Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
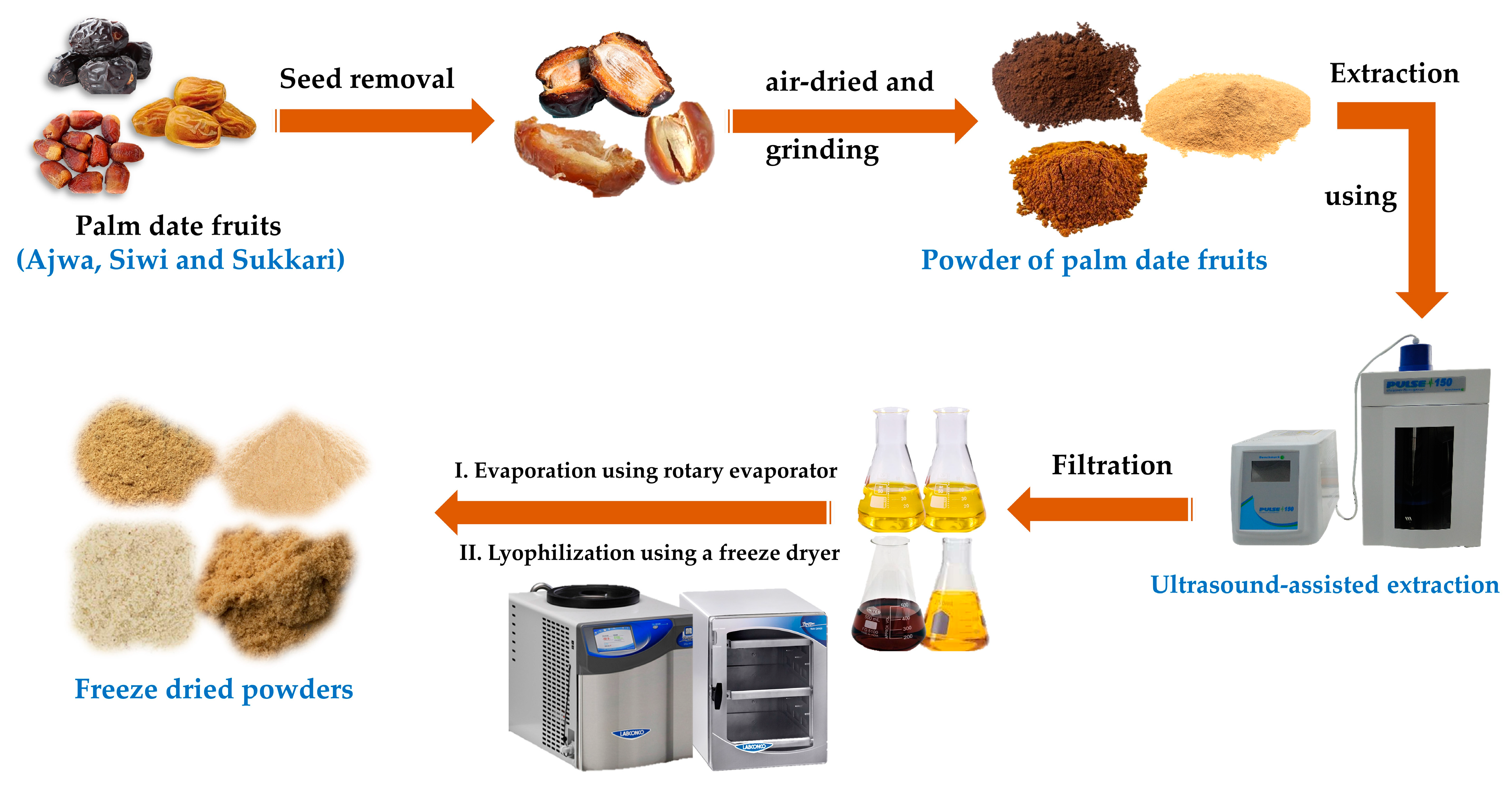
2.3. Extract Preparation
2.4. Chemical Composition
2.5. Mineral Content Determination by ICP-OES
2.6. Determination of Amino Acids by HPLC-MS
2.7. DPPH· Radical Scavenging Activity of Different Extracts of Dates
2.8. Total Phenolic and Flavonoid Contents
2.9. Anticancer Activity of Different Extracts of Dates
2.10. Statistical Analysis
3. Results and Discussion
3.1. Date Fruit Chemical Composition
3.2. Date Fruit Mineral Composition
3.3. Amino Acid Composition of Date Fruits
3.4. Total Phenolics and Total Flavonoids Content (TPC and TFC)
3.5. Antioxidant Potential of Date Extracts
3.6. HPLC-DAD Analysis of Phenolic and Flavonoid Compounds in Ethyl Acetate Extracts
3.7. Anticancer Activity of Various Extracts of Date Varieties
3.8. Interrelationships among Studied Traits and Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix dactylifera L.)–A Review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Jafarian Asl, P.; Niazmand, R.; Razavizadeh, B.M.; Shakeri, M.A.; Jahani, M. Monitoring of Pesticide and Some Metal Residues in Mazafati Date Fruit Cultivar and Risk Assessment to the Health. J. Food Compos. Anal. 2023, 115, 104917. [Google Scholar] [CrossRef]
- Hematian Sourki, A.; Roozitalab, R.; Ghani, A. Functional Properties of Date Powder under Ultrasound, Microwave and Chemical Hydrolysis: Verifying Its Quality and Safety with FTIR Technique. J. Food Meas. Charact. 2023, 17, 1144–1155. [Google Scholar] [CrossRef]
- Tassoult, M.; Kati, D.E.; Fernández-Prior, M.Á.; Bermúdez-Oria, A.; Fernandez-Bolanos, J.; Rodríguez-Gutiérrez, G. Antioxidant Capacity and Phenolic and Sugar Profiles of Date Fruits Extracts from Six Different Algerian Cultivars as Influenced by Ripening Stages and Extraction Systems. Foods 2021, 10, 503. [Google Scholar] [CrossRef]
- Mia, M.A.T.; Mosaib, M.G.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Potentials and Safety of Date Palm Fruit against Diabetes: A Critical Review. Foods 2020, 9, 1557. [Google Scholar] [CrossRef]
- El-Sayed, N.H.; Soliman, A.I.A.; Abdel-Wahab, A.M.A.; El-Said, W.A. Phytochemical and Biological Studies of Date Palm Extracts Phoenix dactylifera Siwi Variety. Egypt. J. Chem. 2020, 63, 1921–1930. [Google Scholar] [CrossRef]
- Mrabet, A.; Hammadi, H.; Rodríguez-Gutiérrez, G.; Jiménez-Araujo, A.; Sindic, M. Date Palm Fruits as a Potential Source of Functional Dietary Fiber: A Review. Food Sci. Technol. Res. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive Compounds from Date Fruit and Seed as Potential Nutraceutical and Functional Food Ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef] [PubMed]
