Protective Action Mechanisms of Launaea mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Collection
2.2. Extract Preparation
2.3. High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry Analysis (UPLC-qTOF-MS)
2.4. Biosynthesis of the Silver Nanoparticles of LMNS
2.5. UV–Vis Spectra and Transmission Electron Microscopy (TEM) Measurements of LMNS
2.6. Bioassays
2.6.1. Drugs and Chemicals
2.6.2. Acute Toxicity
2.6.3. Experimental Animals and Ethical Treatments
2.6.4. Experimental Design
2.6.5. Blood Collection and Tissue Preparation
2.6.6. Assays for Kidney Function
2.6.7. Biochemical Assessment of Renal Tissue
2.6.8. Histopathological Assays
Light Microscopic Examination
Immunohistochemical Examination
2.6.9. Statistical Analysis
3. Results
3.1. LME’ Phytochemical Profiling Using UPLC-QTOF–MS/MS
3.2. Identification of Hydroxycinnamic and Hydroxy Benzoic Acids
3.2.1. Identification of Coumarins
3.2.2. Identification of Flavonoids
3.2.3. Identification of Fatty and Organic Acids
3.3. Chemical Biosynthesis of LMNS
3.3.1. UV–Vis Spectroscopic Analysis of LMNS
3.3.2. TEM Results of LMNS
3.4. Biological Results
3.4.1. LD50 Assay’ Result
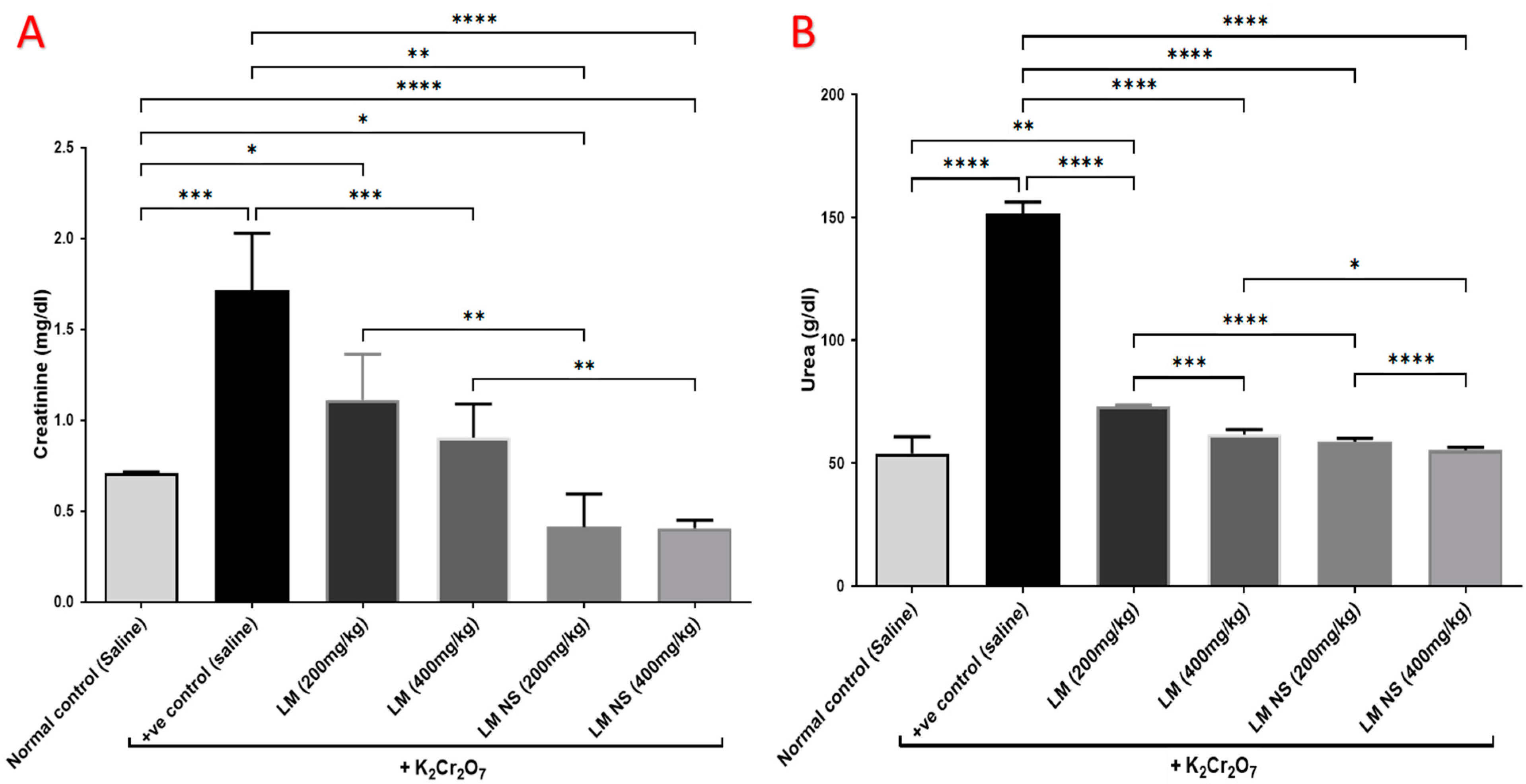
3.4.2. Effect on Urea and Creatinine
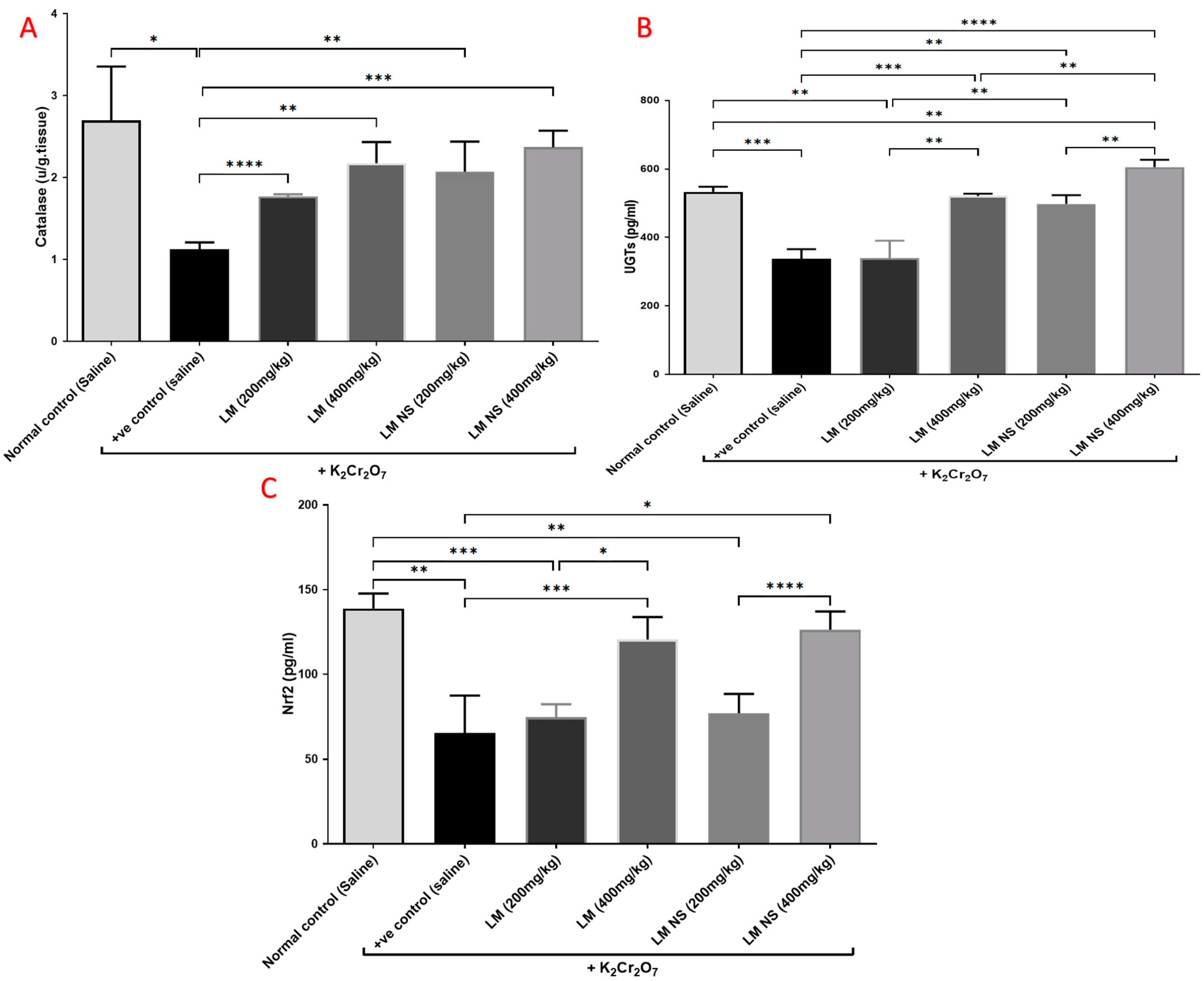
3.4.3. Effect on Catalyzing Enzymes (Catalase and UGTs) and Nrf2
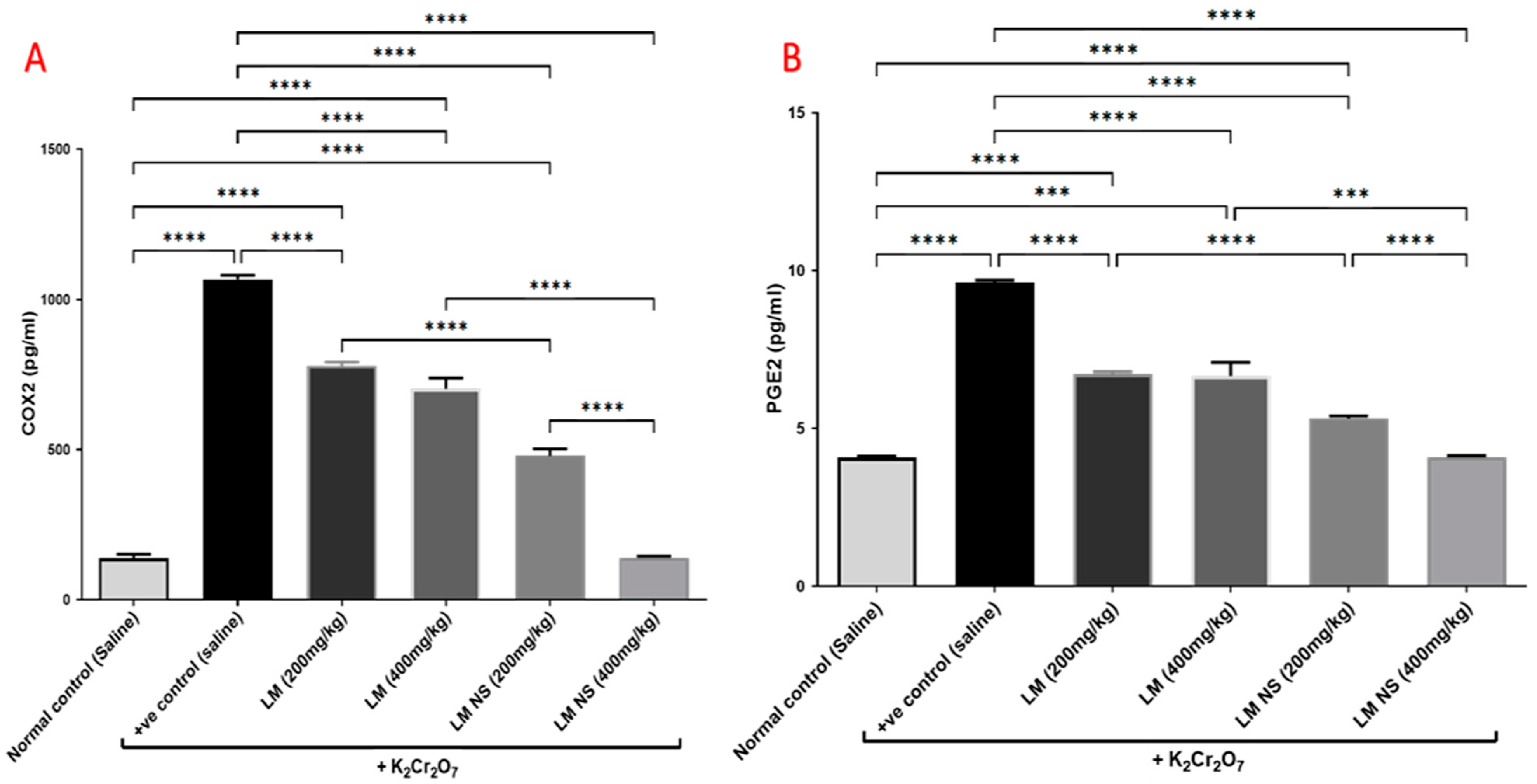
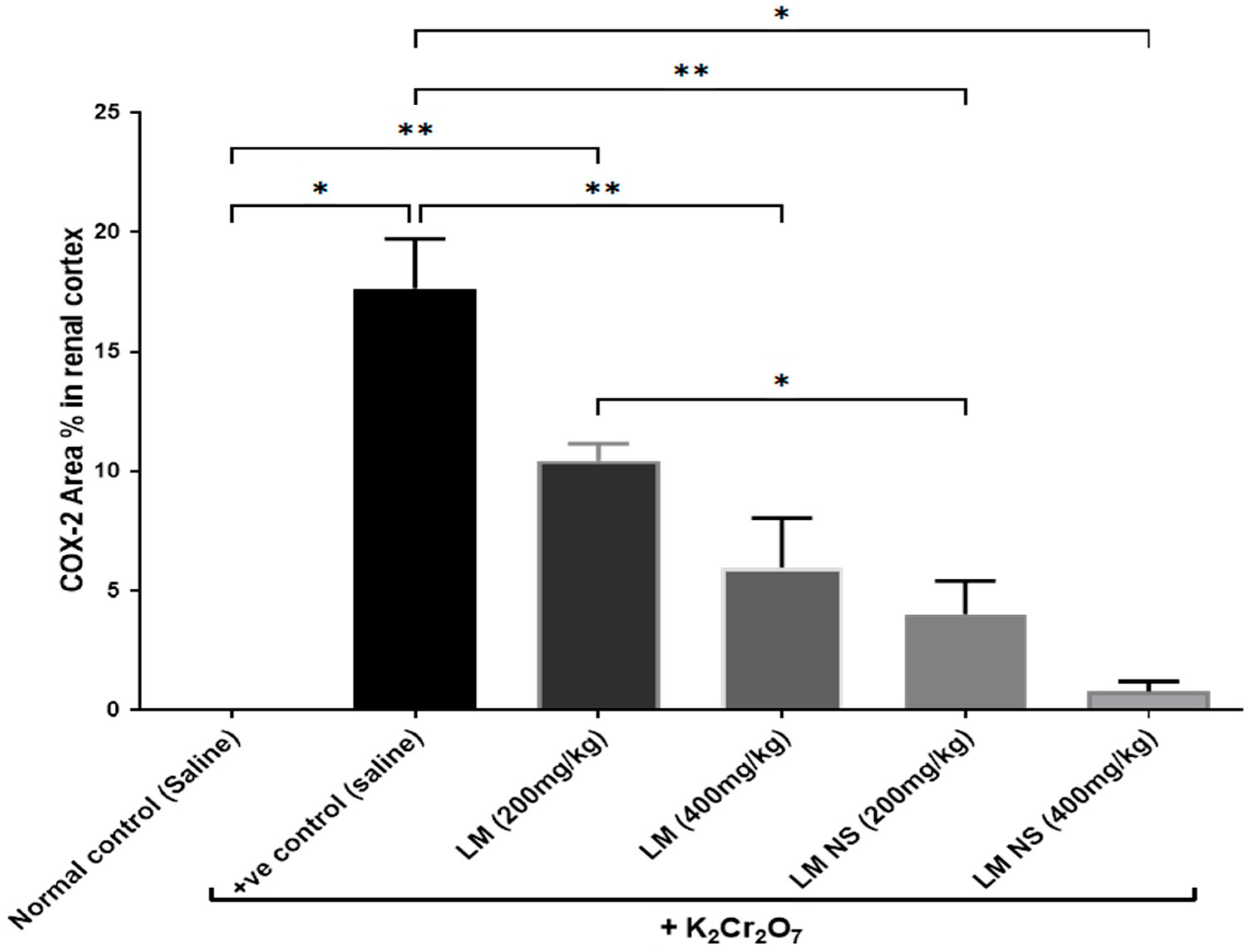
3.4.4. Effect on Inflammation Biomarkers (COX-2 and PGE2)
3.4.5. Effect on the MAPK/ERK Pathway
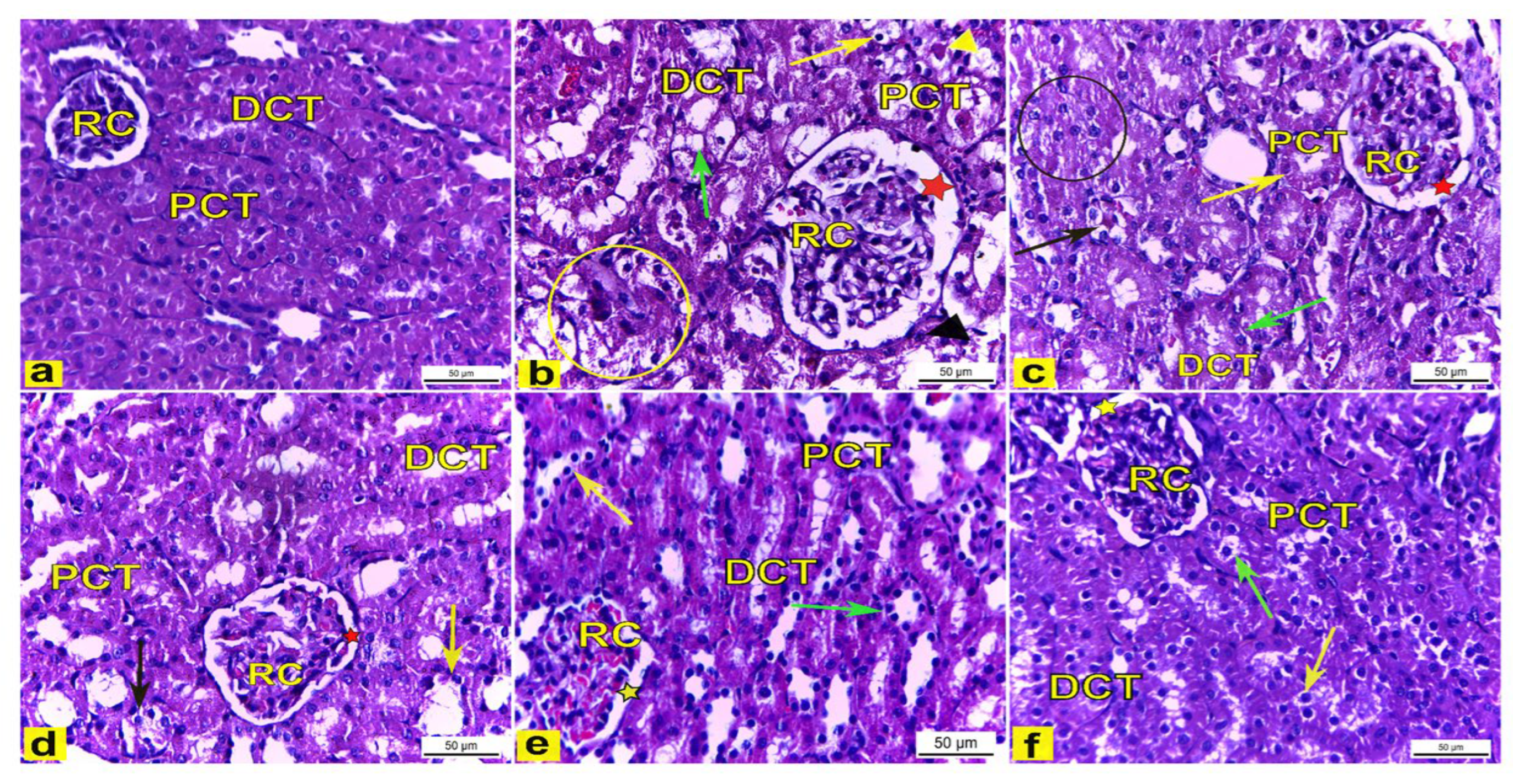
3.4.6. Histopathological Examination
Light Microscopic Observations
Immunohistochemical Examination of Cyclo-Oxygenase 2 (COX-2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Marzouk, R.I.; El-Darier, S.M.; Kamal, S.A.; Nour, I.H. Comparative Taxonomic Study of Launaea Cass. (Asteraceae, Cichorioideae) in Egypt. Taxonomy 2021, 1, 192–209. [Google Scholar] [CrossRef]
- Christensen, C.B.; Soelberg, J.; Stensvold, C.R.; Jäger, A.K. Activity of medicinal plants from Ghana against the parasitic gut protist Blastocystis. J. Ethnopharmacol. 2015, 174, 569–575. [Google Scholar]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Bokhari, J.; Rashid, U.; Jan, S. Phytotoxic characterization of various fractions of Launaea procumbens. Afr. J. Biotechnol. 2011, 10, 5377–5380. [Google Scholar]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Ali, F.; Rehman, M.U.; Tahir, M.; Sharma, S.; Sultana, S. Chrysin abrogates cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br. J. Nutr. 2012, 108, 1574–1585. [Google Scholar] [CrossRef]
- Makasana, A.; Ranpariya, V.; Desai, D.; Mendpara, J.; Parekh, V. Evaluation for the anti-urolithiatic activity of Launaea procumbens against ethylene glycol-induced renal calculi in rats. Toxicol. Rep. 2014, 1, 46–52. [Google Scholar] [CrossRef]
