs, and low in red meat [134]. Moreover, this dietary pattern includes a moderate intake of fish, seafood, eggs, white meat, and dairy products, and a moderate intake of alcohol (mainly red wine); finally, extra virgin olive oil is the main source of added fat [134]. So far, foods typical of Mediterranean-style eating patterns and the related compounds are well-known and under current careful investigation by several research groups for their potential benefit in positively modulating of the endothelial function, inflammation, oxidative stress, lipid profile, and blood pressure, that are crucial risk factors for the development of non-communicable diseases, including CKD [135,136]. Of course, in this context it should be carefully focused on a synergistic or antagonistic action between different bioactive foods or nutraceuticals of the Mediterranean-style eating pattern and, more generally, in plant-based diets, on neuroendocrine immune system modulation and gut microbiota dysbiosis, even more so in the presence of environmental pollution [129,130] caused by PTEs as Cd.
8. Conclusions
The molecular mechanisms of Cd-induced structural and functional damage to the kidney are a research topic of current interest. Experimental and clinical data analyzed in the present narrative review suggest that the multifaceted mechanism of action of nutraceuticals needs to be taken serious to effectively counteract the detrimental molecular cascade in kidney injury caused by environmental PTEs such as Cd.
Author Contributions
Conceptualization, H.R.M., F.B., A.C. and L.M.; Data Curation, D.P., J.F. and G.P.; Writing—Original Draft, H.R.M., D.P. and L.M.; Writing—Review and Editing, H.R.M., G.S., A.M., D.P., J.F. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duffus, J.H. Heavy Metal—A Meaningless Term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals" with "Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Liu, X.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P. The global research trend on cadmium in freshwater: A bibliometric review. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.B.; Yanar, A.; Alkan, E.N. Review of heavy metal accumulation on aquatic environment in Northern East Mediterrenean Sea part I: Some essential metals. Rev. Environ. Health 2017, 32, 119–163. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Pelucelli, A.; Medici, S.; Cappai, R.; Nurchi, V.M.; Zoroddu, M.A. Metal Toxicity and Speciation: A Review. Curr. Med. Chem. 2021, 28, 7190–7208. [Google Scholar] [CrossRef]
- Borsari, M. Encyclopedia of Inorganic and Bioinorganic Chemistry; Online © 2011–2014; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Heinrichs, H.; Schulz-Dobrick, B.; Wedepohl, K.H. Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim. Cosmochim. Acta 1980, 44, 1519–1533. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, F.; Xue, S.; Ma, T.; Yu, L. Heavy Metal Pollution and Source Contributions in Agricultural Soils Developed from Karst Landform in the Southwestern Region of China. Toxics 2022, 10, 568. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Rosén, K.; Eriksson, J.; Vinichuk, M. Uptake and translocation of 109Cd and stable Cd within tobacco plants (Nicotiana sylvestris). J. Environ. Radioact. 2012, 113, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, Q.; Lin, Q.; Zeng, C.; Zhong, C. Cadmium source identification in soils and high-risk regions predicted by geographical detector method. Environ. Pollut. 2020, 263, 114338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Piadé, J.J.; Jaccard, G.; Dolka, C.; Belushkin, M.; Wajrock, S. Differences in cadmium transfer from tobacco to cigarette smoke, compared to arsenic or lead. Toxicol. Rep. 2014, 2, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Jumarie, C. Cadmium transport through type II alveolar cell monolayers: Contribution of transcellular and paracellular pathways in the rat ATII and the human A549 cells. Biochim. Biophys. Acta 2002, 1564, 487–499. [Google Scholar] [CrossRef]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public Health 2017, 14, 1154. [Google Scholar] [CrossRef]