- Ghnimi, S.; Al-Shibli, M.; Al-Yammahi, H.R.; Al-Dhaheri, A.; Al-Jaberi, F.; Jobe, B.; Kamal-Eldin, A. Reducing Sugars, Organic Acids, Size, Color, and Texture of 21 Emirati Date Fruit Varieties (Phoenix dactylifera L.). NFS J. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Rivera, D.; Obón, C.; Alcaraz, F.; Laguna, E.; Johnson, D. Date-Palm (Phoenix, Arecaceae) Iconography in Coins from the Mediterranean and West Asia (485 BC–1189 AD). J. Cult. Herit. 2019, 37, 199–214. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S.J. Reactive Oxygen Species, Antioxidants, and the Mammalian Thioredoxin System. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.M.M.; Osman, M.A.; Al-Tamimi, D.S.; Gassem, M.A.; Al-Khalifa, A.S.; Al-Juhaimi, F.; Mohamed Ahmed, I.A. Antioxidant and Antihyperlipidemic Effects of Ajwa Date (Phoenix dactylifera L.) Extracts in Rats Fed a Cholesterol-Rich Diet. J. Food Biochem. 2019, 43, e12933. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-Cancer Effects of Ajwa Dates (Phoenix dactylifera L.) in Diethylnitrosamine Induced Hepatocellular Carcinoma in Wistar Rats. BMC Complement Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of Ultrasonic-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Sugar Beet Molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Febriyanti, L.; Sudaryat, Y.; Author, C. Cytotoxicity Assay of Water, Ethanol N-Hexane Extract of Dates Fruit (Phoenix dactylifera) Against Murine Leukemia Cancer Cell P388. Syntax. Lit. J. Ilm. Indones. 2021, 6, 288–292. [Google Scholar] [CrossRef]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Valerio, L.G. Medicinal Plants from Peru: A Review of Plants as Potential Agents Against Cancer. Anti-Cancer Agents Med. Chem. 2008, 6, 429–444. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Alfheeaid, H.A. Date Palm Fruit (Phoenix dactylifera) and Its Promising Potential in Developing Functional Energy Bars: Review of Chemical, Nutritional, Functional, and Sensory Attributes. Nutrients 2023, 15, 2134. [Google Scholar] [CrossRef]
- Hamad, I.; Abdelgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.A.; Williams, L.L.; Gad El-Rab, D.A.; Ayivi, R.; Colleran, H.L.; Aljaloud, S.; Ibrahim, S.A. A Review of the Chemical Composition, Nutritional and Health Benefits of Dates for Their Potential Use in Energy Nutrition Bars for Athletes. Cogent Food Agric. 2020, 6, 1809309. [Google Scholar] [CrossRef]