- Asif, M.; Mahrukh; Saadullah, M.; Yaseen, H.S.; Saleem, M.; Yousaf, H.M.; Khan, I.U.; Yaseen, M.; Shams, M.U. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology 2020, 28, 993–1008. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar]
- El-Sharkawy, E.R.; Mahmoud, K. Cytotoxity of two new coumarin derivatives isolated from Launaea mucronata. Nat. Prod. Res. 2016, 30, 394–398. [Google Scholar]
- Elshamy, A.I.; Abdallah, H.M.I.; Farrag, A.R.H.; Riciputi, Y.; Pasini, F.; Taher, R.F.; Raslan, M.A.; Paré, P.W.; Hegazy, M.-E.F. Artichoke Phenolics Confer Protection Against Acute Kidney Injury. Rev. Bras. Farmacogn. 2020, 30, 34–42. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar]
- Al-Naimi, M.; Rasheed, H.; Hussien, N.; Al-Kuraishy, H.; Al-Gareeb, A. Nephrotoxicity: Role and significance of renal biomarkers in the early detection of acute renal injury. J. Adv. Pharm. Technol. Res. 2019, 10, 95–99. [Google Scholar] [CrossRef]
- Ae Bashandy, S.; Salama, A.; Fayed, A.-H.M.; Omara, E.A.; El-Toumy, S.A.; Salib, J.Y. Protective effect of mandarin (Citrus reticulata) peel extract on potassium dichromate induced hepatotoxicity and nephrotoxicity in rats. Plant Arch. 2020, 20 (Suppl. 1), 2231–2242. [Google Scholar]
- Zheng, X.; Li, S.; Li, J.; Lv, Y.; Wang, X.; Wu, P.; Yang, Q.; Tang, Y.; Liu, Y.; Zhang, Z. Hexavalent chromium induces renal apoptosis and autophagy via disordering the balance of mitochondrial dynamics in rats. Ecotoxicol. Environ. Saf. 2020, 204, 111061. [Google Scholar]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Rosmarinus officinalis essential oil modulates renal toxicity and oxidative stress induced by potassium dichromate in rats. J. Trace Elem. Med. Biol. 2021, 67, 126791. [Google Scholar]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar]
- Gan, L.; Zhang, S.; Zhang, Y.; He, S.; Tian, Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep. Biochem. Biotechnol. 2018, 48, 582–588. [Google Scholar]
- Hassan, H.A.; Ayoub, I.M.; Ragab, T.I.M.; Afifi, S.M.; El-Gendy, A.E.-N.G.; Farrag, A.R.H.; Abd-ELGawad, A.M.; Farag, M.; Elshamy, A.; Ammar, N.M. Metabolomics approach of Symphyotrichum squamatum ethanol extract and its nano-Ag formulation protective effect on gastric ulcer via bio-chemical and pathological analyses. Biomarkers 2023, 28, 190–205. [Google Scholar]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.-W.; Kim, S.; Yun, Y.-S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar]
