Eco-Physiological Responses of Avicennia marina (Forssk.) Vierh. to Trace Metals Pollution via Intensifying Antioxidant and Secondary Metabolite Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Sampling
2.2. Assessment of Trace Metals in A. marina Root, Leaves, and Sediments
2.2.1. Geo-Accumulation Index (Igeo)
2.2.2. Contamination Factor (CF) and Pollution Load Index (PLI)
2.2.3. Biological Concentration Factor (BCF)
2.2.4. Translocation Factor (TF)
2.3. Phytochemical and Biochemical Assay
2.3.1. Extraction and Estimation of Pigments, Total Soluble Sugars, and Malondialdehyde
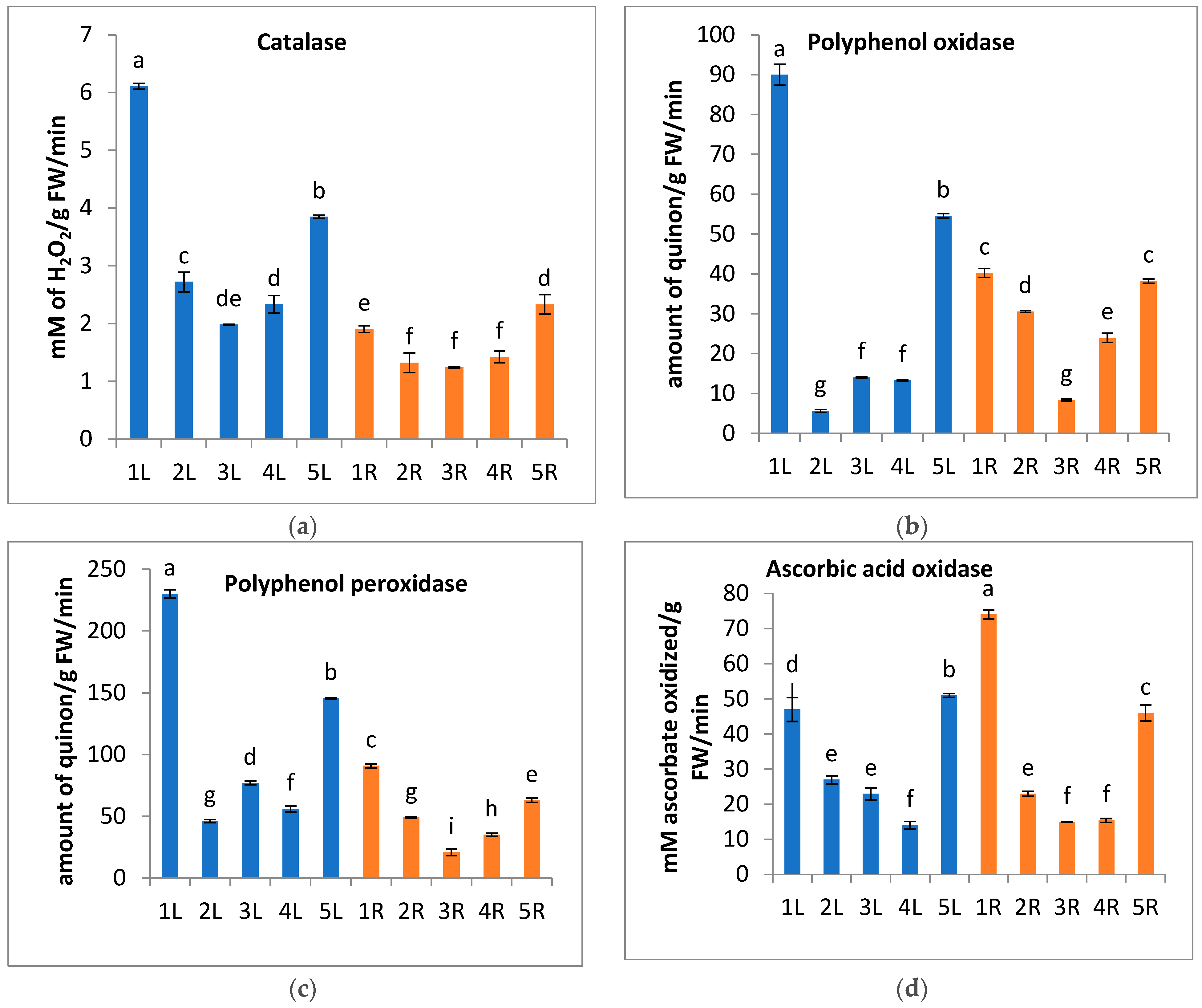
2.3.2. Extraction and Assaying Activity of Certain Enzymes
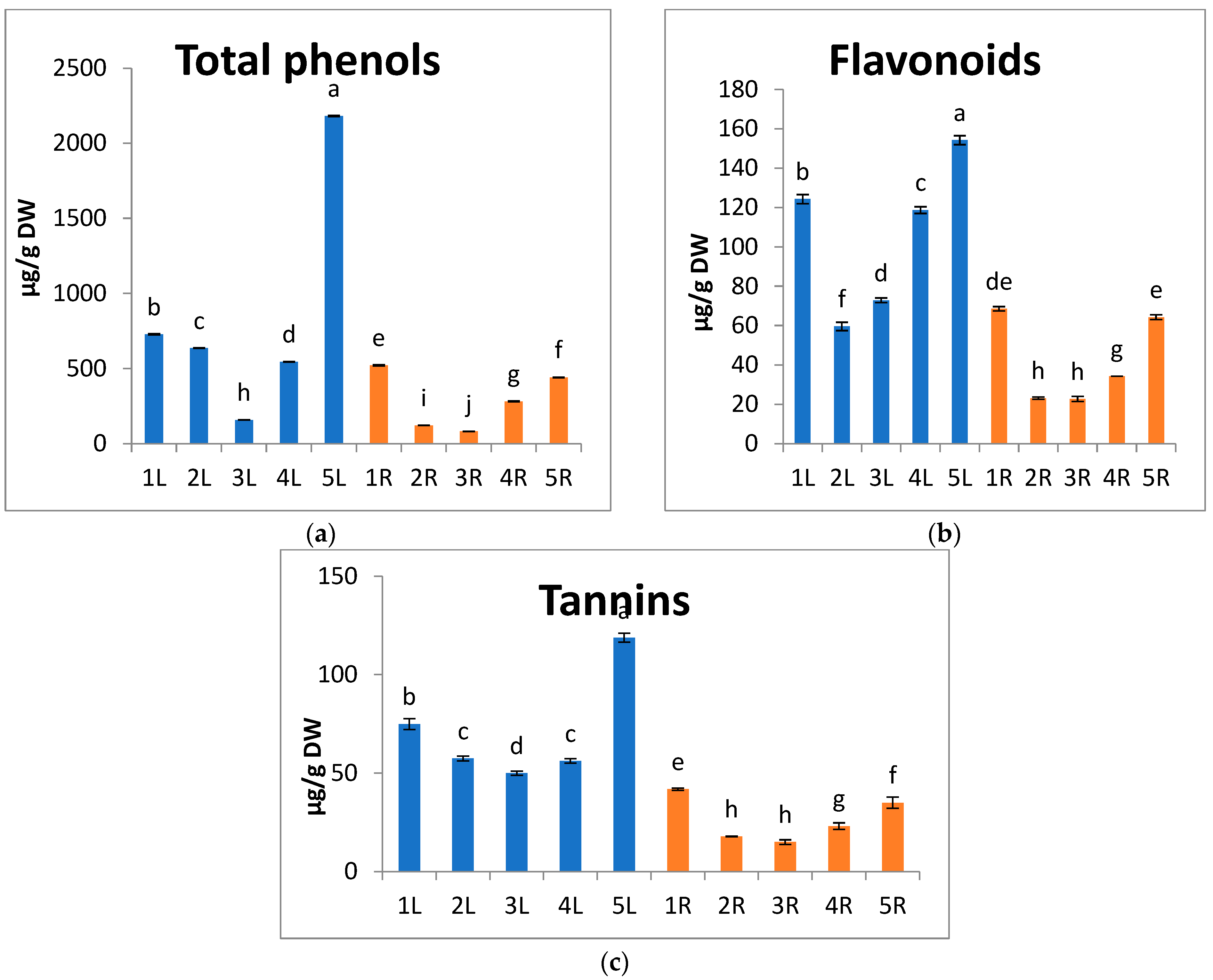
2.3.3. Extraction and Estimation of Total Phenols, Flavonoid, and Tannin Contents
2.3.4. Determination of Proline and Total Antioxidant Capacity (TAC)
3. Results and Discussion
3.1. Trace Metals Concentrations in A. marina Sediments
3.2. Assessment of Pollution Indicators: Geo-Accumulation Index (Igeo), Contamination Factor (CF), and Pollution Load Index (PLI)
3.3. Trace Metals Assessment in Roots and Leaves of A. marina (BCF and TF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semesi, A.K. Mangrove Management and Utilization in Eastern Africa. Ambio 1998, 27, 620–626. [Google Scholar]
- Afonso, F.; Félix, P.M.; Chainho, P.; Heumüller, J.A.; de Lima, R.F.; Ribeiro, F.; Brito, A.C. Assessing Ecosystem Services in Mangroves: Insights from São Tomé Island (Central Africa). Front. Environ. Sci. 2021, 9, 501673. [Google Scholar] [CrossRef]
- Bao, H.; Wu, Y.; Unger, D.; Du, J.; Herbeck, L.S.; Zhang, J. Impact of the Conversion of Mangroves into Aquaculture Ponds on the Sedimentary Organic Matter Composition in a Tidal Flat Estuary (Hainan Island, China). Cont. Shelf Res. 2013, 57, 82–91. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global Patterns in Mangrove Soil Carbon Stocks and Losses. Nat. Clim. Change 2017, 7, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Saderne, V.; Cusack, M.; Almahasheer, H.; Serrano, O.; Masqué, P.; Arias-Ortiz, A.