- Siddeeg, A.; Zeng, X.A.; Ammar, A.F.; Han, Z. Sugar Profile, Volatile Compounds, Composition and Antioxidant Activity of Sukkari Date Palm Fruit. J. Food Sci. Technol. 2019, 56, 754. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Jasass, F.M.; Siddiq, M. Date Fruit Composition and Nutrition. Dates Postharvest Sci. Process. Technol. Health Benefits 2013, 13, 261–283. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R.J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Khadaroo, S.K.; Hosenally, M.; Zengin, G.; Rebezov, M.; Ali Shariati, M.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Simal-Gandara, J. Nutritional, medicinal and functional properties of different parts of the date palm and its fruit (Phoenix dactylifera L.)—A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 17, 1–56. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Nutritional Composition of Fruit of 10 Date Palm (Phoenix dactylifera L.) Cultivars Grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Ralston, R.A.; Lee, J.H.; Truby, H.; Palermo, C.E.; Walker, K.Z. A Systematic Review and Meta-Analysis of Elevated Blood Pressure and Consumption of Dairy Foods. J. Hum. Hypertens. 2011, 26, 3–13. [Google Scholar] [CrossRef]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. Chemical Characterisation and the Anti-Inflammatory, Anti-Angiogenic and Antibacterial Properties of Date Fruit (Phoenix dactylifera L.). J. Ethnopharmacol. 2016, 194, 457–468. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, R.; Khan, M.A.; Upadhyay, S.; Husain, I.; Srivastava, A.N. Cytostatic and Anti-Tumor Potential of Ajwa Date Pulp against Human Hepatocellular Carcinoma HepG2 Cells. Sci. Rep. 2019, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Singh, S.P.; Akhtar, J.; Ali, A.; Arif, M. Chemical Composition of Saudi Arabian Sukkari Variety of Date Seed Oil and Extracts Obtained by Slow Pyrolysis. Indian J. Pharm. Sci. 2018, 80, 940–946. [Google Scholar] [CrossRef]
- Ismail, I.; Altuwairki, D. Chemical Composition and Antimicrobial Efficacy of Date Palm Fruit of Saudi Arabia. World Appl. Sci. J. 2016, 34, 140–146. [Google Scholar] [CrossRef]
- Abuelgassim, A.O.; Abdellatif Eltayeb, M.; Shokry Ataya, F. Palm Date (Phoenix dactylifera) Seeds: A Rich Source of Antioxidant and Antibacterial Activities. Czech J. Food Sci. 2020, 38, 171–178. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Diab, Y.M. Effect of Various Extraction Methods and Solvent Types On Yield, Phenolic and Flavonoid Content and Antioxidant Activity of Spathodea nilotica Leaves. Egypt. J. Chem 2021, 64, 7483–7489. [Google Scholar] [CrossRef]
- Official Methods of Analysis. 21st Edition (2019)—AOAC INTERNATIONAL. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 3 December 2021).