- Abdallah, H.M.I.; Ammar, N.M.; Abdelhameed, M.F.; Gendy, A.E.-N.G.E.; Ragab, T.I.M.; Abd-ElGawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective Mechanism of Acacia saligna Butanol Extract and Its Nano-Formulations against Ulcerative Colitis in Rats as Revealed via Biochemical and Metabolomic Assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Hammam, W.E.; El-Mahdy El-Tantawi, M.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: Evidence from in vivo and in silico studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Awoyomi, O.V.; Adeoye, Y.D.; Oyagbemi, A.A.; Ajibade, T.O.; Asenuga, E.R.; Gbadamosi, I.T.; Ogunpolu, B.S.; Falayi, O.O.; Hassan, F.O.; Omobowale, T.O. Luteolin mitigates potassium dichromate-induced nephrotoxicity, cardiotoxicity and genotoxicity through modulation of Kim-1/Nrf2 signaling pathways. Environ. Toxicol. 2021, 36, 2146–2160. [Google Scholar] [PubMed]
- Ragab, T.I.M.; Zoheir, K.M.A.; Mohamed, N.A.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Abdelhameed, M.F.; Farrag, A.R.H.; Elshamy, A.I. Cytoprotective potentialities of carvacrol and its nanoemulsion against cisplatin-induced nephrotoxicity in rats: Development of nano-encasulation form. Heliyon 2022, 8, e09198. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 0443102791. [Google Scholar]
- Cote, S.L. Currents protocols for light microscopy immunocytochemistry. In Immunohistochemistry II; Wiley and Sons Press: Hoboken, NJ, USA, 1993; pp. 147–168. Available online: https://cir.nii.ac.jp/crid/1572543024937756416 (accessed on 29 May 2023).
- Abib, B.; Afifi, S.M.; El-Din, M.G.S.; Farag, M.A. How do cultivar origin and stepwise industrial processing impact Sesamum indicum seeds’ metabolome and its paste and in relation to their antioxidant effects? A case study from the sesame industry. Food Chem. 2023, 420, 136134. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; Muncipinto, G.; Nicolotti, O.; Carrieri, A.; Rullo, M.; Stefanachi, A.; Leonetti, F.; Altomare, C. A twenty-year journey exploring coumarin-based derivatives as bioactive molecules. Front. Chem. 2022, 10, 1002547. [Google Scholar]
- Abdallah, H.; Farag, M.; Osman, S.; Kim, D.h.; Kang, K.; Pan, C.-H.; Abdel-Sattar, E. Isolation of major phenolics from Launaea spinosa and their protective effect on HepG2 cells damaged with t-BHP. Pharm. Biol. 2016, 54, 536–541. [Google Scholar] [CrossRef]
- Younis, I.Y.; Farag, M.A.; Elgamal, A.M.; Mohsen, E. Untargeted metabolites profiling of volatile and non-volatile components of Egyptian lotus (Nelumbo nucifera Gaertn.) using UHPLC/PDA/ESI-MS and solid-phase microextraction (SPME) GC/MS in relation to its antiaging and anti-inflammatory effects. Ind. Crops Prod. 2023, 197, 116561. [Google Scholar]
- Li, Z.-H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D.-J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Chen, L.-H.; Hu, Q.; Li, G.; Zhang, L.; Qin, L.-Q.; Zuo, H.; Xu, G. Dietary Intake and Biomarkers of α-Linolenic Acid and Mortality: A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2021, 8, 743852. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar]
