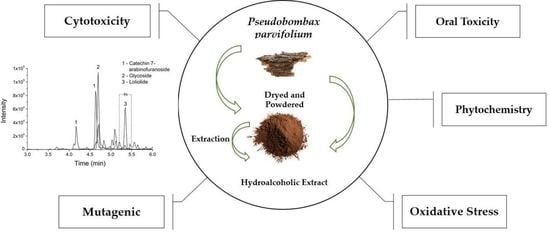
Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Collect and Plant Identification
2.3. Preparation of the P. parvifolium Hydroalcoholic Bark Extract
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Ultra Liquid Chromatography Coupled to Mass Spectrometry (LC–MS/MS)
2.7. Cell Culture
2.8. MTT Cell Viability Assay
2.9. Alamar Blue® Viability Assay
2.10. Mutagenicity Assessment
2.11. SMART (Somatic Mutation and Recombination Test)
2.12. Animals
2.13. Acute Oral Toxicity
2.14. Repeated Dose Toxicity
2.15. Biochemical Parameters
2.16. Preparation of Liver Homogenate
2.17. Oxidative Stress Analysis
2.18. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, H.; Fu, X.; Shao, J.; Tang, Y.; Yu, M.; Li, L.; Huang, L.; Tang, K. Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci. 2023, in press. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârţu, I.; Agoroaei, L. Current trends in toxicity assessment of herbal medicines: A narrative review. Processes 2023, 11, 83. [Google Scholar] [CrossRef]
- Palhares, R.M.; Baratto, L.C.; Scopel, M.; Mügge, F.L.B.; Brandão, M.G.L. Medicinal plants and herbal products from Brazil: How can we improve quality? Front. Pharmacol. 2021, 11, 606623. [Google Scholar] [CrossRef]
- Santos, M.O.; Ribeiro, D.A.; Macêdo, D.G.; Macêdo, M.J.F.; Macedo, J.G.F.; Lacerda, M.N.S.; Macêdo, M.S.; Souza, M.M.A. The Medicinal Plants: Versatility and concordance of use in the caatinga area, Northeastern Brazil. An. Acad. Bras. Cienc. 2018, 90, 2767–2779. [Google Scholar] [CrossRef]
- Duarte, M.C.; Esteves, G.L.; Salatino, M.L.F.; Walsh, K.C.; Baum, D.A. Phylogenetic analyses of Eriotheca and related genera (Bombacoideae, Malvaceae). Syst. Bot. 2011, 36, 690–701. Available online: http://www.bioone.org/doi/full/10.1600/036364411X583655 (accessed on 4 April 2023). [CrossRef]
- Carvalho-Sobrinho, J.G.; Queiroz, L.P. Three new species of Pseudobombax (Malvaceae, Bombacoideae) from Brazil. Novon 2010, 20, 13. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.; Monteiro, J.M.; Neto, E.M.F.L.; Melo, J.G.; Santos, J.P. Medicinal plants of the Caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Islam, M.S. A review study on different plants in Malvaceae family and their medicinal uses. Am. J. Biomed. Sci. Res. 2019, 3, 000641. [Google Scholar] [CrossRef]
- Paiva, D.C.; Santos, C.A.; Diniz, J.C.; Viana, F.A.; Thomazzi, S.M.; Falcão, D.A. Anti-inflammatory and antinociceptive effects of hydroalcoholic extract from Pseudobombax marginatum inner bark from caatinga potiguar. J. Ethnopharmacol. 2013, 149, 416. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.C.G.; Donnici, C.L.; Camargos, E.R.S.; Araujo-Silva, G.; Xavier-Junior, F.H.; Farias, L.M.; Corrêa, L.A.; Luz, J.R.D.; Carvalho, M.A.R.; Almeida, M.G. Interaction of Pseudobombax marginatum Robyns stem bark extract on the cell surface of Bacillus cereus and Staphylococcus aureus. J. Bacteriol. Mycol. 2018, 5, 1063. [Google Scholar]
- Alves, M.B.N.; Barros, N.B.; Lugtenburg, C.A.B.; Barros, R.R. Empirical use of medicinal plants in the treatment of diseases. Braz. J. Dev. 2022, 8, 31491. [Google Scholar] [CrossRef]
- Patrício, K.P.; Minato, A.C.S.; Brolio, A.F.; Lopes, M.A.; Barros, G.R.; Moraes, V.; Barbosa, G.C. Medicinal plant use in primary health care: An integrative review. Ciên. Saúde Colet. 2022, 27, 677. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Mendrick, D.L.; Paine, M.F.; Roe, A.L.; Yeung, C.K. Natural is not synonymous with safe: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020, 113, 104642. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F.; et al. Plants in anticancer drug discovery: From molecular mechanism to chemoprevention. Biomed. Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef]
- ANVISA—National Health Surveillance Agency. Brazilian Pharmacopoeia, 6th ed.; ANVISA: Brasília, Brazil, 2019.