- Perveen, K.; Bokahri, N.A. Comparative Analysis of Chemical, Mineral and in-Vitro Antibacterial Activity of Different Varieties of Date Fruits from Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). 2017 Annual Meeting and Exposition. Available online: http://www.phf.org/events/Pages/American_Public_Health_Association_APHA_2017_Annual_Meeting_and_Exposition.aspx (accessed on 7 December 2021).
- Jajic, I.; Krstovic, S.; Glamocic, D.; Jakšic, S.; Abramovic, B. Validation of an HPLC Method for the Determination of Amino Acids in Feed. J. Serb. Chem. Soc. 2013, 78, 839–850. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoides des fleurs et des feuilles de crataegus monogyna jacq. et de Crataegus laevigata (poiret) dc. En fonction de la periode de vegetation. Plantes Med. Phytother. 1991, 25, 12–16. [Google Scholar]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Mundt, F. Package ‘Factoextra’ Extract and Visualize the Results of Multivariate Data Analyses. 2017. Available online: https://piyanit.nl/wp-content/uploads/2020/10/factoextra.pdf (accessed on 25 May 2023).
- EL-Rify, A.M.N.; Abd El-Hamid, A.A.; Abd El-Majeed, M.H. Effect of Some Treatments on Chemical Composition and Quality Properties of Saidy Date Fruit (Phoenix dactylifera L.) During Storage. Assiut J. Agric. Sci. 2016, 47, 107–124. [Google Scholar] [CrossRef]
- Ramadan, B.R.; Mostafa, T.; Ied, W.A.M.; Mostafa, T.M.A.; Ied, W.A.M. Effect of Drying Methods on Chemical Composition, Mineral and Antioxidants of Saidy Date (Phoenix dactylifera L.) Fruits Residue. J. Food Dairy Sci. 2018, 9, 127–132. [Google Scholar] [CrossRef]
- Selim, K.; Abdel-Bary, M.; Ismaael, O. Effect of Irradiation and Heat Treatments on the Quality Characteristics of Siwy Date Fruit (Phoenix dactylifera L.). AgroLife Sci. J. 2012, 1, 103–111. [Google Scholar]
- Al-Tamim, E.A.A. Comparative Study on the Chemical Composition of Saudi Sukkari and Egyptian Swei Date Palm Fruits. J. Am. Sci. 2014, 10, 149–153. [Google Scholar]
- Khalid Aldhafiri, F. Evaluation of Biochemical Parameters, Phenolic Compounds and Antioxidant Capacity of Some Varieties of Phoenix dactylifera L. (Date Fruits) to Determine the Nutritional Impact Values. Med. J. Nutr. Metab. 2017, 10, 153–164. [Google Scholar] [CrossRef]
- Mohamed, R.M.A.; Fageer, A.S.M.; Eltayeb, M.M.; Mohamed Ahmed, I.A. Chemical Composition, Antioxidant Capacity, and Mineral Extractability of Sudanese Date Palm (Phoenix dactylifera L.) Fruits. Food Sci. Nutr. 2014, 2, 478–489. [Google Scholar] [CrossRef]
- Ali Alghamdi, A. Nutritional Assessment Of Different Date Fruits (Phoenix dactylifera L.) Varieties Cultivated In Hail Province, Saudi Arabia. Biosci. Biotechnol. Res. Commun. 2018, 11, 263–269. [Google Scholar] [CrossRef]
- Trabzuni, D.M.; Ahmed, S.E.B.; Abu-Tarboush, H.M. Chemical Composition, Minerals and Antioxidants of the Heart of Date Palm from Three Saudi Cultivars. Food Nutr. Sci. 2014, 5, 1379–1386. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Hafez, E.E. Biochemical and Nutritional Characterizations of Date Palm Fruits (Phoenix dactylifera L.). J. Appl. Sci. Res. 2010, 6, 1060–1067. [Google Scholar]