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Duarte, C.M. Accumulation of Carbonates Contributes to Coastal Vegetated Ecosystems Keeping Pace with Sea Level Rise in an Arid Region (Arabian Peninsula). J. Geophys. Res. Biogeosci. 2018, 123, 1498–1510. [Google Scholar] [CrossRef] [Green Version]
- Brander, L.M.; Wagtendonk, A.J.; Hussain, S.S.; McVittie, A.; Verburg, P.H.; de Groot, R.S.; van der Ploeg, S. Ecosystem Service Values for Mangroves in Southeast Asia: A Meta-Analysis and Value Transfer Application. Ecosyst. Serv. 2012, 1, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Shaltout, K.H.; El-Bana, M.I.; Eid, E.M. Ecology of the Mangrove Forests along the Egyptian Red Sea Coast; LAP Lambert Academic Publishing: Sunnyvale, CA, USA, 2018; ISBN 978-613-9-95834-4. [Google Scholar]
- Salem, B.B.; Andersen, G.L.; Zahran, M.A. Remote Sensing and Vegetation Map of Egypt. In The Vegetation of Egypt; Zahran, M.A., Willis, A.J., Eds.; Plant and Vegetation; Springer: Dordrecht, The Netherlands, 2009; pp. 319–333. ISBN 978-1-4020-8756-1. [Google Scholar]
- Zahran, M.A.; Willis, A.J. The History of the Vegetation: Its Salient Features and Future Study. In The Vegetation of Egypt; Zahran, M.A., Willis, A.J., Eds.; Plant and Vegetation; Springer: Dordrecht, The Netherlands, 2009; pp. 305–318. ISBN 978-1-4020-8756-1. [Google Scholar]
- Afefe, A. Linking Territorial and Coastal Planning: Conservation Status and Management of Mangrove Ecosystem at the Egyptian-African Red Sea Coast. Aswan Univ. J. Environ. Stud. 2021, 2, 91–114. [Google Scholar] [CrossRef]