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370. [Google Scholar] [CrossRef]
- Silva, L.M.P.; Inácio, M.R.C.; Silva, G.G.C.d.; Silva, J.M.d.S.E.; Luz, J.R.D.d.; Almeida, M.d.G.; Moraes, E.P.; Esposito, D.; Ferreira, L.D.S.; Zucolotto, S.M. The first optimization process from cultivation to flavonoid-rich extract from Moringa oleifera Lam. leaves in Brazil. Foods 2022, 11, 1452. [Google Scholar] [CrossRef]
- Luz, J.R.D.; Barbosa, E.A.; Nascimento, T.E.S.; Rezende, A.A.; Ururahy, M.A.G.; Brito, A.S.; Araujo-Silva, G.; López, J.A.; Almeida, M.G. Chemical characterization of flowers and leaf extracts obtained from Turnera subulata and their immunomodulatory effect on LPS-activated RAW 264.7 macrophages. Molecules 2022, 27, 1084. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332. [Google Scholar] [CrossRef]
- Palhares, L.C.G.F.; Barbosa, J.S.; Scortecci, K.C.; Rocha, H.A.O.; Brito, A.S.; Chavante, S.F. In vitro antitumor and anti-angiogenic activities of a shrimp chondroitin sulfate. Int. J. Biol. Macromol. 2020, 162, 1153. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mol. Mutagen. 1984, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Senes-Lopes, T.F.; López, J.A.; Amaral, V.S.; Brandão-Neto, J.; Rezende, A.A.; Luz, J.R.D.; Guterres, Z.R.; Almeida, M.D.G. Genotoxicity of Turnera subulata and Spondias mombin × Spondias tuberosa extracts from Brazilian Caatinga biome. J. Med. Food 2018, 21, 372. [Google Scholar] [CrossRef]
- Efthimiou, I.; Vlastos, D.; Ioannidou, C.; Tsilimigka, F.; Drosopoulou, E.; Mavragani-Tsipidou, P.; Potsi, G.; Gournis, D.; Antonopoulou, M. Assessment of the genotoxic potential of three novel composite nanomaterials using human lymphocytes and the fruit fly Drosophila melanogaster as model systems. Adv. Chem. Eng. 2022, 9, 100230. [Google Scholar] [CrossRef]
- ANVISA—National Health Surveillance Agency. RE n° 90/2004. Standards for toxicological studies of herbal products. In Brasília, DF: Official Gazette of the Federative Republic of Brazil, Executive Branch; ANVISA: Brasília, Brazil, 2004. [Google Scholar]
- OECD—Organisation for Economic Co-Operation and Development. Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2001. [Google Scholar]
- Batista, D.; Luz, J.R.D.; Nascimento, T.E.S.; Senes-Lopes, T.F.; Galdino, O.A.; Silva, S.V.; Ferreira, M.P.; Azevedo, M.A.S.; Brandão-Neto, J.; Araujo-Silva, G.; et al. Licania rigida leaf extract: Protective effect on oxidative stress, associated with cytotoxic, mutagenic and preclinical aspects. J. Toxicol. Environ. Health A 2021, 85, 276. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Co-Operation and Development. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2008. [Google Scholar]
- Yagi, K.A. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882. [Google Scholar]
