Differences in the Production of Extracellular Polymeric Substances (EPS) and Other Metabolites of Plenodomus (Leptosphaeria) Infecting Winter Oilseed Rape (Brassica napus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterisation of Strains
2.2. Pathogenicity Test
2.3. Dynamics of the Production of Extracellular Polymeric Substances (EPS)
2.4. Fungal Strain Growth Dynamics
2.5. β-Glucanase and Invertase Activity
2.6. Metabolic Activity of Strains—Screening Studies
2.6.1. Proteolytic Activity
2.6.2. Cellulolytic Activity
2.6.3. Phosphate-Solubilising Capacity
2.6.4. Amylolytic Activity
2.6.5. Siderophore Synthesis Capacity
2.7. Determination of IAA Concentration
2.8. Statistical Analysis
3. Results
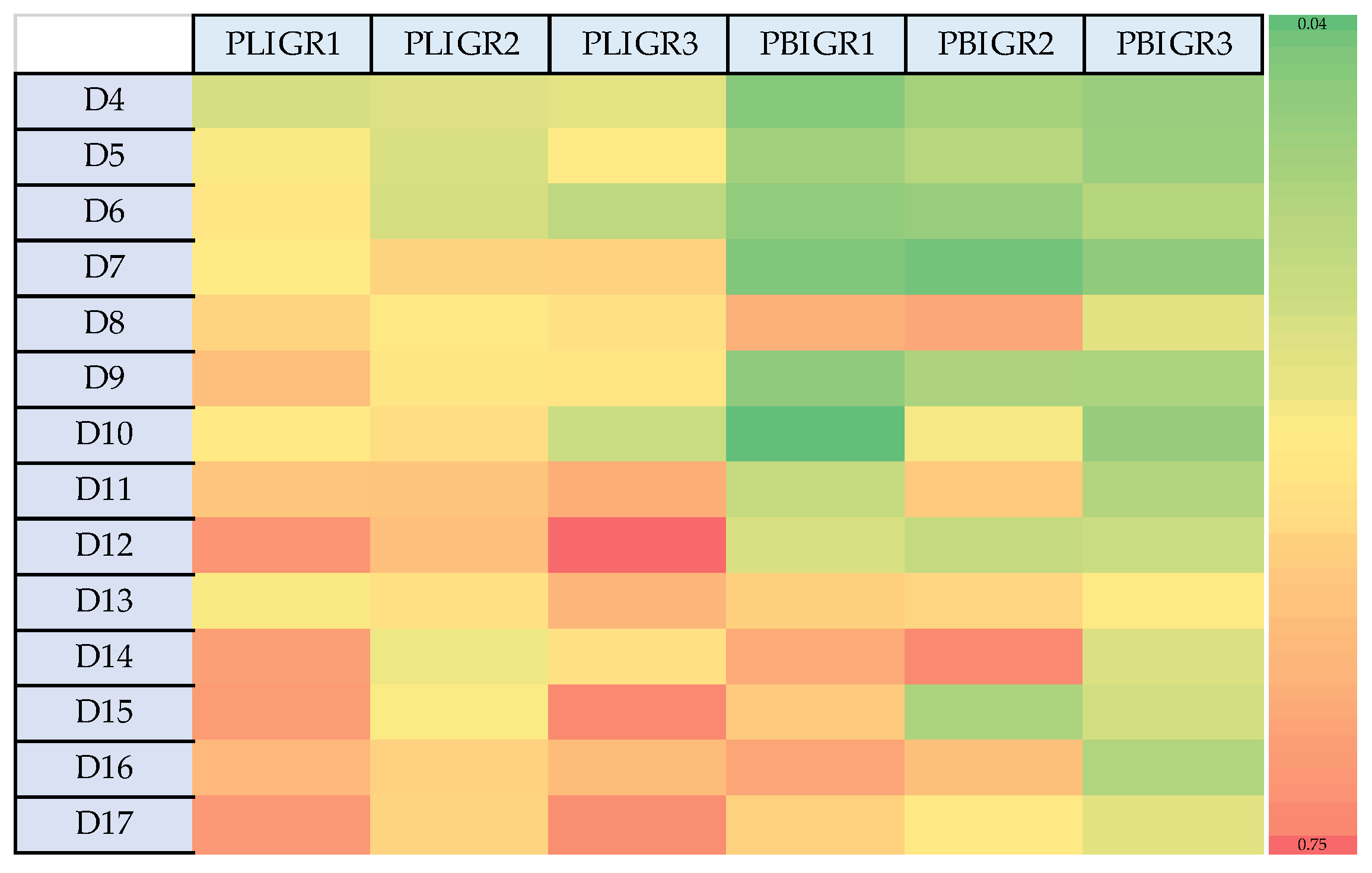
3.1. Dynamics of EPS Synthesis
3.2. Fungal Strain Growth Dynamics
3.3. Pathogenicity Tests
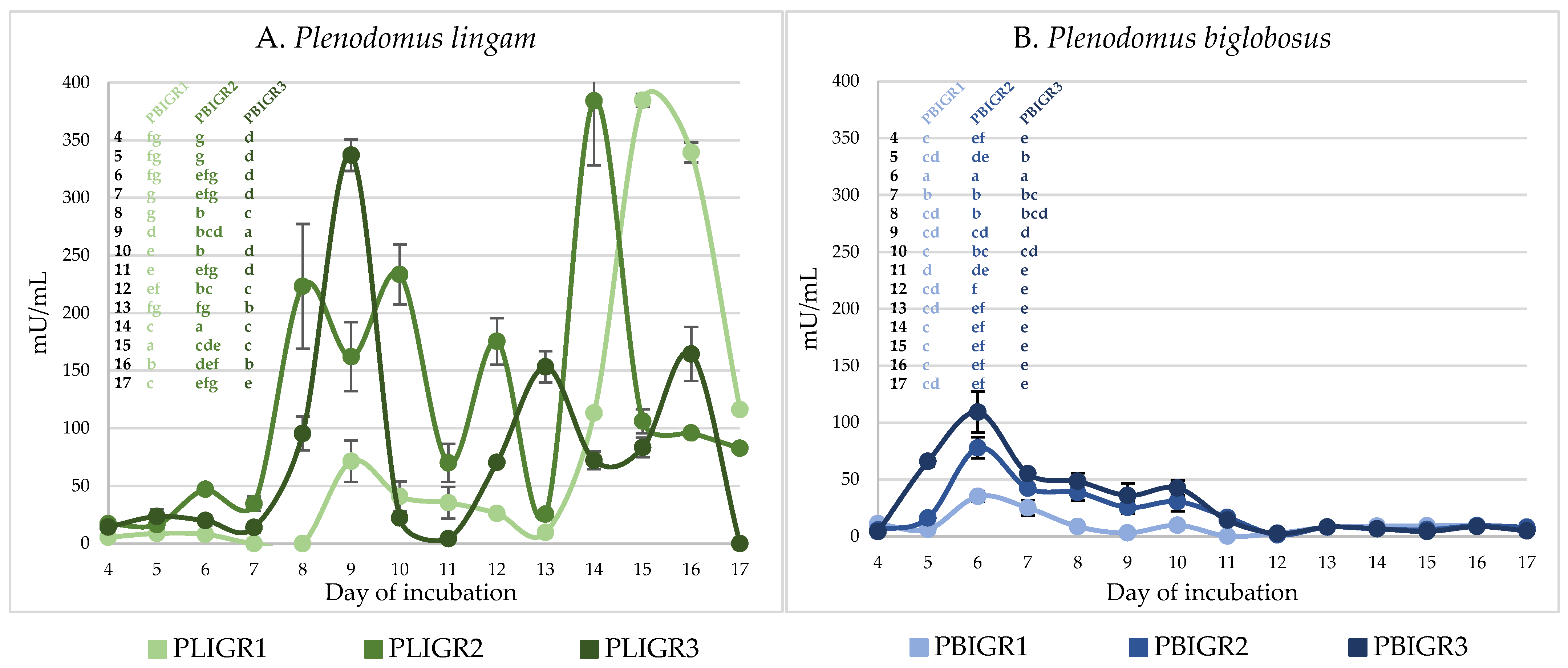
3.4. Enzymatic Activity
3.4.1. β-Glucanase Activity
3.4.2. Invertase Activity
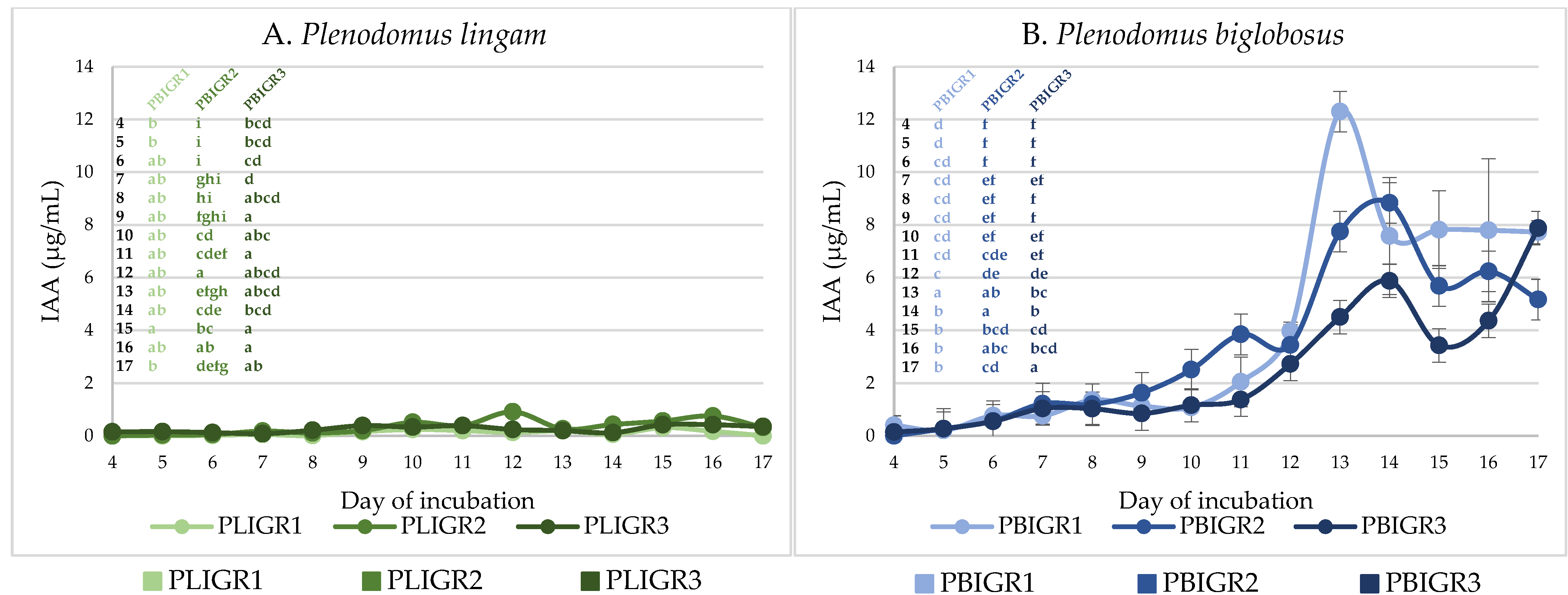
3.5. IAA Concentration
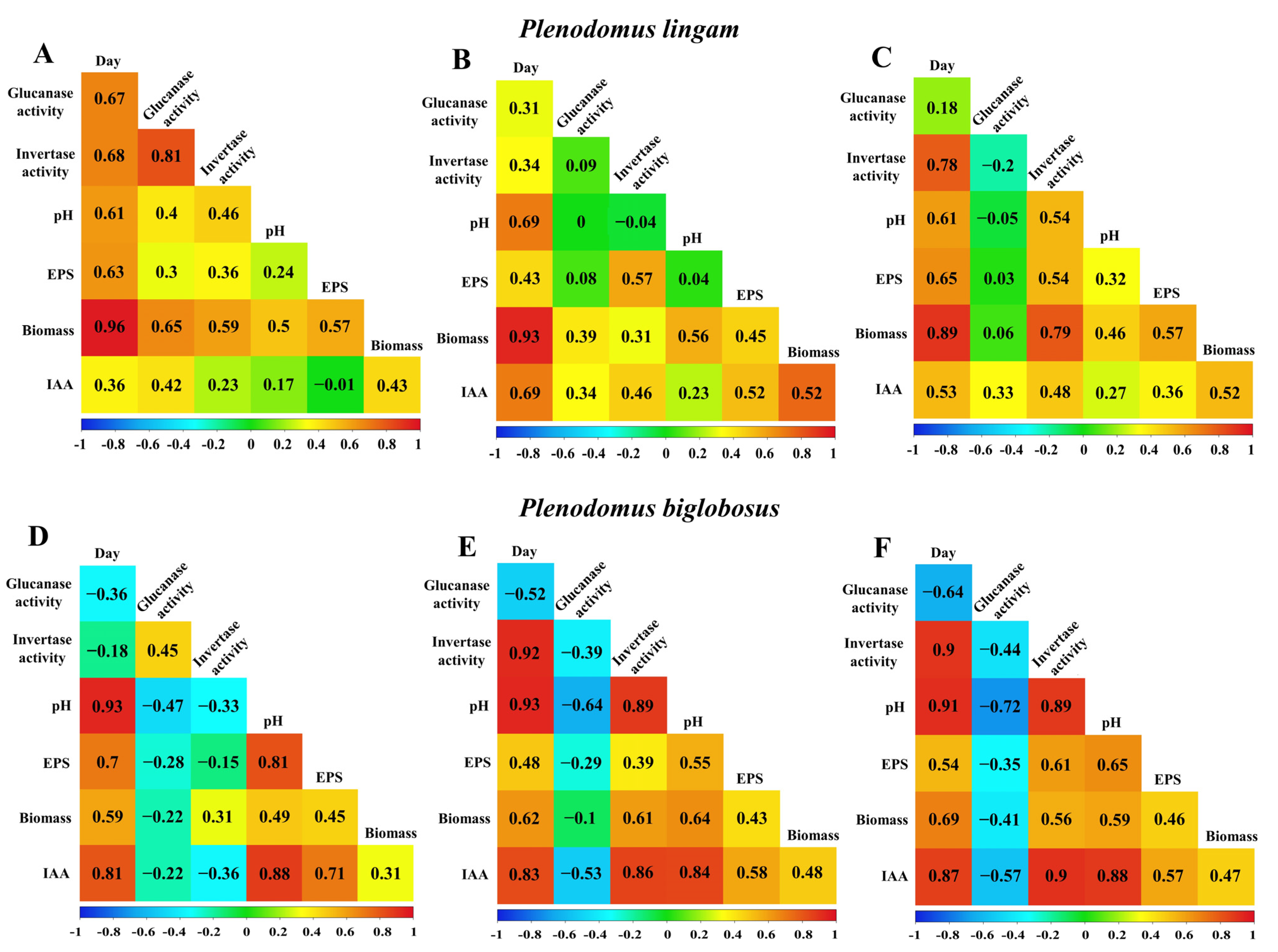
3.6. Correlations between EPS Synthesis and Enzymatic Activity
3.7. Ability of the Tested Strains to Synthesise Diverse Secondary Metabolites Facilitating Environmental Adaptation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A Review. Front. Plant Sci. 2021, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pocsi, I. Secondary metabolites in fungus-plant interactions. Front Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.I.; Hamilton, O.; Jonge, R.; Bolton, M.D. Fungal social influencers: Secondary metabolites as a platform for shaping the plant-associated community. Plant J. 2021, 108, 632–645. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Félix, C.; Meneses, R.; Gonçalves, M.F.M.; Tilleman, L.; Duarte, A.S.; Jorrín-Novo, J.V.; Van de Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Esteves, A.C.; et al. A multi-omics analysis of the grapevine pathogen Lasiodiplodia theobromae reveals that temperature affects the expression of virulence- and pathogenicity-related genes. Sci. Rep. 2019, 9, 13144. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Reineke, G.; Heinze, B.; Schirawski, J.; Buettner, H.; Kahmann, R.; Basse, C.W. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol. Plant Pathol. 2008, 9, 339–355. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Destructuring plant biomass: Focus on fungal and extremophilic cell wall hydrolases. Plant Sci. 2015, 234, 180–193. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Słomka, A.; Janczarek, M.; Rodzik, B. Activities of cell wall degrading enzymes in autolyzing cultures of three Fusarium culmorum isolates: Growth-promoting, deleterious and pathogenic to rye (Secale cereale). Mycologia 2011, 103, 929–945. [Google Scholar] [CrossRef]
- Ye, X.F.; Xu, C.S.; Xie, T.T.; Zhang, Y.; Zhao, Y.Q.; Xia, C.Y.; Li, Z.K.; Huang, Y.; Fan, J.Q.; Cao, H.; et al. Myxobacterial outer membrane beta-1,6-glucanase induced the cell death of Fusarium oxysporum by destroying the cell wall integrity. Appl. Environ. Microbiol. 2023, 89, e0123622. [Google Scholar] [CrossRef]