- Koopsamy Naidoo, S.V.; Bester, M.J.; Arbi, S.; Venter, C.; Dhanraj, P.; Oberholzer, H.M. Oral exposure to cadmium and mercury alone and in combination causes damage to the lung tissue of Sprague-Dawley rats. Environ. Toxicol. Pharmacol. 2019, 69, 86–94. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef]
- Bolan, S.; Seshadri, B.; Keely, S.; Kunhikrishnan, A.; Bruce, J.; Grainge, I.; Talley, N.J.; Naidu, R. Bioavailability of arsenic, cadmium, lead and mercury as measured by intestinal permeability. Sci. Rep. 2021, 11, 14675. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Sampson, B. Dermal toxicity and percutaneous absorption of cadmium in rats and mice. Lab. Anim. Sci. 1996, 46, 549–554. [Google Scholar]
- Liaw, F.Y.; Chen, W.L.; Kao, T.W.; Chang, Y.W.; Huang, C.F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci. Rep. 2017, 7, 1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant-cadmium (II) with human serum albumin. Environ. Sci. Pollut. Res. Int. 2014, 21, 6994–7005. [Google Scholar] [CrossRef]
- Sabolić, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Fan, R.F.; Tang, K.K.; Wang, Z.Y.; Wang, L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology 2021, 464, 152999. [Google Scholar] [CrossRef]
- Micali, A.; Pallio, G.; Irrera, N.; Marini, H.; Trichilo, V.; Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Laurà, R.; et al. Flavocoxid, a Natural Antioxidant, Protects Mouse Kidney from Cadmium-Induced Toxicity. Oxid. Med. Cell Longev. 2018, 2018, 9162946. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Arab, H.H.; Ashour, A.M.; Eid, A.H.; Arafa, E.A.; Al Khabbaz, H.J.; Abd El-Aal, S.A. Targeting oxidative stress, apoptosis, and autophagy by galangin mitigates cadmium-induced renal damage: Role of SIRT1/Nrf2 and AMPK/mTOR pathways. Life Sci. 2022, 291, 120300. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, Y.; Weaver, V.; Tellez-Plaza, M.; Guallar, E.; Lee, B.; Navas-Acien, A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ. Health Perspect. 2011, 119, 1800–1805. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012 Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef]
- Gibb, H.J.; Barchowsky, A.; Bellinger, D.; Bolger, P.M.; Carrington, C.; Havelaar, A.H.; Oberoi, S.; Zang, Y.; O’Leary, K.; Devleesschauwer, B. Estimates of the 2015 global and regional disease burden from four foodborn-arsenic, cadmium, lead and methylmercury. Environ. Res. 2019, 174, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Adams, S.V.; Newcomb, P.A. Cadmium blood and urine concentrations as measures of exposure, NHANES 1999–2010. J. Exp. Sci. Environ. Epidemiol. 2014, 24, 163–170. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Vacchi-Suzzi, C.; Eriksen, K.T.; Levine, K.; McElroy, J.; Tjønneland, A.; Raaschou-Nielsen, O.; Harrington, J.M.; Meliker, J.R. Dietary intake estimates and urinary cadmium levels in danish postmenopausal women. PLoS ONE 2015, 10, e0138784. [Google Scholar] [CrossRef]
- Kawata, T. Cadmium intake and chronic kidney disease. Clin. Nutr. 2018, 37, 1779. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Singh, J.; Sinha, S. Classification, Regulatory Acts and Applications of Nutraceuticals for Health. Int. J. Pharma. Biosci. 2012, 2, 177–187. [Google Scholar]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Rysz, J.; Franczyk, B.; Kujawski, K.; Sacewicz-Hofman, I.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Are Nutraceuticals Beneficial in Chronic Kidney Disease? Pharmaceutics 2021, 13, 231. [Google Scholar] [CrossRef]
- Salis, S. Role of nutraceuticals and probiotics in chronic kidney disease. J. Renal Nutr. Metab. 2018, 4, 47. [Google Scholar] [CrossRef]
- Pari, L.; Murugavel, P.; Sitasawad, S.L.; Kumar, K.S. Cytoprotective and antioxidant role of diallyl tetrasulfide on cadmium induced renal injury: An in vivo and in vitro study. Life Sci. 2007, 80, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Lee, J.Y.; Tokumoto, M.; Satoh, M. Cadmium renal toxicity via apoptotic pathways. Biol. Pharm. Bull. 2012, 35, 1892–1897. [Google Scholar] [CrossRef]