- Frei, H.; Würgler, F.E. Statistical methods to decide whether mutagenicity test data from Drosophila assay indicate a positive, negative, or inconclusive result. Mutat. Res. 1988, 203, 297. [Google Scholar] [CrossRef]
- Kastenbaum, M.A.; Bowman, K.O. Tables for determining the statistical significance of mutation frequencies. Mutat. Res. 1970, 9, 527. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, K.H.; Shin, Y.C.; Jang, B.-H.; Ko, S.-G. Utilization of traditional medicine in primary health care in low- and middle-income countries: A systematic review. Health Policy Plan. 2020, 35, 1070–1083. [Google Scholar] [CrossRef]
- Theodoridis, S.; Drakou, E.G.; Hickler, T.; Thines, M.; Nogues-Bravo, D. Evaluating natural medicinal resources and their exposure to global change. Lancet Planet. Health 2023, 7, e155–e163. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnopharmacology: Quo vadis? Challenges for the future. Rev. Bras. Farmacogn. 2014, 24, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Menezes, M.A.G.; Neto, F.B.O.; Bertino, L.M.; Silva, F.F.M.; Alves, L.A. Quantification of anthocyanins of embiratanha (Pseudobombax marginatum). Holos 2015, 1, 30. [Google Scholar] [CrossRef] [Green Version]
- Chaves, T.P.; Santana, C.P.; Véras, G.; Brandão, D.O.; Felismino, D.C.; Medeiros, A.C.D.; Trovão, D.M.B.M. Seasonal variation in the production of secondary metabolites and antimicrobial activity of two plant species used in Brazilian traditional medicine. Afr. J. Biotechnol. 2013, 12, 847–853. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Ningthoujam, S.S.; Talukdar, A.D.; Upadhyaya, H.; Tundis, R.; Das, S.K.; Patra, J.K. Systematics, phytochemistry, biological activities and health promoting effects of the plants from the subfamily Bombacoideae (family Malvaceae). Plants 2021, 10, 651. [Google Scholar] [CrossRef]
- Refaat, J.; Desoky, S.Y.; Ramadan, M.A.; Kamel, M.S. Bombacaceae: A phytochemical review. Pharm. Biol. 2013, 51, 100–130. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Melro, J.C.L.; Fonseca, S.A.; Silva Júnior, J.M.; Franco, S.P.B.; Souza, M.A.; Costa, J.G.; Pimentel, Y.F.C.; Bomfim, M.R.P.; Almeida, E.M.; Matos-Rocha, T.J.; et al. Ethnodirigid study of medicinal plants used by the population assisted by the “Programa de Saúde da Família” (Family Health Program) in Marechal Deodoro—AL, Brazil. Braz. J. Biol. 2020, 80, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.M.F.; Pinheiro, L.S.; Pereira, C.K.S.; Matias, W.N.; Gomes, R.A.; Chaves, O.S.; Souza, M.F.V.; Almeida, R.N.; Assis, T.S. Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Terán, F.; Mendrano-Martinez, A.; Navarro-Ocaña, A. Antioxidant and free radical scavenging activities of plant extracts used in traditional medicine in Mexico. Afric. J. Biotechnol. 2008, 7, 1886. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y.; et al. (-)-Loliolide isolated from Sargassum horneri suppressed oxidative stress and inflammation by activating Nrf2/HO-1 signaling in IFN-γ/TNF-α-stimulated HaCaT keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Valli, V.; Tomás-Cobos, L.; Tomás-Chisbert, T.; Murgui-Bosch, L.; Danesi, F.; Bordoni, A. Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives? BMC Complement. Altern. Med. 2017, 17, 453. [Google Scholar] [CrossRef] [Green Version]