- Silva, D.P.D.; Cardoso, M.S.; Macedo, A.J. Endophytic Fungi as a Source of Antibacterial Compounds—A Focus on Gram-Negative Bacteria. Antibiotics 2022, 11, 1509. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nadales, E.; Nogueira, M.F.; Baldin, C.; Castanheira, S.; El Ghalid, M.; Grund, E.; Lengeler, K.; Marchegiani, E.; Mehrotra, P.V.; Moretti, M.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Genet. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.H.; Heinekamp, T.; Brakhage, A.A. Human and plant fungal pathogens: The role of secondary metabolites. PLoS Pathog. 2014, 10, e1003859. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Yu, Y. Phytotoxins, elicitors and other secondary metabolites from phytopathogenic “blackleg” fungi: Structure, phytotoxicity and biosynthesis. Nat. Prod. Commun. 2009, 4, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Priest, E.; Naglik, J.R.; Richardson, J.P. Fungal toxins and host immune responses. Front. Microbiol. 2021, 12, 697. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Chumala, P.; Yu, Y. The phytopathogenic fungi Leptosphaeria maculans and Leptosphaeria biglobosa: Chemotaxonomical characterization of isolates and metabolite production in different culture media. Can. J. Microbiol. 2007, 53, 364–371. [Google Scholar] [CrossRef]
- Kerdraon, L.; Barret, M.; Balesdent, M.; Suffert, F.; Laval, V. Impact of a resistance gene against a fungal pathogen on the plant host residue microbiome: The case of the Leptosphaeria maculans–Brassica napus pathosystem. Mol. Plant Pathol. 2020, 21, 1545–1558. [Google Scholar] [CrossRef]
- Montesano, M.; Kõiv, V.; Mäe, A.; Palva, E.T. Novel receptor-like protein kinases induced by Erwinia carotovora and short oligogalacturonides in potato. Mol. Plant Pathol. 2001, 2, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on fungal phytotoxins and their role in grapevine trunk diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Schmidt, F.; Fischer, J.; Riemann, M.; Thines, E.; Nick, P. The fungal elicitor eutypine from Eutypa lata activates basal immunity through its phenolic side chains. Hortic. Res. 2022, 9, uhac120. [Google Scholar] [CrossRef] [PubMed]
- Möbius, N.; Hertweck, C. Fungal Phytotoxins as Mediators of Virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- El Oirdi, M.; El Rahman, T.A.; Rigano, L.; El Hadrami, A.; Rodriguez, M.C.; Daayf, F.; Vojnov, A.; Bouarab, K. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 2011, 23, 2405–2421. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Luft, L.; Seibel, J.; Kuhn, R.C.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environ. Technol. 2019, 41, 2742–2749. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Nowak, A.; Komaniecka, I.; Choma, A.; Jarosz-Wilkołazka, A.; Osińska-Jaroszuk, M.; Tyśkiewicz, R.; Wiater, A.; Rogalski, J. Differences in production, composition, and antioxidant activities of exopolymeric substances (EPS) obtained from cultures of endophytic Fusarium culmorum strains with different effects on cereals. Molecules 2020, 25, 616. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Pacholak, A.; Gao, Z.L.; Gong, X.Y.; Kaczorek, E.; Cui, Y.W. The metabolic pathways of polyhydroxyalkanoates and exopolysaccharides synthesized by Haloferax mediterranei in response to elevated salinity. J. Proteom. 2021, 232, 104065. [Google Scholar] [CrossRef]
- Kornmann, H.; Duboc, P.; Marison, I.; von Stockar, U. Influence of nutritional factors on the nature, yield, and composition of exopolysaccharides produced by Gluconacetobacter xylinus I-2281. Appl. Environ. Microb. 2003, 69, 6091–6098. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, U.; Sulyok, M.; Wiwart, M.; Suchowilska, E.; Giedrojć, W.; Gontarz, D.; Kandler, W.; Krska, R. Secondary metabolites of pathogenic fungi in Triticum durum grain protected with Debaryomyces hansenii in two different locations in Poland. Agronomy 2023, 13, 721. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Winiarczyk, K.; Baturo, A.; Łukanowski, A. Colonization of root tissues and protection against fusarium wilt of rye (Secale cereale) by nonpathogenic rhizosphere strains of Fusarium culmorum. Biol. Control. 2008, 45, 297–307. [Google Scholar] [CrossRef]
- Frąc, M.; Kaczmarek, J.; Jędryczka, M. Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the causal agents of phoma leaf spotting and stem canker of oilseed rape (Brassica napus) in agricultural ecosystems. Pathogens 2022, 11, 50. [Google Scholar] [CrossRef]
- Almeida, F.B.; Rodrigues, L.M.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Roodi, D.; Millner, J.P.; McGill, C.R.; Johnson, R.D.; Hea, S.-Y.; Brookes, J.J.; Glare, T.R.; Card, S.D. Development of plant–fungal endophyte associations to suppress phoma stem canker in Brassica. Microorganisms 2021, 9, 2387. [Google Scholar] [CrossRef] [PubMed]
- Borhan, M.H.; Van de Wouw, A.P.; Larkan, N.J. Molecular interactions between Leptosphaeria maculans and Brassica species. Annu. Rev. Phytopathol. 2022, 60, 237–257. [Google Scholar] [CrossRef]
- Fitt, B.D.; Huang, Y.-J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Phytopathology 2006, 44, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.; Jedryczka, M. Characterization of two coexisting pathogen populations of Leptosphaeria spp., the cause of stem canker of brassicas. Acta Agrobot. 2011, 64, 3–14. [Google Scholar] [CrossRef]
- West, J.S.; Balesdent, M.H.; Rouxel, T.; Narcy, J.P.; Huang, Y.J.; Roux, J.; Steed, J.M.; Fitt, B.D.L.; Schmit, J. Colonization of winter oilseed rape tissues by ATox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathol. 2002, 51, 311–321. [Google Scholar] [CrossRef]
- Huang, Y.J.; Fitt, B.D.L.; Jedryczka, M.; Dakowska, S.; West, J.S.; Gladders, P.; Steed, J.M.; Li, Z.Q. Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). Eur. J. Plant Pathol. 2005, 111, 263–277. [Google Scholar] [CrossRef]