- Alzahrani, D.A.; Selim, E.-M.M.; El-Sherbiny, M.M. Ecological Assessment of Trace metals in the Grey Mangrove (Avicennia marina) and Associated Sediments along the Red Sea Coast of Saudi Arabia. Oceanologia 2018, 60, 513–526. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Khattab, R.A.; Ali, I.; Binnaser, Y.S.; Aqeel, A. Evaluation of the Trace metals Threat to the Yanbu Shoreline, Red Sea, Saudi Arabia. Mar. Freshw. Res. 2018, 69, 1557–1568. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Wu, H.; Ning, C.; Lin, G. Distribution and Ecological Risk Assessment of Trace metals in Surface Sediments of a Typical Restored Mangrove–Aquaculture Wetland in Shenzhen, China. Mar. Pollut. Bull. 2017, 124, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Afefe, A.A.; Abbas, M.S.; Soliman, A.S.; Khedr, A.-H.A.; Hatab, E.-B.E. Physical and Chemical Characteristics of Mangrove Soil under Marine Influence. A Case Study on the Mangrove Forests at Egyptian-African Red Sea Coast. Egypt. J. Aquat. Biol. Fish. 2019, 23, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Chakraborty, P.; Nath, B.N. Lead Distribution in Coastal and Estuarine Sediments around India. Mar. Pollut. Bull. 2015, 97, 36–46. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A. Monitoring Expression Profiles of Antioxidant Genes to Salinity, Iron, Oxidative, Light and Hyperosmotic Stresses in the Highly Salt Tolerant Grey Mangrove, Avicennia marina (Forsk.) Vierh. by MRNA Analysis. Plant Cell Rep. 2006, 25, 865–876. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B.; Zhang, Z. Environmental Factors Causing Stress in Avicennia marina Mangrove in Rabigh Lagoon Along the Red Sea: Based on a Multi-Approach Study. Front. Mar. Sci. 2021, 8, 646993. [Google Scholar] [CrossRef]
- Thatoi, H.N.; Patra, J.K.; Das, S.K. Free Radical Scavenging and Antioxidant Potential of Mangrove Plants: A Review. Acta Physiol. Plant. 2014, 36, 561–579. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Allah, A.K.; El-Banna, M. Environmental Characteristics of the Mangrove Sites along the Egyptian Red Sea Coast. 2005. Available online: https://www.researchgate.net/publication/280597380_Environmental_Characteristics_of_the_Mangrove_Sites_along_the_Egyptian_Red_Sea_Coast (accessed on 4 March 2022).