- Pitchakarn, P.; Inthachat, W.; Karinchai, J.; Temviriyanukul, P. Human hazard assessment using Drosophila wing spot test as an alternative in vivo model for genotoxicity testing—A review. Int. J. Mol. Sci. 2021, 22, 9932. [Google Scholar] [CrossRef]
- UNECE—United Nations Economic Commission for Europe. The Purple Book. In Globally Harmonized System of Classification and Labelling of Chemicals (GHS); UNECE: Geneva, Switzerland, 2019. [Google Scholar]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C.A. Ecotoxicity of plant extracts and essential oils: A review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Sponchiado, G.; Adam, M.L.; Silva, C.D.; Soley, B.S.; Mello-Sampayo, C.; Cabrini, D.A.; Correr, C.J.; Otuki, M.F. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. J. Ethnopharmacol. 2016, 178, 289–296. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef] [Green Version]
- Fitria, L.; Gunawan, I.C.P.; Sanjaya, W.B.T.; Meidianing, M.I.M. Single-dose acute oral toxicity study of chloroform extract of snake plant (Sansevieria trifasciata Prain.) leaf in Wistar rats (Rattus norvegicus Berkenhout, 1769). J. Trop. Biodivers. Biotechnol. 2022, 7, jtbb69389. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan Afr. Med. J. 2009, 3, 17. [Google Scholar]
- Guex, C.G.; Cassanego, G.B.; Dornelles, R.C.; Casoti, R.; Engelmann, A.M.; Somacal, S.; Maciel, R.M.; Duarte, T.; Borges, W.S.; Andrade, C.M.; et al. Tucumã (Astrocaryum aculeatum) extract: Phytochemical characterization, acute and subacute oral toxicity studies in Wistar rats. Drug Chem. Toxicol. 2022, 45, 810. [Google Scholar] [CrossRef] [PubMed]
- Croom, E. Metabolism of xenobiotics of human environments. In Progress in Molecular Biology and Translational Science; Hodgson, E., Ed.; Academic Press: Cambridge, UK, 2012; Volume 112, pp. 31–88. [Google Scholar] [CrossRef]
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. Plants used in Mexican traditional medicine for the management of urolithiasis: A review of preclinical evidence, bioactive compounds, and molecular mechanisms. Molecules 2022, 27, 2008. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.E. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Rodríguez-Yoldi, M.J. Anti-inflammatory and antioxidant properties of plant extracts. Antioxidants 2021, 10, 921. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological efficacy of medicinal plant extracts in preventing oxidative damage. Oxid. Med. Cell. Longev. 2018, 2018, 7904349. [Google Scholar] [CrossRef]