- Jedryczka, M.; Fitt, B.D.L.; Kachlicki, P.; Lewartowska, E.; Balesdent, M.H.; Rouxel, T. Comparison between Polish and United Kingdom populations of Leptosphaeria maculans, cause of stem canker of winter oilseed rape. J. Plant Dis. Prot. 1999, 106, 608–617. [Google Scholar]
- Bagi, B.; Csaba, N.; Tóth, A.; Palkovics, L.; Petróczy, M. Plenodomus biglobosus on oilseed rape in Hungary. Phytopathol Mediterr. 2020, 59, 345–351. [Google Scholar] [CrossRef]
- King, K.M.; West, J.S. Detection of the Phoma pathogens Plenodomus biglobosus subclades ‘brassicae’ and ‘canadensis’ on wasabi, and ‘canadensis’ in Europe. Eur. J. Plant Pathol. 2022, 162, 751–756. [Google Scholar] [CrossRef]
- Voigt, K.; Jędryczka, M.; Wöstemeyer, J. Strain typing of Polish Leptosphaeria maculans isolates supports at the genomic level the multi-species concept of aggressive and non-aggressive strains. Microbiol. Res. 2001, 156, 169–177. [Google Scholar] [CrossRef]
- Kachlicki, P.; Stobiecki, M.; Jędryczka, M. The benzoic acid—The phytotoxic metabolite of Tox0 strain of the fungus Phoma lingam. Oilseed Crop. 1996, 17, 193–198. [Google Scholar]
- Balesdent, M.H.; Gall, C.; Robin, P.; Rouxel, T. Intraspecific variation in soluble mycelia protein and esterase patterns of Leptosphaeria maculans French isolates. Mycol. Res. 1992, 96, 677–684. [Google Scholar] [CrossRef]
- Sippell, D.W.; Hall, R. Glucose phosphate isomerase polymorphisms distinguish weakly virulent from highly virulent-strains of Leptosphaeria maculans. Can. J. Plant Pathol. 1995, 17, 1–6. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Hall, R.; Goodwin, P.H. Co-infection and induction of systemic acquired resistance by weakly and highly virulent isolates of Leptosphaeria maculans in oilseed rape. Physiol. Mol. Plant Pathol. 1996, 49, 61–72. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, Z.; Fitt, B.D.L.; Evans, N.; Foster, S.J.; Huang, Y.J.; Latunde-Dada, A.O.; Lucas, J.A. Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathol. 2006, 55, 401–412. [Google Scholar] [CrossRef]
- Jędryczka, M.; Burzyński, A.; Brachaczek, A.; Langwiński, W.; Song, P.; Kaczmarek, J. Loop-mediated isothermal amplification as a good tool to study changing Leptosphaeria populations in oilseed rape plants and air samples. Acta Agrobot. 2014, 67, 93–100. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Stonard, J.F.; Howlett, B.J.; West, J.S.; Fitt, B.D.L.; Atkins, S.D. Determining frequencies of avirulent alleles in airborne Leptosphaeria maculans inoculum using quantitative PCR. Plant Pathol. 2010, 59, 809–818. [Google Scholar] [CrossRef]