- El Daba, A.E.M.S.; Abd El Wahab, M. Geo-Environmental Study on Mangrove Swamps in Some Localities along the Red Sea Coast of Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Zahran, M.A. Climate-Vegetation: Afro-Asian Mediterranean and Red Sea Coastal Lands; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 978-90-481-8595-5. [Google Scholar]
- Atlas, W. Cairo, Egypt-Climate & Monthly Weather Forecast, Weather Atlas. Available online: https://www.weather-atlas.com/en/egypt/cairo-climate (accessed on 30 May 2022).
- Hamed, M.M.; Nashwan, M.S.; Shahid, S. Climatic Zonation of Egypt Based on High-Resolution Dataset Using Image Clustering Technique. Prog. Earth Planet. Sci. 2022, 9, 35. [Google Scholar] [CrossRef]
- Libes, S.M. Introduction to Marine Biogeochemistry; Elsevier/Academic: Amsterdam, The Netherlands, 2009; ISBN 978-0-12-088530-5. [Google Scholar]
- El-Sorogy, A.; Al-Kahtany, K.; Youssef, M.; Al-Kahtany, F.; Al-Malky, M. Distribution and Metal Contamination in the Coastal Sediments of Dammam Al-Jubail Area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2018, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 1st ed.; American Public Health Association: Washington, DC, USA, 2005; ISBN 978-0-87553-047-5. [Google Scholar]
- Muller, G. Trace Metals in the Sediment of the Rhine-Changesseity. Umsch. Wiss. Available online: https://www.scienceopen.com/document?vid=4b875795-5729-4c05-9813-64951e2ca488 (accessed on 6 March 2023).
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. GSA Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Geochemical Evolution of the Continental Crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control: A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Mountouris, A.; Voutsas, E.; Tassios, D. Bioconcentration of Trace metals in Aquatic Environments: The Importance of Bioavailability. Mar. Pollut. Bull. 2002, 44, 1136–1141. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Lee, S.S.; Awad, Y.M.; Lim, K.J.; Yang, J.E.; Ok, Y.S. Soil Pollution Assessment and Identification of Hyperaccumulating Plants in Chromated Copper Arsenate (CCA) Contaminated Sites, Korea. Chemosphere 2012, 87, 872–878. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Prud’homme, M.-P.; Gonzalez, B.; Billard, J.-P.; Boucaud, J. Carbohydrate Content, Fructan and Sucrose Enzyme Activities in Roots, Stubble and Leaves of Ryegrass (Lolium perenne L.) as Affected by Source/Sink Modification after Cutting. J. Plant Physiol. 1992, 140, 282–291. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in Anthrone Method for Determination of Carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-Induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An Update on Magnesium Homeostasis Mechanisms in Plants. Metallomics 2013, 5, 1170. [Google Scholar] [CrossRef] [PubMed]
- Reyhan, K.; Matpan Bekler, F.; Acer, O.; Alkan, H.; Dogru, M. Purification and Characterization of Polyphenol Oxidase from Purslane. Food Sci. Technol. 2017, 37, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koricheva, J.; Roy, S.; Vranjic, J.A.; Haukioja, E.; Hughes, P.R.; Hänninen, O. Antioxidant Responses to Simulated Acid Rain and Heavy Metal Deposition in Birch Seedlings. Environ. Pollut. 1997, 95, 249–258. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology: A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Harborne, A.J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Springer: London, UK; New York, NY, USA, 1998; ISBN 978-0-412-57270-8. [Google Scholar]