- Kellerer, T.; Kleigrewe, K.; Brandl, B.; Hofmann, T.; Hauner, H.; Skurk, T. Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Are Associated with Diet, BMI, and Age. Front. Nutr. 2021, 8, 691401. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Urrutia, A.; Arregui, F.J. Effect of both protective and reducing agents in the synthesis of multicolor silver nanoparticles. Nanoscale Res. Lett. 2013, 8, 101. [Google Scholar] [CrossRef]
- Alhumaydhi, F.A.; Aljohani, A.S.M.; Elsharkawy, E.R. UPLC/ESI-MS Phytochemical Screening of Deverra tortuosa Haematological and Histopathological Studies and Streptozotocin-Induced Diabetes in Rat. Evid.-Based Complement. Altern. Med. 2021, 2021, 4718854. [Google Scholar] [CrossRef]
- Turkmen, R.; Demirel, H.H.; Akosman, M.; Fırat, F. An investigation of the effect of chlorogenic acid on potassium dichromate-induced oxidative stress in rats. Res. Sq. 2022, 1–27. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Briones-Herrera, A.; Medina-Campos, O.N.; Reyes-Fermín, L.M.; Martínez-Klimova, E.; León-Contreras, J.C.; Hernández-Pando, R.; Tapia, E.; Pedraza-Chaverri, J. Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem. Toxicol. 2020, 145, 111774. [Google Scholar] [CrossRef]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of Chromium-Induced Oxidative Stress by Combined Treatment of Selected Plant-Growth-Promoting Rhizobacteria and Earthworms via Modulating the Expression of Genes Related to Reactive Oxygen Species Metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 309. [Google Scholar]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef] [PubMed]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/ HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Theyab, A.; Al-Ghamdy, A.O.; Algahtani, M.; Mufti, A.H.; Alsharif, K.F.; Abdella, E.M.; Habotta, O.A.; Omran, M.M.; Lokman, M.S. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ. Sci. Pollut. Res. 2022, 29, 12208–12221. [Google Scholar]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 283. [Google Scholar]
- Nasrin, S.; Watson, C.J.W.; Bardhi, K.; Fort, G.; Chen, G.; Lazarus, P. Inhibition of UDP-Glucuronosyltransferase Enzymes by Major Cannabinoids and Their Metabolites. Drug Metab. Dispos. 2021, 49, 1081–1089. [Google Scholar] [CrossRef]
- Fan, Y.; Rong, Y.; Li, P.; Dong, W.; Zhang, D.; Zhang, L.; Cui, M. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem. Toxicol. 2013, 55, 172–181. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2016, 30, 299–308. [Google Scholar] [CrossRef]
- Meech, R.; Hu, D.G.; McKinnon, R.A.; Mubarokah, S.N.; Haines, A.Z.; Nair, P.C.; Rowland, A.; Mackenzie, P.I. The UDP-Glycosyltransferase (UGT) Superfamily: New Members, New Functions, and Novel Paradigms. Physiol. Rev. 2019, 99, 1153–1222. [Google Scholar] [CrossRef]