- Rambabu, K.; Bharath, G.; Hai, A.; Banat, F.; Hasan, S.W.; Taher, H.; Zaid, H.F.M. Nutritional Quality and Physico-Chemical Characteristics of Selected Date Fruit Varieties of the United Arab Emirates. Processes 2020, 8, 256. [Google Scholar] [CrossRef]
- Kawashima, L.M.; Valente Soares, L.M. Mineral Profile of Raw and Cooked Leafy Vegetables Consumed in Southern Brazil. J. Food Compos. Anal. 2003, 16, 605–611. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Ndamitso, M.M.; Mathew, J.T.; Etsunyakpa, M.B.; Tsado, A.N.; Muhammad, S.S. Nutritional and Anti-Nutritional Composition of Date Palm (Phoenix dactylifera L.) Fruits Sold in Major Markets of Minna Niger State, Nigeria. Afr. J. Pure Appl. Chem. 2015, 9, 167–174. [Google Scholar] [CrossRef]
- Ali, H.S.M.; Al-Khalifa, A.S.; Brückner, H. Taurine Is Absent from Amino Components in Fruits of Opuntia Ficus-Indica. Springerplus 2014, 3, 663. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A Review on Chemistry and Pharmacology of Ajwa Date Fruit and Pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Saleh, E.A.; Tawfik, M.S.; Abu-Tarboush, H.M. Phenolic Contents and Antioxidant Activity of Various Date Palm (Phoenix dactylifera L.) Fruits from Saudi Arabia. Food Nutr. Sci. 2011, 2, 1134–1141. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Awad, M.A.; El-Dengawy, E.R.F.A.; Abdel-Mageed, H.M.; El-Badry, M.O.; Salah, H.A.; Abdel-Aty, A.M.; Fahmy, A.S. Total Phenolic and Flavonoid Contents and Antioxidant Activities of Sixteen Commercial Date Cultivars Grown in Saudi Arabia. RSC Adv. 2016, 6, 44814–44819. [Google Scholar] [CrossRef]
- Eid, N.M.S.; Al-Awadi, B.; Vauzour, D.; Oruna-Concha, M.J.; Spencer, J.P.E. Effect of Cultivar Type and Ripening on the Polyphenol Content of Date Palm Fruit. J. Agric. Food Chem. 2013, 61, 2453–2460. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous Optimization of the Ultrasound-Assisted Extraction for Phenolic Compounds Content and Antioxidant Activity of Lycium ruthenicum Murr. Fruit Using Response Surface Methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Phenolic Content, Reducing Power and Membrane Protective Activities of Solanum xanthocarpum Root Extracts. Vegetos 2013, 26, 301–307. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M.; Podsędek, A. Antioxidant and Antiradical Properties of Green Tea Extract Compounds. Int. J. Electrochem. Sci 2017, 12, 6600–6610. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. de Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- George, S.E.; Ramalakshmi, K.; Rao, L.J.M. A Perception on Health Benefits of Coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef]
- Rodriguez De Sotillo, D.; Hadley, M.; Wolf-Hall, C. Potato Peel Extract a Nonmutagenic Antioxidant with Potential Antimicrobial Activity. J. Food Sci. 1998, 63, 907–910. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Bai, Y.; Zhao, J.; Zhang, Y.; Zhang, L. Chlorogenic Acid against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Eur. J. Pharmacol. 2009, 623, 119–124. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic Acid Exhibits Anti-Obesity Property and Improves Lipid Metabolism in High-Fat Diet-Induced-Obese Mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Tee-Ngam, P.; Nunant, N.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and Rapid Determination of Ferulic Acid Levels in Food and Cosmetic Samples Using Paper-Based Platforms. Sensors 2013, 13, 13039. [Google Scholar] [CrossRef] [PubMed]