- Ejikeme, C.M.; Ezeonu, C.S.; Eboatu, A.N. Determination of physical and phytochemical constituents of some tropical timbers indigenous to Niger Delta area of Nigeria. Eur. Sci. J. ESJ 2014, 10, 247–270. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Alharbi, T.; Alfaifi, H.; El-Sorogy, A. Metal Pollution in Al-Khobar Seawater, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2017, 119, 407–415. [Google Scholar] [CrossRef]
- Nour, H.; Abd El Wahab, M.; El-Sorogy, A.S. Trace metals Distribution in Some Mangrove Sediments of the Southern Red Sea Coast, Egypt. In 8th International Conference of Geography Arab World; Cairo University: Giza, Egypt, 2006; pp. 25–32. [Google Scholar]
- Al-Taani, A.A.; Batayneh, A.; Nazzal, Y.; Ghrefat, H.; Elawadi, E.; Zaman, H. Status of Trace Metals in Surface Seawater of the Gulf of Aqaba, Saudi Arabia. Mar. Pollut. Bull. 2014, 86, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R. Abundance of Chemical Elements in the Continental Crust: A New Table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Praveena, S.M.; Radojevic, M.; Abdullah, M.H.; Aris, A.Z. Application of sediment quality guidelines in the assessment of mangrove surface sediment in Mengkabong lagoon, Sabah, Malaysia. J. Environ. Health Sci. Eng. 2008, 5, 35–42. [Google Scholar]
- Muller, G. Dei Schwermetallbelstung Der Sedimente Des Neckars and Seiner Nebenfusse: Eine Estandsaufnahme. Chem. Ztg. 1981, 105, 157–164. [Google Scholar]
- Banerjee; Adarsh Kumar; Subodh Kumar Maiti; Abhiroop Chowdhury Seasonal Variation in Heavy Metal Contaminations in Water and Sediments of Jamshedpur Stretch of Subarnarekha River, India. Environ. Earth Sci. 2016, 75, 265. [CrossRef]
- Alloway, B.J.; Jackson, A.P.; Morgan, H. The Accumulation of Cadmium by Vegetables Grown on Soils Contaminated from a Variety of Sources. Sci. Total Environ. 1990, 91, 223–236. [Google Scholar] [CrossRef]
- Bastakoti, U.; Robertson, J.; Alfaro, A.C. Spatial Variation of Trace metals in Sediments within a Temperate Mangrove Ecosystem in Northern New Zealand. Mar. Pollut. Bull. 2018, 135, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ghatak, S.; Sikdar, S.K. L-Lactate Mediates Neuroprotection against Ischaemia by Increasing TREK1 Channel Expression in Rat Hippocampal Astrocytes in Vitro. J. Neurochem. 2016, 138, 265–281. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.-Y.; Wang, Y.-S. Physiological and Biochemical Responses in the Leaves of Two Mangrove Plant Seedlings (Kandelia candel and Bruguiera gymnorrhiza) Exposed to Multiple Trace metals. J. Hazard. Mater. 2010, 182, 848–854. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Khattab, R.A.; Ali, I.; Binnaser, Y.S.; Aqeel, A. Assessment of Trace metals Contamination in the Sediments and Mangroves (Avicennia marina) at Yanbu Coast, Red Sea, Saudi Arabia. Mar. Pollut. Bull. 2019, 149, 110669. [Google Scholar] [CrossRef]
- Mackay, D.; Fraser, A. Bioaccumulation of Persistent Organic Chemicals: Mechanisms and Models. Environ. Pollut. 2000, 110, 375–391. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Mohamed, H.M. Effect of Microbial Inoculation and EDTA on the Uptake and Translocation of Heavy Metal by Corn and Sunflower. Chemosphere 2009, 76, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Almahasheer, H.; Serrano, O.; Duarte, C.M.; Irigoien, X. Remobilization of Trace metals by Mangrove Leaves. Front. Mar. Sci. 2018, 5, 484. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Ecological Risk Assessment of Heavy Metal Contamination in Mangrove Habitats, Using Biochemical Markers and Pollution Indices: A Case Study of Avicennia marina L. in the Rabigh Lagoon, Red Sea. Saudi J. Biol. Sci. 2020, 27, 1174–1184. [Google Scholar] [CrossRef]