- Jacques, N.; Balesdent, M.; Rouxel, T.; Laval, V. New specific quantitative real-time PCR assays shed light on the epidemiology of two species of the Leptosphaeria maculans–Leptosphaeria biglobosa species complex. Plant Pathol. 2021, 70, 643–654. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Jędryczka, M.; Fitt, B.D.L.; Lucas, J.A.; Latunde-Dada, A.O. Analyses of air samples for ascospores of Leptosphaeria maculans and L. biglobosa with light microscopic and molecular techniques. J. Appl. Genet. 2009, 50, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.; Jedryczka, M.; Cools, H.J.; Fitt, B.D.; Lucas, J.A.; Latunde-Dada, A.O. Quantitative PCR analysis of abundance of airborne propagules of Leptosphaeria species in air samples from different regions of Poland. Aerobiologia 2012, 28, 199–212. [Google Scholar] [CrossRef]
- Padmathilake, K.R.E.; Fernando, W.G.D. Less Virulent Leptosphaeria biglobosa immunizes the canola plant to resist highly virulent L. maculans, the blackleg pathogen. Plants 2022, 11, 996. [Google Scholar] [CrossRef]
- Stachowiak, A.; Olechnowicz, J.; Jedryczka, M.; Rouxel, T.; Balesdent, M.H.; Happstadius, I.; Gladders, P.; Latunde-Dada, A.; Evans, N. Frequency of avirulence alleles in field populations of Leptosphaeria maculans in Europe. Eur. J. Plant Pathol. 2006, 114, 67–75. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Louvard, K.; Pinochet, X.; Rouxel, T. A large-scale survey of races of Leptosphaeria maculans occurring on oilseed rape in France. In Sustainable Strategies for Managing Brassica napus (Oilseed Rape) Resistance to Leptosphaeria maculans (Phoma Stem Canker); Fitt, B.D.L., Evans, N., Howlett, B.J., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E. Hydrolysis of fungal and plant cell walls by enzymatic complexes from cultures of Fusarium isolates with dierent aggressiveness to rye (Secale cereale). Arch. Microbiol. 2012, 194, 653–665. [Google Scholar] [CrossRef]
- Wolska-Mitaszko, B.; Jaroszuk-Ściseł, J.; Pszeniczna, K. Isoforms of trehalase and invertase of Fusarium oxysporum. Mycol. Res. 2007, 111, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTKZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.A.S.I.; Ahmed, M.F.; Parthasarathi, N. A comparative study on the protease producing bacteria isolated from dairy effluents of Chennai region, identification, characterization, and application of enzyme in detergent formulation. Asian J. Microbiol. Biotechnol. Environ. Sci. 2014, 16, 41–46. [Google Scholar]
- Mahdi, S.; Mohd, N.; Arbakariya, A.; Rosfarizan, M. Screening, isolation and selection of cellulolytic fungi from oil palm empty fruit bunch fibre. Biotechnology 2011, 10, 108–113. [Google Scholar] [CrossRef]
- Kurek, E.; Niedźwiedzki, E.; Protasowicki, M.; Słomka, A.; Ozimek, E. The effects of biofertilizer “Juwei” C.B.I. produced in China on growth and yield of maize cultivated on sandy soils in western Pomerania. Soil Sci. Ann. 2004, 55, 121–128. [Google Scholar]
- Khokhar, I.; Mukhtar, I.; Mushtaq, S. Isolation and screening of amylolytic filamentous fungi. J. Appl. Sci. Environ. Manag. 2011, 15, 203. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Salkowski, E. Ueber das verhalten der skatolcarbonsaüre im organismus. Z. Physiol. Chem. 1885, 9, 23–33. [Google Scholar] [CrossRef]