- Yueh, M.-F.; Tukey, R.H. Nrf2-Keap1 Signaling Pathway Regulates Human UGT1A1 Expression in Vitro and in Transgenic UGT1 Mice. J. Biol. Chem. 2007, 282, 8749–8758. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, H.; Li, S.; Han, B.; Liu, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Zhang, Z. Sulforaphane prevents chromium-induced lung injury in rats via activation of the Akt/GSK-3β/Fyn pathway. Environ. Pollut. 2020, 259, 113812. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.-L.; Shu, L.; Su, Z.-Y.; Kong, A.-N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Abd El-Twab, S.M. Gallic acid attenuates chromium-induced thyroid dysfunction by modulating antioxidant status and inflammatory cytokines. Environ. Toxicol. Pharmacol. 2016, 48, 225–236. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 inhibitors as promising anti-inflammatory agents: An update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar]
- Albarakati, A.J.A. Protocatechuic acid counteracts oxidative stress and inflammation in carrageenan-induced paw edema in mice. Environ. Sci. Pollut. Res. 2022, 29, 56393–56402. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K.; Mohamad, H.F.; Ahmad, A. Luteolin and apigenin derived glycosides from Alphonsea elliptica abrogate LPS-induced inflammatory responses in human plasma. J. Ethnopharmacol. 2021, 275, 114120. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef]
- Yin, F.; Yan, J.; Zhao, Y.; Guo, K.-J.; Zhang, Z.-L.; Li, A.-P.; Meng, C.-Y.; Guo, L. Bone marrow mesenchymal stem cells repair Cr (VI)-injured kidney by regulating mitochondria-mediated apoptosis and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2019, 176, 234–241. [Google Scholar]
- Wang, B.-J.; Sheu, H.-M.; Guo, Y.-L.; Lee, Y.-H.; Lai, C.-S.; Pan, M.-H.; Wang, Y.-J. Hexavalent chromium induced ROS formation, Akt, NF-κB, and MAPK activation, and TNF-α and IL-1α production in keratinocytes. Toxicol. Lett. 2010, 198, 216–224. [Google Scholar]
- Yoshitake, R.; Saeki, K.; Eto, S.; Shinada, M.; Nakano, R.; Sugiya, H.; Endo, Y.; Fujita, N.; Nishimura, R.; Nakagawa, T. Aberrant expression of the COX2/PGE2 axis is induced by activation of the RAF/MEK/ERK pathway in BRAFV595E canine urothelial carcinoma. Sci. Rep. 2020, 10, 7826. [Google Scholar]
- Salama, A.A.A.; Elgohary, R.; Fahmy, M.I. Protocatechuic acid ameliorates lipopolysaccharide-induced kidney damage in mice via downregulation of TLR-4-mediated IKBKB/NF-κB and MAPK/Erk signaling pathways. J. Appl. Toxicol. 2023, 1–11. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Kim, J.; Shin, J.Y.; Kang, H.J.; Jang, S. Il Effect of luteolin and apigenin on the production of IL-31 and IL-33 in lipopolysaccharides-activated microglia cells and their mechanism of action. Nutrients 2020, 12, 811. [Google Scholar] [PubMed]
- Hanan, E.L.; Mokhtar, M.D.; Alshymaa, O.H.; Ahmed, M.S.; Samaa, S.; Abd El-Fatah, M.D.; Heba, O.M.; Helmy, M.D. Structural Changes Induced by Potassium Dichromate in Renal Cortex of Adult Male Albino Rats and the Possible Protective Role of Selenium. Med. J. Cairo Univ. 2019, 87, 661–675. [Google Scholar] [CrossRef]