- Cota-Arriola, O.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Robles-Sánchez, R.M.; Ezquerra-Brauer, J.M.; Ruíz-García, J.; Vega-Acosta, J.R.; Cortez-Rocha, M.O. Preparation of Chitosan Matrices with Ferulic Acid: Physicochemical Characterization and Relationship on the Growth of Aspergillus Parasiticus. CYTA J. Food 2017, 15, 65–74. [Google Scholar] [CrossRef]
- Moldovan, M.; Lahmar, A.; Bogdan, C.; Parauan, S.; Tomuţă, I.; Crişan, M. Formulation and Evaluation of a Water-in-Oil Cream Containing Herbal Active Ingredients and Ferulic Acid. Clujul Med. 2017, 90, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharm. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-Proliferative Activity of Essential Oil Extracted from Thai Medicinal Plants on KB and P388 Cell Lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Abd Ghafar, S.A.; Ismail, M.; Saiful Yazan, L.; Fakurazi, S.; Ismail, N.; Chan, K.W.; Md Tahir, P. Cytotoxic Activity of Kenaf Seed Oils from Supercritical Carbon Dioxide Fluid Extraction towards Human Colorectal Cancer (HT29) Cell Lines. Evid.-Based Complement. Altern. Med. 2013, 2013, 549705. [Google Scholar] [CrossRef]
- Nordin, M.L.; Abdul Kadir, A.; Zakaria, Z.A.; Abdullah, R.; Abdullah, M.N.H. In Vitro Investigation of Cytotoxic and Antioxidative Activities of Ardisia Crispa against Breast Cancer Cell Lines, MCF-7 and MDA-MB-231. BMC Complement. Altern. Med. 2018, 18, 87. [Google Scholar] [CrossRef]
- El, D.M.; Sheikh, D.M.E.; El-Kholany, E.A.; Kamel, S.M. Nutritional Value, Cytotoxicity, Anti-Carcinogenic and Beverage Evaluation of Roasted Date Pits. World J. Dairy Food Sci. 2014, 9, 308–316. [Google Scholar] [CrossRef]
- Sairi, A.M.M.; Ismail, S.I.; Sukor, A.; Rashid, N.M.N.; Saad, N.; Jamian, S.; Abdullah, S. Cytotoxicity and Anticancer Activity of Donkioporiella Mellea on MRC5 (Normal Human Lung) and A549 (Human Lung Carcinoma) Cells Lines. Evid.-Based Complement. Altern. Med. 2020, 2020, 7415672. [Google Scholar] [CrossRef]
- Mirza, M.B.; Elkady, A.I.; Al-Attar, A.M.; Syed, F.Q.; Mohammed, F.A.; Hakeem, K.R. Induction of Apoptosis and Cell Cycle Arrest by Ethyl Acetate Fraction of Phoenix dactylifera L. (Ajwa Dates) in Prostate Cancer Cells. J. Ethnopharmacol. 2018, 218, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sayqal, A. Fingerprinting Analysis Using Crystal Based Mid-Infrared Spectroscopy to Differentiate between Saudi Dates Fruits for Food Adulteration. J. Umm Al-Qura Univ. Appl. Sci. 2020, 6, 6–9. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Khadri, H.; Azam, M.; Khan, M.A.; Rahmani, A.H.; Alrumaihi, F.; Khateef, R.; Ansari, M.A.; Alatawi, E.A.; Alsugoor, M.H.; et al. Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Anti-Biofilm, and Cytotoxic Potential. Appl. Sci. 2022, 12, 4537. [Google Scholar] [CrossRef]