- Lauwers, R.; De Wals, P. Environmental pollution by cadmium and mortality from renal diseases. Lancet 1981, 317, 383. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nishijo, M.; Morikawa, Y.; Tabata, M.; Miura, K.; Takahara, H.; Okumura, Y.; Yoshita, K.; Kawano, S.; Nishi, M.; et al. Increased urinary β2-microglobulin and mortality rate by cause of death in a Cadmium-polluted area. Environ. Health Prev. Med. 1996, 1, 144–148. [Google Scholar] [CrossRef]
- Nishijo, M.; Morikawa, Y.; Nakagawa, H.; Tawara, K.; Miura, K.; Kido, T.; Ikawa, A.; Kobayashi, E.; Nogawa, K. Causes of death and renal tubular dysfunction in residents exposed to cadmium in the environment. Occup. Environ. Med. 2006, 63, 545–550. [Google Scholar] [CrossRef]
- Kazantzis, G. Cadmium, osteoporosis and calcium metabolism. Biometals 2004, 17, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Khandelwal, S. Influence of cadmium on murine thymocytes: Potentiation of apoptosis and oxidative stress. Toxicol. Lett. 2006, 165, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free. Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Kayama, F.; Yoshida, T.; Elwell, M.R.; Luster, M.I. Cadmium- induced renal damage and proinflammatory cytokines: Possible role of IL-6 in tubular epithelial cell regeneration. Toxicol. Appl. Pharmacol. 1995, 134, 26–34. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Vu, T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium hepatotoxicity and nephrotoxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Thevenod, F. Nephrotoxicity and the proximal tubules: Insights from cadmium. Nephron Physiol. 2003, 93, 87–93. [Google Scholar] [CrossRef]
- Fouad, A.A.; Jresat, I. Protective effect of telmisartan against cadmium-induced nephrotoxicity in mice. Life Sci. 2011, 89, 29–35. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Mohamed, W.R.; Ahmed, O.S.; Abdel-Daim, M.M.; Sayed, A.M. The role of inflammation in cadmium nephrotoxicity: NF-κB comes into view. Life Sci. 2022, 308, 120971. [Google Scholar] [CrossRef] [PubMed]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Grape seedproanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem. Cell Biol. 2015, 93, 210–226. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Sandoval, J.M.; Egido, J.; Mayoral, P.; Arevalo, M.A.; Fernández-Tagarro, M.; Lopez-Novoa, J.M.; Pérez-Barriocanal, F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Kidney Cadmium Toxicity, Diabetes and High Blood Pressure: The Perfect Storm. Tohoku, J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef]
- Oh, S.H.; Choi, J.E.; Lim, S.C. Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 2006, 220, 1–12. [Google Scholar] [CrossRef]
- Yang, C.W.; Faulkner, G.R.; Wahba, I.M.; Christianson, T.A.; Bagby, G.C.; Jin, D.C.; Abboud, H.E.; Andoh, T.F.; Bennett, W.M. Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am. J. Transplantat. 2002, 2, 391–399. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Choi, D.E.; Jeong, J.Y.; Lim, B.J.; Lee, K.W.; Shin, Y.T.; Na, K.R. Pretreatment with darbepoetin attenuates renal injury in a rat model of cisplatin-induced nephrotoxicity. Korean J. Intern. Med. 2009, 24, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Lynch, S.M. Cadmium alters the localization of N-cadherin, E-cadherin, and b-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003, 189, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Kayama, F. Cadmium and cisplatin damage erythropoietin-producing proximal renal tubular cells. Arch. Toxicol. 2006, 80, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Kayama, F.; Oguma, E.; Willmore, W.G.; Hradecky, P.; Bunn, H.F. Cadmium and platinum suppression of erythropoietin production in cell culture: Clinical implications. Blood 2000, 96, 3743–3747. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, J.; Chen, Y.; Shi, C.; Zhang, Q.; Ning, Z.; Yu, Y.; Li, Y. Urinary cadmium in relation to bone damage: Cadmium exposure threshold dose and health-based guidance value estimation. Ecotoxicol. Environ. Saf. 2021, 226, 112824. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, V.M.; Besharat, Z.M.; Antonioni, A.; Cella, V.; Lenzi, A.; Ferretti, E.; Migliaccio, S. The endocrine disruptor cadmium: A new player in the pathophysiology of metabolic diseases. J. Endocrinol. Investig. 2021, 44, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicol. Appl. Pharmacol. 2005, 202, 68–83. [Google Scholar] [CrossRef]