- Sahu, B.D.; Koneru, M.; Bijargi, S.R.; Kota, A.; Sistla, R. Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Biol. Interact. 2014, 223, 69–79. [Google Scholar] [PubMed]
- Salama, A.A.A.; Mostafa, R.E.; Elgohary, R. Effect of L-carnitine on potassium dichromate-induced nephrotoxicity in rats: Modulation of PI3K/AKT signaling pathway. Res. Pharm. Sci. 2022, 17, 153–163. [Google Scholar]
- Flores, A.; Pérez, J.M. Cytotoxicity, apoptosis, and in vitro DNA damage induced by potassium chromate. Toxicol. Appl. Pharmacol. 1999, 161, 75–81. [Google Scholar]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar]
- Cheng, D.; Li, H.; Zhou, J.; Wang, S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Food Funct. 2019, 10, 681–690. [Google Scholar]
- Veeren, B.; Bringart, M.; Turpin, C.; Rondeau, P.; Planesse, C.; Ait-Arsa, I.; Gimié, F.; Marodon, C.; Meilhac, O.; Gonthier, M.-P. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines 2021, 9, 358. [Google Scholar]
- Diniz, L.R.L.; Elshabrawy, H.A.; Souza, M.T.S.; Duarte, A.B.S.; Madhav, N.; de Sousa, D.P. Renoprotective Effects of Luteolin: Therapeutic Potential for COVID-19-Associated Acute Kidney Injuries. Biomolecules 2022, 12, 1544. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants 2021, 10, 1643. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, S.-B.; Yu, H.Y.; Kim, Y.H.; Kim, J. Antioxidant Efficacy of Esculetin against Tert-Butyl Hydroperoxide-Induced Oxidative Stress in HEK293 Cells. Curr. Issues Mol. Biol. 2022, 44, 5986–5994. [Google Scholar] [CrossRef]
- Sen, Z.; Weida, W.; Jie, M.; Li, S.; Dongming, Z.; Xiaoguang, C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine 2019, 57, 385–395. [Google Scholar]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A.; Rajanan, A.; Suresh, A.J.; Raut, P.S.; Kundu, S.; Sahu, B.D. Natural coumarins: Preclinical evidence-based potential candidates to alleviate diabetic nephropathy. Phytomedicine Plus 2022, 2, 100379. [Google Scholar]
- Basu, A.; Namporn, T.; Ruenraroengsak, P. Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 1611. [Google Scholar] [CrossRef]
- Mabrouk Zayed, M.M.; Sahyon, H.A.; Hanafy, N.A.N.; El-Kemary, M.A. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics 2022, 14, 1160. [Google Scholar] [CrossRef]
- Sánchez-Jaramillo, E.A.; Gasca-Lozano, L.E.; Vera-Cruz, J.M.; Hernández-Ortega, L.D.; Gurrola-Díaz, C.M.; Bastidas-Ramírez, B.E.; Vargas-Guerrero, B.; Mena-Enríquez, M.; Martínez-Limón, F.d.J.; Salazar-Montes, A.M. Nanoparticles Formulation Improves the Antifibrogenic Effect of Quercetin on an Adenine-Induced Model of Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 5392. [Google Scholar] [CrossRef]
- Saber, T.M.; Farag, M.R.; Cooper, R.G. Ameliorative effect of extra virgin olive oil on hexavalent chromium-induced nephrotoxicity and genotoxicity in rats. Rev. Méd. Vét. 2015, 166, 11–19. [Google Scholar]