- Sharma, K.D.; Agrawal, M. Biological Effects of heavy metals: An Overview. J. Environ. Biol. 2005, 26 (Suppl. S2), 301–313. [Google Scholar]
- Memon, A.; Aktoprakligil, D.; Özdemir, A.; Vertii, A. Heavy Metal Accumulation and Detoxification Mechanisms in Plants. Turk. J. Bot. 2001, 25, 111–121. [Google Scholar]
- Hu, C.; Yang, X.; Gao, L.; Zhang, P.; Li, W.; Dong, J.; Li, C.; Zhang, X. Comparative Analysis of Heavy Metal Accumulation and Bioindication in Three Seagrasses: Which Species Is More Suitable as a Bioindicator? Sci. Total Environ. 2019, 669, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sun, T.; Adams, M.P.; Zhou, Y.; Zhang, X.; Xu, S.; Gu, R. Seasonal Dynamics of Trace Elements in Sediment and Seagrass Tissues in the Largest Zostera Japonica Habitat, the Yellow River Estuary, Northern China. Mar. Pollut. Bull. 2018, 134, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Borg, J.A.; Di Martino, V. Levels of Trace metals in Wetland and Marine Vascular Plants and Their Biomonitoring Potential: A Comparative Assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef]
- Devi Rama, S.; Prasad, M.N.V. Copper Toxicity in Ceratophyllum demersum L. (Coontail), a Free Floating Macrophyte: Response of Antioxidant Enzymes and Antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N.; Bisht, S.S. Modulation of Oxidative Stress Responsive Enzymes by Excess Cobalt. Plant Sci. 2002, 162, 381–388. [Google Scholar] [CrossRef]
- Aldoobie, N.F.; Beltagi, M.S. Physiological, Biochemical and Molecular Responses of Common Bean (Phaseolus vulgaris L.) Plants to Trace metals Stress. Afr. J. Biotechnol. 2013, 12, 4614–4622. [Google Scholar] [CrossRef] [Green Version]
- D’angiolillo, F.; Mammano, M.M.; Fascella, G. Pigments, Polyphenols and Antioxidant Activity of Leaf Extracts from Four Wild Rose Species Grown in Sicily. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M. Carotenoid Oxidation Products as Stress Signals in Plants. Plant J. Cell Mol. Biol. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant Enzyme Responses of Plants to Heavy Metal Stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Mazhoudi, S.; Chaoui, A.; Habib Ghorbal, M.; El Ferjani, E. Response of Antioxidant Enzymes to Excess Copper in Tomato (Lycopersicon Esculentum, Mill.). Plant Sci. 1997, 127, 129–137. [Google Scholar] [CrossRef]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper Toxicity in Young Maize (Zea mays L.) Plants: Effects on Growth, Mineral and Chlorophyll Contents, and Enzyme Activities. Plant Soil Neth. 1996, 182, 287–300. [Google Scholar] [CrossRef]
- Allen, R.D. Dissection of Oxidative Stress Tolerance Using Transgenic Plants. Plant Physiol. 1995, 107, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Jung, C.; Maeder, V.; Funk, F.; Frey, B.; Sticher, H.; Frossard, E. Release of Phenols from Lupinus albus L. Roots Exposed to Cu and Their Possible Role in Cu Detoxification. Plant Soil 2003, 252, 301–312. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of Iron with Phenolic Compounds from Soybean Nodules and Other Legume Tissues: Prooxidant and Antioxidant Properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milić, B.L.; Djilas, S.M.; Čanadanović-Brunet, J.M. Antioxidative Activity of Phenolic Compounds on the Metal-Ion Breakdown of Lipid Peroxidation System. Food Chem. 1998, 61, 443–447. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Siddique, A.; Dubey, A.P. Phyto-Toxic Effect of Heavy Metal (CdCl2) on Seed Germination, Seedling Growth and Antioxidant Defence Metabolism in Wheat (Triticum aestivum L.) Variety HUW-234. Int. J. Bio-Resour. Stress Manag. 2017, 8, 261–267. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Compatible Solutes Reduce ROS-Induced Potassium Efflux in Arabidopsis Roots. Plant Cell Environ. 2007, 30, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzam, M.; Hossain, M.A.; Fujita, M. Selenium in Higher Plants: Physiological Role, Antioxidant Metabolism and Abiotic Stress Tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar] [CrossRef] [Green Version]




| No. | Location Name | Geographical Coordinates | Description | Field Photo | |
|---|---|---|---|---|---|
| North | East | ||||
| 1 | 17 Km South Safaga | 26°36′55.9″ | 34°00′43.1″ | It is located 17 km south of Safaga city, which has many tourist villages and the seaport of Safaga. |  |
| 2 | El-Quah | 26°24′16.2″ | 34°06′35.2″ | It is located 44 km south of Safaga City. This location has many sand dunes inhabited by Zygophyllum and Tamarix species. Mangrove swamps in this location are healthy and of high density. |  |
| 3 | 33 Km South El-Qosier | 25°52′22.1″ | 34°25′02.1″ | Mangrove swamps in this site are completely located inside the mangrove bay hotel (33 km south of Qosier along the road to Marsa Alam). They are protected from grazing camels. |  |
| 4 | South Marsa-alam (Wadi El-Gimal) | 24°22′58.1″ | 35°15′39.0″ | A small stand of A. marina is grown in this location. Mangroves are healthy and highly protected. No grazing or human activities are noted in this location. |  |
| 5 | Wadi Al- Qul’an delta | 24°21′27.1″ | 35°18′22.2″ | This site is located in Wadi Al- Qul’an village. There are many human activities on this site, such as diving, camping, village sewage, and many solid wastes, in addition to grazing by camels. |  |
| Location | Trace Metal Concentration (μg g−1) | ||||
|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | |
| 1 | 330.43 ± 1.40 | 1.45 ± 0.03 | 236.23 ± 1.66 | 82.27 ± 0.75 | 437.40 ± 1.60 |
| 2 | 130.30± 1.28 | 0.45 ± 0.03 | 33.63 ± 1.31 | 15.63 ± 0.4 | 114.00 ± 2.00 |
| 3 | 63.17 ± 2.02 | 0.50 ± 0.02 | 18.28 ± 0.26 | 5.48 ± 0.02 | 69.50 ± 1.50 |
| 4 | 45.43 ± 1.86 | 0.45 ± 0.02 | 36.47 ± 1.50 | 5.92 ± 0.16 | 95.00 ± 2.00 |
| 5 | 267.50 ± 1.50 | 0.92 ± 0.18 | 229.17 ± 1.76 | 89.37 ± 0.95 | 384.30 ± 1.70 |
| Average | 167.40 | 0.75 | 110.65 | 39.79 | 220.00 |
| Background continental crust (Taylor 1964) | 55 | 0.20 | 75 | 12.5 | 70 |
| Location | Cu | Cd | Ni | Pb | Zn | Reference |
|---|---|---|---|---|---|---|
| Egyptian Red Sea Coast | 167.40 | 0.75 | 110.65 | 39.79 | 220.00 | Current study |
| Red Sea, Egypt | 0.38 | 0.09 | 3.16 | 2.56 | 7.66 | [52] |
| Gulf of Aqaba | 7.60–10.80 | 0.06–0.07 | ---- | 3.7–6.8 | 7.00–7.70 | [53] |
| Arabian Gulf, Saudi Arabia | 182.97 | 0.23 | 75.01 | 5.35 | 52.68 | [51] |
| 17 km South Safaga | 33.47 | 0.23 | 18.56 | 6.14 | 53.08 | [23] |
| El-Quah location | 35.93 | 0.58 | 30.97 | 5.46 | 68.91 | [23] |
| Background continental crust | 55 | 0.2 | 75 | 12.5 | 70 | [54] |
| Sites | Averages of Igeo of the Studied Trace Metals | ||||
|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | |
| 1 | 2.00 | 2.27 | 1.06 | 2.13 | 2.05 |
| 2 | 0.65 | 0.58 | −1.74 | −0.25 | 0.11 |
| 3 | −0.37 | 0.73 | −2.61 | −1.77 | −0.59 |
| 4 | −0.85 | 0.58 | −1.62 | −1.68 | −0.14 |