- Bulgheroni, A.; Kinsner-Ovaskainen, A.; Hoffmann, S.; Hartung, T.; Prieto, P. Estimation of acute oral toxicity using the no observed adverse effect level (NOAEL) from the 28 day repeated dose toxicity studies in rats. Regul. Toxicol. Pharmacol. 2009, 53, 16–19. [Google Scholar] [CrossRef]
- Ungur, R.A.; Borda, I.M.; Codea, R.A.; Ciortea, V.M.; Năsui, B.A.; Muste, S.; Sarpataky, O.; Filip, M.; Irsay, L.; Crăciun, E.C.; et al. A Flavonoid-rich extract of Sambucus nigra L. reduced lipid peroxidation in a rat experimental model of gentamicin nephrotoxicity. Materials 2022, 15, 772. [Google Scholar] [CrossRef]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular biomarkers in drug-induced liver injury: Challenges and future perspectives. Front. Pharmacol. 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, D.P.; Araújo, F.F.; Neri-Numa, A.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of polyphenols in the management of dyslipidemia: A focus on clinical studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Puangpraphant, S.; Cuevas-Rodríguez, E.O.; Oseguera-Toledo, M. Anti-inflammatory and antioxidant phenolic compounds. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Levy, N., Ed.; Academic Press: Cambridge, UK, 2022; pp. 165–180. [Google Scholar] [CrossRef]
- Dereli, F.T.G. Plant-based bioactive components: Phytochemicals: A review. In Bioactive Components; Thakur, M., Belwal, T., Eds.; Springer: Singapore, 2023; pp. 27–33. [Google Scholar] [CrossRef]
- Silva, J.D.N.; Monção, N.B.N.; Farias, R.R.S.; Citó, A.M.D.G.L.; Chaves, M.H.; Araújo, M.R.S.; Lima, D.J.B.; Pessoa, C.; Lima, A.; Araújo, E.C.D.C.; et al. Toxicological, chemopreventive and cytotoxic potentialities of rare vegetal species and supporting findings for the Brazilian Unified Health System (SUS). J. Toxicol. Environ. Health Part A 2020, 83, 525. [Google Scholar] [CrossRef]
- Rajčević, N.; Bukvički, D.; Dodoš, T.; Marin, P.D. Interactions between natural products—A review. Metabolites 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]



| Compound | Cosine | Mass Diffence | Mass | Molecular Formula | Ion Fragments | Adduct | |
|---|---|---|---|---|---|---|---|
| 1 | Catechin 7-arabinofuranoside | 0.87 | 0 | 423.13 | C20H22O10 | 291.08, 165.05, 147.04, 139.04, 123.05 | [M + H]+ |
| 2 | Glycoside | 0.88 | 0 | 600.265 | C28H38O13 | 411.17, 249.11, 187.07, 131.08, 69.03 | [M + NH4]+ |
| 3 | Loliolide | 0.87 | 0 | 197.116 | C11H16O3 | 179.11, 161.09, 135.12, 133.10, 107.09 | [M + H]+ |
| Genotypes and Treatments (mg/mL) | Number of Flies | Spots per Fly (Number of Spots) Estatistical Diagnosis a | Spots with Mwh Clone c (n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small Single Spots | Large Single Spots | Twin Spots | Total Spots | |||||||||||