- Scimeca, M.; Feola, M.; Romano, L.; Rao, C.; Gasbarra, E.; Bonanno, E.; Brandi, M.L.; Tarantino, U. Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environ. Toxicol. 2017, 32, 1333–1342. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Jin, T.; Gu, S.; Xiao, H.; Qiu, J. Cadmium induces differentiation of RAW264.7 cells into osteoclasts in the presence of RANKL. Food Chem. Toxicol. 2011, 49, 2392–2397. [Google Scholar] [CrossRef]
- Nambunmee, K.; Honda, R.; Nishijo, M.; Swaddiwudhipong, W.; Nakagawa, H.; Ruangyuttikarn, W. Bone resorption acceleration and calcium reabsorption impairment in a Thai population with high cadmium exposure. Toxicol. Mech. Methods. 2010, 20, 7–13. [Google Scholar] [CrossRef]
- Wallin, M.; Sallsten, G.; Fabricius-Lagging, E.; Öhrn, C.; Lundh, T.; Barregard, L. Kidney cadmium levels and associations with urinary calcium and bone mineral density: A cross-sectional study in Sweden. Environ. Health. 2013, 12, 22. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Beshir, S.; Shahy, E.M.; Shaheen, W. Effect of Occupational Cadmium Exposure on Parathyroid Gland. Open Access Maced. J. Med. Sci. 2016, 4, 302–306. [Google Scholar] [CrossRef]
- Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. Int. J. Mol. Sci. 2021, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Pilat-Marcinkiewicz, B.; Brzóska, M.; Moniuszko-Jakoniuk, J. Thyroid and parathyroid function and structure in male rats chronically exposed to cadmium. Pol. J. Environ. Stud. 2008, 17, 113–120. [Google Scholar]
- Alfvén, T.; Elinder, C.G.; Carlsson, M.D.; Grubb, A.; Hellström, L.; Persson, B.; Pettersson, C.; Spång, G.; Schütz, A.; Järup, L. Low-level cadmium exposure and osteoporosis. J. Bone Miner. Res. 2000, 15, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Åkesson, A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef]
- Chen, X.; Wang, K.; Wang, Z.; Gan, C.; He, P.; Liang, Y.; Jin, T.; Zhu, G. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone 2014, 63, 76–80. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B.R. Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J. Bone Metab. 2016, 23, 223–231. [Google Scholar] [CrossRef]
- Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Karlsson, M.K.; Lorentzon, M.; Ohlsson, C.; Mellström, D. Low-Level Cadmium Exposure Is Associated with Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study. J. Bone Miner. Res. 2016, 31, 732–741. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, P.; Huang, R.; Liang, X.; Wang, P.; Tan, J.; Chen, Z.; Dun, Z.; Wang, J.; Jiang, Q.; et al. Cadmium Exposure and Osteoporosis: A Population-Based Study and Benchmark Dose Estimation in Southern China. J. Bone Miner. Res. 2017, 32, 1990–2000. [Google Scholar] [CrossRef]
- Kim, E.S.; Shin, S.; Lee, Y.J.; Ha, I.H. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch. Osteoporos. 2021, 16, 22. [Google Scholar] [CrossRef]
- Elonheimo, H.; Lange, R.; Tolonen, H.; Kolossa-Gehring, M. Environmental Substances Associated with Osteoporosis-A Scoping Review. Int. J. Environ. Res. Public Health. 2021, 18, 738. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Song, D.; Wu, Z.; Yang, L.; Wang, P.; Zhang, R.; Zhu, X. Resveratrol protects MC3T3-E1 cells against cadmium-induced suppression of osteogenic differentiation by modulating the ERK1/2 and JNK pathways. Ecotoxicol. Environ. Saf. 2021, 214, 112080. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Roszczenko, A.; Galażyn-Sidorczuk, M.; Majewska, K. Zinc supplementation can protect from enhanced risk of femoral neck fracture in male rats chronically exposed to cadmium. Exp. Toxicol. Pathol. 2011, 63, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Hernández-Navarro, D.; Pérez-Pastén, R.; Chamorro-Cevallos, G. Spirulina (Arthrospira) protects against cadmium-induced teratogenic damage in mice. J. Med. Food. 2011, 14, 398–404. [Google Scholar] [CrossRef]