- Yadav, K.L.; Rahi, D.K.; Soni, S.K. An indigenous hyperproductive species of Aureobasidium pullulans RYLF-10: Influence of fermentation conditions on exopolysaccharide (EPS) production. Appl. Biochem. Biotechnol. 2014, 172, 1898–1908. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Fenice, M.; Federici, F.; Petruccioli, M. Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res. Microbiol. 2002, 153, 585–592. [Google Scholar] [CrossRef]
- Orlandelli, R.C.; Vasconcelos, A.F.D.; Azevedo, J.L.; da Silva, M.d.L.C.; Pamphile, J.A. Screening of endophytic sources of exopolysaccharides: Preliminary characterization of crude exopolysaccharide produced by submerged culture of Diaporthe sp. JF766998 under different cultivation time. Biochim. Open 2016, 2, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.; Brachaczek, A.; Jedryczka, M. Concentration of ascospores of Leptosphaeria maculans and L. biglobosa in the region of Wielkopolska in autumn 2011–2013. Prog. Plant Prot. 2015, 55, 20–24. [Google Scholar] [CrossRef]
- Mendes-Pereira, E.; Balesdent, M.H.; Brun, H.; Rouxel, T. Molecular phylogeny of the Leptosphaeria maculans-L. biglobosa species complex. Mycol. Res. 2003, 107, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Furtado, B.U.; Szymańska, S.; Hrynkiewicz, K. A Window into fungal endophytism in Salicornia europaea: Deciphering fungal characteristics as plant growth promoting agents. Plant Soil 2019, 445, 577–594. [Google Scholar] [CrossRef]
- Abe, C.A.L.; Faria, C.B.; de Castro, F.F.; de Souza, S.R.; dos Santos, F.C.; da Silva, C.N.; Tessmann, D.J.; Barbosa-Tessmann, I.P. Fungi isolated from maize (Zea mays L.) grains and production of associated enzyme activities. Int. J. Mol. Sci. 2015, 16, 15328–15346. [Google Scholar] [CrossRef]
- Hai, Y.; Jenner, M.; Tang, Y. Fungal siderophore biosynthesis catalysed by an iterative nonribosomal peptide synthetase. Chem. Sci. 2020, 11, 11525–11530. [Google Scholar] [CrossRef]
- Pecoraro, L.; Wang, X.; Shah, D.; Song, X.; Kumar, V.; Shakoor, A.; Tripathi, K.; Ramteke, P.W.; Rani, R. Biosynthesis pathways, transport mechanisms and biotechnological applications of fungal siderophores. J. Fungi 2021, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Kurek, E.; Trytek, M. Efficiency of indoleacetic acid, gibberellic acid and ethylene synthesized in vitro by Fusarium culmorum strains with different effects on cereal growth. Biologia 2014, 69, 281–292. [Google Scholar] [CrossRef]
- Voigt, K.; Cozijnsen, A.J.; Kroymann, J.; Pöggeler, S.; Howlett, B.J. Phylogenetic relationships between members of the crucifer pathogenic Leptosphaeria maculans species complex as shown by mating type (MAT1-2), actin, and β-tubulin sequences. Mol. Phylogenet. Evol. 2005, 37, 541–557. [Google Scholar] [CrossRef]
- Rouxel, T.; Balesdent, M.H. The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Mol. Plant Pathol. 2005, 6, 225–241. [Google Scholar] [CrossRef]