| (1–2 Cells) b | (>2 Cells) b | |||||||||||||
| m = 2 | m = 5 | m = 5 | m = 2 | |||||||||||
| ST * | ||||||||||||||
| Negative Control | 40 | 0.28 | (11) | 0.05 | (02) | 0.00 | (00) | 0.33 | (13) | 13 | ||||
| 1.25 | 40 | 0.20 | (08) | − | 0.00 | (00) | i | 0.00 | (00) | i | 0.20 | (08) | − | 8 |
| 2.5 | 40 | 0.25 | (10) | i | 0.00 | (00) | i | 0.00 | (00) | i | 0.25 | (10) | − | 10 |
| 5 | 40 | 0.33 | (13) | i | 0.00 | (00) | i | 0.03 | (01) | i | 0.35 | (14) | i | 14 |
| DXR (0.125) | 40 | 2.85 | (114) | + | 2.33 | (93) | + | 2.43 | (97) | + | 7.60 | (304) | + | 304 |
| HB * | ||||||||||||||
| Negative Control | 40 | 0.45 | (18) | 0.05 | (02) | 0.00 | (00) | 0.50 | (20) | 20 | ||||
| 1.25 | 40 | 0.35 | (14) | − | 0.03 | (01) | i | 0.00 | (00) | i | 0.38 | (15) | − | 15 |
| 2.5 | 40 | 0.38 | (15) | − | 0.05 | (02) | i | 0.05 | (02) | i | 0.48 | (19) | − | 19 |
| 5 | 40 | 0.45 | (18) | − | 0.08 | (03) | i | 0.03 | (01) | i | 0.55 | (22) | − | 22 |
| DXR (0.125) | 40 | 5.43 | (217) | + | 4.15 | (166) | + | 3.85 | (154) | + | 13.43 | (537) | + | 537 |
| Organ (g) | Acute Oral Toxicity | Repeated Dose Oral Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2000 mg/kg | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | |
| Male | Female | Male | ||||||||
| Kidney (R) | 0.40 ± 0.04 | 0.40 ± 0.05 | 1.45 ± 0.04 | 1.44 ± 0.16 | 1.42 ± 0.51 | 1.45 ± 0.01 | 1.38 ± 0.17 | 1.35 ± 0.13 | 1.30 ± 0.12 | 1.30 ± 0.05 |
| Kidney (L) | 0.41 ± 0.06 | 0.38 ± 0.05 | 1.36 ± 0.09 | 1.41 ± 0.11 | 1.45 ± 0.31 | 1.45 ± 0.01 | 1.25 ± 0.08 | 1.24 ± 0.13 | 1.30 ± 0.12 | 1.30 ± 0.10 |
| Liver | 3.41 ± 0.54 | 3.12± 0.32 | 9.54 ± 0.98 | 9.56 ± 0.56 | 9.45 ± 1.12 | 8.99 ± 1.32 | 8.54 ± 1.45 | 9.00 ± 1.43 | 8.00 ± 1.34 | 8.32 ± 1.42 |
| Spleen | 0.24 ± 0.04 | 0.18± 0.01 | 1.34 ± 0.54 | 1.35 ± 0.13 | 1.33 ± 0.44 | 1.47 ± 0.05 | 1.26 ± 0.25 | 1.28 ± 0.05 | 1.20 ± 0.01 | 1.27 ± 0.05 |
| Heart | 0.31 ± 0.02 | 0.30± 0.03 | 1.21 ± 0.02 | 1.24 ± 0.12 | 1.25 ± 0.05 | 1.30 ± 0.05 | 1.16 ± 0.17 | 1.14 ± 0.05 | 1.10 ± 0.02 | 1.03 ± 0.01 |
| Lung | 0.52 ± 0.07 | 0.55 ± 0.01 | 2.01 ± 0.54 | 1.99 ± 0.01 | 2.14 ± 0.01 | 1.49 ± 0.01 | 1.93 ± 0.30 | 1.78 ± 0.03 | 2.00 ± 0.14 | 1.80 ± 0.01 |
| Stomach | 0.95 ± 0.14 | 0.98 ± 0.05 | 3.12 ± 1.02 | 2.99 ± 0.02 | 2.90 ± 0.05 | 3.22 ± 0.45 | 3.18 ± 0.80 | 3.42 ± 0.78 | 3.35 ± 0.43 | 3.40 ± 0.33 |
| Biochemical Parameters | Acute Oral Toxicity | Repeated Dose Oral Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2000 mg/kg | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | |
| Male | Female | Male | ||||||||
| Gluc (mg/dL) | 122 ± 21.9 | 120 ± 1.30 | 124.34 ± 42.32 | 120.05 ± 22.12 | 122.09 ± 25.02 | 120.98 ± 17.32 | 121.73 ± 32.0 | 121.10 ± 16.13 | 115.43 ± 15.34 | 115.94 ± 12.31 |
| Trig (mg/dL) | 53 ± 5.65 | 55.6 ± 3.21 | 23.80 ± 6.87 | 24.99 ± 1.03 | 24.23 ± 1.09 | 23.98 ± 6.23 | 38.21 ± 5.12 | 36.12 ± 3.34 | 34.03 ± 1.76 | 35.22 ± 1.63 |