- Rajak, C.; Singh, N.; Parashar, P. Metal toxicity and natural antidotes: Prevention is better than cure. Environ. Sci. Pollut. Res. Int. 2020, 27, 43582–43598. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sanchez, C.; Jerkic, M.; Santiago, J.M.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N.Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, W.M.; Niuc, Y.J.; Guoc, H.C.; Liuc, X.H.; Houc, Y.C.; Zhaoc, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Env. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.C.; Wang, Y.; Lin, H.Y.; Su, K.; Feng, X.S.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef]
- Pari, L.; Murugavel, P. Role of diallyltetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ. Toxicol. Pharmacol. 2005, 20, 493–500. [Google Scholar] [CrossRef]
- Hagar, H.; Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-Environ. Toxicol. Pharmacol. 2014, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Wang, L.C. Effect of taurine on toxicity of cadmium in rats. Toxicology 2001, 167, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Sil, P.C. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 2009, 36, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Batoye, S.; Jindal, R. Protective efficacy of naringenin against cadmium-induced redox imbalance in Labeo rohita: An integrated biomarker approach. Environ. Sci. Pollut. Res. Int. 2021, 29, 25591–25604. [Google Scholar] [CrossRef]
- Huang, J.; Ma, X.T.; Xu, D.D.; Yao, B.J.; Zhao, D.Q.; Leng, X.Y.; Liu, J. Xianling Gubao Capsule Prevents Cadmium-Induced Kidney Injury. Biomed. Res. Int. 2021, 2021, 3931750. [Google Scholar] [CrossRef]
- Iserhienrhien, L.O.; Okolie, N.P. Protective effect of Geophila obvallata (Shumach) Didr leaf extract and its fractions against cadmium-induced nephrotoxicity in male Wistar rats. Toxicol. Rep. 2021, 9, 87–93. [Google Scholar] [CrossRef]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nishigaki, Y.; Palaniswami, R.; Nishigaki, I. In vitro studies on mangiferin protection against cadmium-induced human renal endothelial damage and cell death via the MAP kinase and NF-κB pathways. J. Recept. Signal Transduct. Res. 2016, 36, 57–66. [Google Scholar] [CrossRef]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Luo, K.; Liu, Y.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol. 2013, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhang, S.P.; Liu, C.W.; Cai, Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol. In Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jin, X.; Fan, R.; Xing, M.; Guo, J.; Zhang, Z.; Zhang, J.; Xu, S. Cadmium-mediated miR-30a-GRP78 leads to JNK-dependent autophagy in chicken kidney. Chemosphere 2019, 215, 710–715. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Hamm, R.; Kuete, V. Harmful and Protective Effects of Terpenoids from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 557–576. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Nutraceuticals as modulators of gut microbiota: Role in therapy. Br. J. Pharmacol. 2020, 177, 1351–1362. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Davis, L.M.; Martínez, I.; Walter, J.; Hutkins, R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int. J. Food Microbiol. 2010, 144, 285–292. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Meijers, B.; Farré, R.; Dejongh, S.; Vicario, M.; Evenepoel, P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med. Food. 2017, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, X.; Lei, C.; Huang, Q.; Song, W.; Fang, R.; Li, C.; Li, X.; Mo, H.; Sun, N.; et al. High-Fat Diet Affects Heavy Metal Accumulation and Toxicity to Mice Liver and Kidney Probably via Gut Microbiota. Front. Microbiol. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Pérez-Torres, A.; Caverni-Muñoz, A.; González García, E. Mediterranean Diet and Chronic Kidney Disease (CKD): A Practical Approach. Nutrients 2022, 15, 97. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).