- Stoev, S.D.; Grozeva, N.; Simeonov, R.; Borisov, I.; Hubenov, H.; Nikolov, Y.; Tsaneva, M.; Lazarova, S. Experimental cadmium poisoning in sheep. Exp. Toxicol. Pathol. 2003, 55, 309–314. [Google Scholar]
- Hegazy, R.; Salama, A.; Mansour, D.; Hassan, A. Renoprotective effect of lactoferrin against chromium-induced acute kidney injury in rats: Involvement of IL-18 and IGF-1 inhibition. PLoS ONE 2016, 11, e0151486. [Google Scholar]
- Salama, A.; Hegazy, R.; Hassan, A. Intranasal Chromium Induces Acute Brain and Lung Injuries in Rats: Assessment of Different Potential Hazardous Effects of Environmental and Occupational Exposure to Chromium and Introduction of a Novel Pharmacological and Toxicological Animal Model. PLoS ONE 2016, 11, e0168688. [Google Scholar] [CrossRef]





| No. | Rt. | m/z | [M−H]−/[M+H]+ | MS/MS | Error (ppm) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 0.951 | 191.0561 | C7H11O6− | 127 | −2.54 | Quinic acid |
| 2 | 1.003 | 133.0142 | C4H5O5− | 115 | 4.08 | Malic acid |
| 3 | 1.059 | 290.0881 | C11H16NO8− | 200, 128 | −8.11 | Deoxy-dehydro-N-acetyl neuraminic acid |
| 4 | 1.493 | 167.0197 | C4H7O7− | 124 | −8.18 | Tartaric acid |
| 5 | 2.569 | 163.0401 | C9H7O3− | 145, 119 | −1.42 | P-coumaric acid |
| 6 | 2.843 | 169.0142 | C7H5O5− | 125 | −2.05 | Gallic acid |
| 7 | 3.558 | 341.0878 | C15H17O9− | 179, 161 | −0.57 | Caffeoyl hexose |
| 8 | 7.426 | 315.0722 | C13H15O9− | 153 | −4.89 | Protocatechuic acid-O-hexoside |
| 9 | 7.565 | 137.0244 | C7H5O3− | 119 | −2.05 | p-hydroxy benzoic acid |
| 10 | 8.137 | 311.0409 | C13H11O9− | 179, 149 | −0.78 | Caffeoyl tartaric acid |
| 11 | 8.402 | 137.0244 | C7H6O3− | 93 | −0.6 | Salicylic acid |
| 12 | 8.537 | 353.0878 | C16H17O9− | 191 | −1.68 | Chlorogenic acid |
| 13 | 8.610 | 325.0929 | C15H17O8− | 191, 173 | −1.56 | Coumaroyl hexoside |
| 14 | 8.680 | 177.019 | C9H5O4− | 89 | −1.5 | Aesculetin |
| 15 | 8.710 | 339.0722 | C15H15O9− | 177 | −0.72 | Aesculin |
| 16 | 8.854 | 313.0929 | C14H17O8− | 179 | 0.35 | Unknown caffeic acid derivative |
| 17 | 9.353 | 179.035 | C9H7O4− | 135 | −1.76 | Caffeic acid |
| 18 | 9.722 | 515.0831 | C24H19O13− | 353 | −0.36 | Chlorogenic acid hexoside |
| 19 | 9.827 | 611.1607 | C27H31O16+ | 449, 287 | 2.56 | Luteolin- dihexoside |
| 20 | 10.134 | 595.1657 | C27H31O15+ | 449, 287 | 3.44 | Luteolin-O-rutinoside |
| 21 | 10.152 | 461.0725 | C21H17O12− | 285 | 0.11 | Luteolin-O-glucuronic acid |
| 22 | 10.277 | 447.0933 | C21H19O11− | 285 | 1.87 | Luteolin-O-hexoside |
| 23 | 10.291 | 443.1042 | C15H23O15− | 285 | 4.15 | Unknown luteolin derivative |
| 24 | 10.349 | 195.0652 | C10H11O4+ | 179 | 0.95 | Ferulic acid |
| 25 | 10.451 | 515.1195 | C25H23O12− | 353, 191 | 0.97 | Di-caffeoylquinic acid |
| 26 | 10.464 | 197.1172 | C11H16O3+ | 179, 133 | 1.64 | Unknown phenolic acid |