| Chol (mg/dL) | 54.5 ± 6.06 | 56.2 ± 0.18 | 64.63 ± 10.32 | 63.12 ± 2.43 | 64.09 ± 5.22 | 60.74 ± 3.98 | 60.21 ± 9.42 | 62.32 ± 7.02 | 60.04 ± 6.95 | 58.93 ± 4.58 |
| ALT (U/L) | 97 ± 4.24 | 99 ± 2.20 | 86.34 ± 15.64 | 88.32 ± 15.03 | 86.92 ± 13.33 | 85.65 ± 10.08 | 95.23 ± 11.54 | 96.65 ± 10.01 | 95.52 ± 8.98 | 93.13 ± 5.01 |
| AST (U/L) | 250 ± 23.3 | 225 ± 9.31 | 109.98 ± 14.39 | 110.65 ± 9.38 | 110.24 ± 6.39 | 110.76 ± 5.98 | 100.23 ± 23.3 | 100.32 ± 5.35 | 102.98 ± 1.54 | 100.76 ± 3.99 |
| γ-GT (U/L) | 12 ± 1.4 | 12.1 ± 0.02 | 10.98 ± 0.12 | 10.99 ± 1.05 | 10.12 ± 0.55 | 10.77 ± 1.01 | 9.89 ± 0.32 | 10.23 ± 1.61 | 10.32 ± 0.6 | 9.32 ± 0.12 |
| TB (mg/dL) | 0.38 ± 0.01 | 0.43 ± 0.01 | 1.46 ± 0.02 | 1.50 ± 0.01 | 1.40 ± 0.25 | 1.45 ± 0.24 | 1.35 ± 0.15 | 1.39 ± 0.01 | 1.35 ± 0.02 | 1.31 ± 0.43 |
| DB (mg/dL) | 0.10 ± 0.07 | 0.103 ± 0.05 | 1.37 ± 0.01 | 1.38 ± 0.05 | 1.38 ± 0.01 | 1.30 ± 0.05 | 1.43 ± 0.05 | 1.44 ± 0.01 | 1.40 ± 0.05 | 1.38 ± 0.12 |
| IB (mg/dL) | 0.28 ± 0.01 | 0.30 ± 0.09 | 0.09 ± 0.001 | 0.10 ± 0.001 | 0.08 ± 0.001 | 0.10 ± 0.005 | 0.08 ± 0.002 | 0.09 ± 0.001 | 0.08 ± 0.001 | 0.08 ± 0.001 |
| Urea (mg/dL) | 54.5 ± 7.7 | 30.2 ± 2.06 * | 35.23 ± 7.21 | 25.87 ± 5.81 * | 25.77 ± 4.81 * | 20.54 ± 1.02 * | 35.23 ± 7.21 | 24.87 ± 5.61 * | 24.77 ± 2.81 * | 20.22 ± 1.12 * |
| Uric acid (mg/dL) | 1.16 ± 0.35 | 1.12 ± 0.01 | 1.15 ± 0.21 | 1.25 ± 0.08 | 1.26 ± 0.13 | 2.12 ± 0.01 | 1.16 ± 0.41 | 1.10 ± 0.01 | 1.13 ± 0.05 | 1.14 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senes-Lopes, T.F.d.; Luz, J.R.D.d.; Guterres, Z.d.R.; Barbosa, E.A.; Batista, D.; Galdino, O.A.; Ururahy, M.A.G.; Gomes dos Santos, E.C.; López, J.A.; Araujo-Silva, G.; et al. Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress. Metabolites 2023, 13, 748. https://doi.org/10.3390/metabo13060748
Senes-Lopes TFd, Luz JRDd, Guterres ZdR, Barbosa EA, Batista D, Galdino OA, Ururahy MAG, Gomes dos Santos EC, López JA, Araujo-Silva G, et al. Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress. Metabolites. 2023; 13(6):748. https://doi.org/10.3390/metabo13060748
Chicago/Turabian StyleSenes-Lopes, Tiago Felipe de, Jefferson Romáryo Duarte da Luz, Zaira da Rosa Guterres, Eder A. Barbosa, Débora Batista, Ony Araújo Galdino, Marcela Abbott Galvão Ururahy, Elizabeth Cristina Gomes dos Santos, Jorge A. López, Gabriel Araujo-Silva, and et al. 2023. "Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress" Metabolites 13, no. 6: 748. https://doi.org/10.3390/metabo13060748
APA StyleSenes-Lopes, T. F. d., Luz, J. R. D. d., Guterres, Z. d. R., Barbosa, E. A., Batista, D., Galdino, O. A., Ururahy, M. A. G., Gomes dos Santos, E. C., López, J. A., Araujo-Silva, G., & Almeida, M. d. G. (2023). Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress. Metabolites, 13(6), 748. https://doi.org/10.3390/metabo13060748