- Cabras, A.; Mannoni, M.A.; Serra, S.; Andolfi, A.; Fiore, M.; Evidente, A. Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic of Pinus radiata. Eur. J. Plant Pathol. 2006, 115, 187–193. [Google Scholar] [CrossRef]
- Nowak, A.; Tyśkiewicz, R.; Wiater, A.; Jaroszuk-Ściseł, J. (1→3)-α-d-glucooligosaccharides as elicitors influencing the activity of plant resistance pathways in wheat tissues. Agronomy 2022, 12, 1170. [Google Scholar] [CrossRef]
- Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A report on fungal (1→3)-α-d-glucans: Properties, functions and application. Molecules 2019, 24, 3972. [Google Scholar] [CrossRef]
- Kim, P.D.; Šašek, V.; Burketová, L.; Čopíková, J.; Synytsya, A.; Jindřichová, B.; Valentová, O. Cell Wall Components of Leptosphaeria maculans Enhance Resistance of Brassica napus. J. Agric. Food Chem. 2013, 61, 5207–5214. [Google Scholar] [CrossRef]
- Ghozlan, M.H.; Eman, E.-A.; Tokgöz, S.; Lakshman, D.K.; Mitra, A. Plant defense against necrotrophic pathogens. J. Plant Sci. 2020, 11, 2122–2138. [Google Scholar] [CrossRef]
- Ullah, C.; Schmidt, A.; Reichelt, M.; Tsai, C.J.; Gershenzon, J. Lack of antagonism between salicylic acid and jasmonate signalling pathways in poplar. New Phytol. 2022, 235, 701–717. [Google Scholar] [CrossRef] [PubMed]






| No. | Cultivar Name | Type of Variety and Resistance | Breeder | Year of Introduction to NLI APV 1 |
|---|---|---|---|---|
| 1 | Birdy | open pollinated | KWS Momont Recherche SARL | 2016 |
| 2 | Bono | HR Smolice, Grupa IHAR | 2020 | |
| 3 | Californium | Monsanto Technology LLC | 2002/2021 * | |
| 4 | Gemini | HR Strzelce, Grupa IHAR | 2019 | |
| 5 | SY Ilona | Syngenta Participations AG | 2016 | |
| 6 | Absolut F1 | hybrid, Rlm7 | Limagrain Europe S.A.S. | 2018 |
| 7 | LG Anarion F1 | Limagrain Europe S.A.S. | 2020 | |
| 8 | LG Areti F1 | Limagrain Europe S.A.S. | 2020 | |
| 9 | Luciano KWS F1 | KWS Saat SE & Co. KGaA | 2019 | |
| 10 | Dominator F1 | hybrid, APR37 (RlmS) | Deutsche Saatveredelung AG (DSV) | 2019 |
| 11 | Akilah F1 | Deutsche Saatveredelung AG (DSV) | 2020 | |
| 12 | Kicker F1 | Norddeutsche Pflanzenzucht Hans-Georg Lembke KG (NPZ) | 2017 |
| Plenodomus lingam | Plenodomus biglobosus | ||||
|---|---|---|---|---|---|
| Highest EPS Yield | Lowest EPS Yield | Highest EPS Yield | Lowest EPS Yield | ||
| No. | Cultivar Name | PLIGR3 | PLIGR2 | PBIGR2 | PBIGR3 |
| 1 | Birdy | 3.50 ± 0.51 | 4.05 ± 0.39 | 4.00 ± 0.34 | 2.00 ± 0.56 |
| 2 | Bono | 4.58 ± 0.51 | 4.58 ± 0.51 | 2.00 ± 0.65 | 2.00 ± 0.32 |
| 3 | Californium | 4.00 ± 0.32 | 4.70 ± 0.80 | 2.00 ± 0.34 | 2.00 ± 0.33 |
| 4 | Gemini | 4.00 ± 0.32 | 3.00 ± 0.32 | 2.00 ± 0.65 | 2.00 ± 0.00 |
| 5 | SY Ilona | 4.16 ± 0.38 | 3.70 ± 0.57 | 2.00 ± 0.33 | 2.00 ± 0.47 |
| 6 | Absolut F1 | 2.15 ± 0.49 | 1.30 ± 0.80 | 3.57 ± 0.51 | 3.40 ± 0.50 |
| 7 | LG Anarion F1 | 1.00 ± 0.46 | 1.33 ± 0.91 | 3.68 ± 1.03 | 3.25 ± 0.44 |