| 27 | 10.745 | 433.1129 | C21H21O10+ | 271 | 2.37 | Apigenin-O-hexoside |
| 28 | 10.670 | 445.0776 | C21H17O11− | 269 | −1.04 | Unknown apigenin derivative |
| 29 | 10.758 | 447.0922 | C21H19O11+ | 271 | 2.44 | Apigenin-O-glucuronic acid |
| 30 | 10.934 | 187.0976 | C9H15O4− | 143 | −0.09 | Nonanedioic acid |
| 31 | 11.004 | 211.0965 | C11H15O4+ | 179 | 8.02 | Unknown caffeic acid derivative |
| 32 | 11.129 | 227.1278 | C12H19O4+ | 209 | 1.26 | Hydroxy jasmonic acid |
| 33 | 11.618 | 461.1078 | C22H21O11+ | 271 | 1.6 | Unknown apigenin derivative |
| 34 | 11.823 | 285.0405 | C15H9O6− | 257, 199 | −2.58 | Luteolin |
| 35 | 11.889 | 571.0882 | C30H19O12− | 285 | −1.57 | Unknown Luteolin derivative |
| 36 | 12.182 | 209.1536 | C13H21O2+ | 173 | 1.47 | Tridecatrienoic acid |
| 37 | 12.515 | 269.0455 | C15H9O5− | 117 | −2.42 | Apigenin |
| 38 | 12.540 | 331.2479 | C18H35O5+ | 313 | 2.42 | Trihydroxy-octadecenoic acid |
| 39 | 12.549 | 337.0354 | C18H9O7− | 269 | 5.85 | Unknown apigenin derivative |
| 40 | 13.087 | 301.202 | C16H29O5− | 283 | 0.49 | Hydroxy hexadecanedioic acid |
| 41 | 13.22 | 287.2228 | C16H31O4− | 269 | −1.79 | Dihydroxy hexadecanoic acid |
| 42 | 13.782 | 941.5162 | C45H83O16P2− | - | 7.3 | Phosphatidylinositol phosphate (18:0/18:2) |
| 43 | 14.648 | 318.3003 | C18H40NO3+ | 303 | 1.8 | Amino octadecanetriol |
| 44 | 17.273 | 295.2268 | C18H31O3− | 277 | 1.6 | Hydroxy octadecdienoic acid |
| 45 | 19.08 | 271.2279 | C16H31O3− | 225 | −1.22 | Hydroxy-palmitic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Fadaly, A.A.; Younis, I.Y.; Abdelhameed, M.F.; Ahmed, Y.H.; Ragab, T.I.M.; El Gendy, A.E.-N.G.; Farag, M.A.; Elshamy, A.I.; Elgamal, A.M. Protective Action Mechanisms of Launaea mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses. Metabolites 2023, 13, 786. https://doi.org/10.3390/metabo13070786
El-Fadaly AA, Younis IY, Abdelhameed MF, Ahmed YH, Ragab TIM, El Gendy AE-NG, Farag MA, Elshamy AI, Elgamal AM. Protective Action Mechanisms of Launaea mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses. Metabolites. 2023; 13(7):786. https://doi.org/10.3390/metabo13070786
Chicago/Turabian StyleEl-Fadaly, Amany A., Inas Y. Younis, Mohamed F. Abdelhameed, Yasmine H. Ahmed, Tamer I. M. Ragab, Abd El-Nasser G. El Gendy, Mohamed A. Farag, Abdelsamed I. Elshamy, and Abdelbaset M. Elgamal. 2023. "Protective Action Mechanisms of Launaea mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses" Metabolites 13, no. 7: 786. https://doi.org/10.3390/metabo13070786
APA StyleEl-Fadaly, A. A., Younis, I. Y., Abdelhameed, M. F., Ahmed, Y. H., Ragab, T. I. M., El Gendy, A. E.-N. G., Farag, M. A., Elshamy, A. I., & Elgamal, A. M. (2023). Protective Action Mechanisms of Launaea mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses. Metabolites, 13(7), 786. https://doi.org/10.3390/metabo13070786