| 5 | 1.69 | 1.58 | 1.02 | 2.25 | 1.87 |
| Sites | CF of the Studied Trace Metals | PL Index | ||||
|---|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | ||
| 1 | 6.00 | 7.25 | 3.14 | 6.58 | 6.24 | 5.63 |
| 2 | 2.36 | 2.25 | 0.44 | 1.25 | 1.62 | 1.37 |
| 3 | 1.15 | 2.50 | 0.24 | 0.43 | 0.99 | 0.79 |
| 4 | 0.82 | 2.25 | 0.48 | 0.46 | 1.35 | 0.89 |
| 5 | 4.86 | 4.50 | 3.05 | 7.17 | 5.49 | 4.83 |
| Average | 3.03 | 3.75 | 1.47 | 3.17 | 3.13 | 2.70 |
| Sites | Trace Metals Concentration in Roots | Trace Metals Concentration in Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | Cu | Cd | Ni | Pb | Zn | |
| 1 | 90 ± 1.7 a | 1.5 ± 0.2 a | 180 ± 2.8 a | 89 ± 1.7 a | 92 ± 1.2 a | 120 ± 1.7 a | 1.6 ± 00.17 a | 191 ± 2.9 a | 93 ± 1.7 a | 107 ± 3.4 a |
| 2 | 27.6 ± 0.58 c | 0.022 ± 0.001 c | 50 ± 1.7 b | 6.3 ± 0.17 b | 40.5 ± 2.3 c | 20.5 ± 0.6 c | 0.21 ± 0.006 c | 40 ± 1.1 b | 7.4 ± 0.3 b | 29.3 ± 2.3 c |
| 3 | 19.4 ± 1.1 d | 0.025 ± 0.001 c | 11.3 ± 0.2 c | 4.1 ± 0.06 b | 31.2 ± 0.6 d | 14.5 ± 0.3 c | 0.015 ± 0.00 c | 10.2 ± 0.12 c | 4 ± 0.05 b | 26.8 ± 1.3 c |
| 4 | 17.5 ± 0.28 d | 0.033 ± 0.002 c | 15.5 ± 0.3 c | 4.7 ± 0.11 b | 26.7 ± 1.1 d | 15.7 ± 1.2 c | 0.021 ± 0.001 c | 13 ± 0.6 c | 4 ± 0.01 b | 25.8 ± 1.7 c |
| 5 | 70 ± 2.3 b | 0.92 ± 0.01 b | 183 ± 1.5 a | 91 ± 1.7 a | 83 ± 1.7 b | 81.6 ± 4 b | 0.94 ± 0.023 b | 187 ± 1.7 a | 92 ± 2.3 a | 91.5 ± 2.3 b |
| Average | 44.9 | 0.5 | 87.96 | 39.02 | 54.68 | 50.46 | 0.5572 | 88.24 | 40.08 | 56.08 |
| Sites | Bioconcentration Factors (BCF) in Roots | Bioconcentration Factors (BCF) in Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | Cu | Cd | Ni | Pb | Zn | |
| 1 | 0.27 | 1.03 | 0.76 | 1.08 | 0.21 | 0.36 | 1.1 | 0.80 | 1.13 | 0.24 |
| 2 | 0.21 | 0.04 | 1.49 | 0.40 | 0.35 | 0.28 | 0.06 | 1.19 | 0.47 | 0.25 |
| 3 | 0.30 | 0.05 | 0.61 | 0.75 | 0.44 | 0.22 | 0.03 | 0.55 | 0.73 | 0.38 |
| 4 | 0.38 | 0.07 | 0.42 | 0.80 | 0.28 | 0.51 | 0.04 | 0.35 | 0.68 | 0.27 |
| 5 | 0.26 | 1.02 | 0.79 | 1.01 | 0.21 | 0.30 | 1.04 | 0.81 | 1.02 | 0.23 |
| Sites | Translocation Factor (TF) | ||||
|---|---|---|---|---|---|
| Cu | Cd | Ni | Pb | Zn | |
| 1 | 1.33 | 1.06 | 1.06 | 1.04 | 1.16 |
| 2 | 0.74 | 9.54 | 0.8 | 1.17 | 0.72 |
| 3 | 0.74 | 0.6 | 0.90 | 0.97 | 0.85 |
| 4 | 0.89 | 0.63 | 0.83 | 0.85 | 0.96 |
| 5 | 1.16 | 1.02 | 1.02 | 1.01 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, B.M.; Abdulmajeed, A.M.; Jabbour, A.A.; Hashim, A.M. Eco-Physiological Responses of Avicennia marina (Forssk.) Vierh. to Trace Metals Pollution via Intensifying Antioxidant and Secondary Metabolite Contents. Metabolites 2023, 13, 808. https://doi.org/10.3390/metabo13070808
Alharbi BM, Abdulmajeed AM, Jabbour AA, Hashim AM. Eco-Physiological Responses of Avicennia marina (Forssk.) Vierh. to Trace Metals Pollution via Intensifying Antioxidant and Secondary Metabolite Contents. Metabolites. 2023; 13(7):808. https://doi.org/10.3390/metabo13070808
Chicago/Turabian StyleAlharbi, Basmah M., Awatif M. Abdulmajeed, Alae A. Jabbour, and Ahmed M. Hashim. 2023. "Eco-Physiological Responses of Avicennia marina (Forssk.) Vierh. to Trace Metals Pollution via Intensifying Antioxidant and Secondary Metabolite Contents" Metabolites 13, no. 7: 808. https://doi.org/10.3390/metabo13070808
APA StyleAlharbi, B. M., Abdulmajeed, A. M., Jabbour, A. A., & Hashim, A. M. (2023). Eco-Physiological Responses of Avicennia marina (Forssk.) Vierh. to Trace Metals Pollution via Intensifying Antioxidant and Secondary Metabolite Contents. Metabolites, 13(7), 808. https://doi.org/10.3390/metabo13070808