| 8 | LG Areti F1 | 1.50 ± 0.51 | 1.00 ± 0.34 | 3.68 ± 0.49 | 2.00 ± 0.84 |
| 9 | Luciano KWS F1 | 1.58 ± 0.84 | 1.30 ± 0.92 | 3.40 ± 0.60 | 3.50 ± 0.69 |
| 10 | Dominator F1 | 3.00 ± 0.46 | 2.30 ± 0.57 | 3.50 ± 0.51 | 2.50 ± 0.61 |
| 11 | Akilah F1 | 3.56 ± 0.51 | 3.00 ± 0.32 | 4.20 ± 0.70 | 5.00 ± 0.46 |
| 12 | Kicker F1 | 2.84 ± 0.96 | 3.60 ± 0.82 | 4.50 ± 0.76 | 3.50 ± 0.69 |
| Total score | 35.89 | 33.86 | 38.50 | 33.15 | |
| Fungal Strains | Growth Rate Ratio (ΔT) (mm/Day) | ||||
|---|---|---|---|---|---|
| A. Siderophores (CAS Agar) | B. Amylolytic (AM Agar) | C. Cellulolytic (CMC Agar) | D. Phosphate Solubilisation (PS Agar) | E. Proteolytic (SM Agar) | |
| PLIGR1 | 0.035 ± 0.003 | 0.240 ± 0.01 | 0.492 ± 0.08 | 3.377 ± 0.35 | 6.503 ± 0.35 |
| PLIGR2 | 0.035 ± 0.004 | 0.726 ± 0.03 | 0.407 ± 0.01 | 1.587 ± 0.11 | 7.246 ± 0.31 |
| PLIGR3 | 0.035 ± 0.002 | 0.240 ± 0.02 | 0.407 ± 0.02 | 1.266 ± 0.19 | 5.458 ± 0.12 |
| PBIGR1 | 0.467 ± 0.09 | 0.570 ± 0.02 | 0.116 ± 0.01 | 7.305 ± 0.29 | 12.486 ± 0.4 |
| PBIGR2 | 0.407 ± 0.03 | 0.361 ± 0.03 | 0.407 ± 0.02 | 5.289 ± 0.22 | 5.128 ± 0.17 |
| PBIGR3 | 0.790 ± 0.02 | 0.361 ± 0.03 | 0.327 ± 0.02 | 5.524 ± 0.21 | 10.140 ± 0.65 |
| Fungal Strains | Efficiency of Activity | ||||
|---|---|---|---|---|---|
| A. Siderophores (CAS Agar) | B. Amylolytic (AM Agar) | C. Cellulolytic (CMC Agar) | D. Phosphate Solubilisation (PS Agar) | E. Proteolytic (SM Agar) | |
| PLIGR1 | +++ | ++ | ++ | − | − |
| PLIGR2 | +++ | + | ++ | − | − |
| PLIGR3 | +++ | ++ | ++ | − | − |
| PBIGR1 | +++++ | +++ | ++++ | − | − |
| PBIGR2 | +++++ | ++ | +++ | − | − |
| PBIGR3 | ++++ | ++ | +++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Kutyła, M.; Kaczmarek, J.; Jaroszuk-Ściseł, J.; Jędryczka, M. Differences in the Production of Extracellular Polymeric Substances (EPS) and Other Metabolites of Plenodomus (Leptosphaeria) Infecting Winter Oilseed Rape (Brassica napus L.). Metabolites 2023, 13, 759. https://doi.org/10.3390/metabo13060759
Nowak A, Kutyła M, Kaczmarek J, Jaroszuk-Ściseł J, Jędryczka M. Differences in the Production of Extracellular Polymeric Substances (EPS) and Other Metabolites of Plenodomus (Leptosphaeria) Infecting Winter Oilseed Rape (Brassica napus L.). Metabolites. 2023; 13(6):759. https://doi.org/10.3390/metabo13060759
Chicago/Turabian StyleNowak, Artur, Mateusz Kutyła, Joanna Kaczmarek, Jolanta Jaroszuk-Ściseł, and Małgorzata Jędryczka. 2023. "Differences in the Production of Extracellular Polymeric Substances (EPS) and Other Metabolites of Plenodomus (Leptosphaeria) Infecting Winter Oilseed Rape (Brassica napus L.)" Metabolites 13, no. 6: 759. https://doi.org/10.3390/metabo13060759
APA StyleNowak, A., Kutyła, M., Kaczmarek, J., Jaroszuk-Ściseł, J., & Jędryczka, M. (2023). Differences in the Production of Extracellular Polymeric Substances (EPS) and Other Metabolites of Plenodomus (Leptosphaeria) Infecting Winter Oilseed Rape (Brassica napus L.). Metabolites, 13(6), 759. https://doi.org/10.3390/metabo13060759