- Pasha, A.Z.; Bukhari, S.A.; Mustafa, G.; Anjum, F.; Mahr-Un-Nisa Qari, S.H. Evaluation of Modified Date Palm (Phoenix dactylifera L.) Mucilage as a Potential Pharmaceutical Excipient. J. Food Qual. 2022, 2022, 3923812. [Google Scholar] [CrossRef]
- Añón, M.T.; Ubeda, A.; Alcaraz, M.J.H. Protective Effects of Phenolic Compounds on CCl4-Induced Toxicity in Isolated Rat Hepatocytes. Z. Nat. C 1992, 47, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Paranthaman, R. HPLC and HPTLC Determination of Caffeine in Raw and Roasted Date Seeds (Phoenix Dactylifera L). J. Chromatogr. Sep. Tech. 2012, 1, 1–4. [Google Scholar] [CrossRef]


| Component (%) | Ajwa | Siwi | Sukkari |
|---|---|---|---|
| Moisture | 17.31 ±0.46 b | 19.18 ± 0.50 a | 18.13 ± 0.38 ab |
| Total sugars | 75.24 ± 1.75 b | 80.25 ± 2.02 a | 79.42 ± 2.90 a |
| Non-reducing sugars | 3.88 ± 0.02 b | 04.89 ± 0.01 a | 3.780 ± 0.00 b |
| reducing sugars | 71.55 ± 1.25 b | 75.36 ± 2.01 a | 75.64 ± 1.05 a |
| Ash | 3.86 ± 0.01 a | 2.14 ± 0.05 b | 2.480 ± 0.00 b |
| Crud protein | 3.25 ± 0.03 a | 1.970 ± 0.01 b | 2.260 ± 0.01 b |
| Crud fibers | 3.51 ± 0.04 a | 2.580 ± 0.00 b | 2.130 ± 0.02 b |
| Crud lipids | 0.97 ± 0.000 a | 1.230 ± 0.05 a | 1.050 ± 0.04 a |
| Variety | Ca | Na | Mg | K | Zn | Fe | Cu | P |
|---|---|---|---|---|---|---|---|---|
| Ajwa | 128.79 ± 3.22 a | 45.35 ± 1.24 a | 151.17 ± 2.32 a | 1118.64 ± 4.27 a | 1.33 ± 0.01 a | 3.33 ± 1.00 b | 0.695 ± 0.51 a | 231.30 ± 6.25 a |
| Siwi | 55.73 ± 1.87 c | 37.15 ± 2.64 ab | 92.88 ± 1.11 b | 1040.26 ± 3.86 b | 0.93 ± 0.00 b | 6.81 ± 1.69 a | 0.612 ± 0.22 a | 167.18 ± 2.55 b |
| Sukkari | 64.12 ± 2.02 b | 30.54 ± 1.01 b | 91.597 ± 3.52 b | 1038.11 ± 3.45 b | 1.16 ± 0.02 ab | 6.11 ± 2.04 a | 0.37 ± 0.00 b | 109.92 ± 2.07 c |
| Amino Acid | Ajwa | Siwi | Sukkari |
|---|---|---|---|
| Aspartic acid | 105.521 ± 0.011 | 65.530 ± 0.31 | 122.018 ± 0.06 |
| Glutamic acid | 9.804 ± 0.0301 | 11.861 ± 0.00 | 8.250 ± 0.058 |
| Serine | 3.181 ± 0.0810 | 4.118 ± 0.036 | 3.059 ± 0.074 |
| Histidine | 0.9580 ± 0.0216 | 1.065 ± 0.005 | 1.568 ± 0.0289 |
| Glycine | 5.8510 ± 0.172 | 5.922 ± 0.289 | 5.407 ± 0.102 |
| Threonine | 2.6920 ± 0.034 | 2.080 ± 0.025 | 2.340 ± 0.041 |
| Arginine | 4.1491 ± 0.023 | 4.463 ± 0.082 | 6.909 ± 0.075 |
| Alanine | 5.501 ± 0.0000 | 5.506 ± 0.001 | 7.471 ± 0.007 |
| Tyrosine | 2.816 ± 0.0271 | 3.016 ± 0.013 | 10.805 ± 0.063 |
| Cystine | n.d. | n.d. | n.d. |
| Valine | 6.661 ± 0.0121 | 6.360 ± 0.021 | 9.366 ± 0.061 |
| Methionine | 8.222 ± 0.0502 | 8.334 ± 0.032 | 9.619 ± 0.016 |
| Phenylalanine | 17.212 ± 0.0070 | 15.868 ± 0.005 | 18.891 ± 0.091 |
| Isoleucine | 3.037 ± 0.0453 | 3.319 ± 0.055 | 5.931 ± 0.062 |
| Leucine | 42.145 ± 0.0130 | 41.639 ± 0.034 | 4.516 ± 0.057 |
| Lysine | n.d. | n.d. | n.d. |
| Proline | 673.744 ± 0.028 | 796.344 ± 0.018 | 681.476 ± 0.075 |
| Variety | Extract | Extract Yield (%) | TPC (mg GAE·g−1 Extract) | TFC (mg RE·g−1 Extract) |
|---|---|---|---|---|
| Ajwa | EtOAc | 0.901 ± 0.17 j | 23.97 ± 0.29 b | 13.48 ± 0.29 b |
| EtOH/H2O 70% | 44.36 ± 0.34 d | 18.62 ± 0.29 c | 10.28 ± 0.29 c | |
| MeOH/H2O 80% | 46.58 ± 0.25 c | 14.84 ± 0.29 e | 6.66 ± 0.29 e | |
| H2O | 32.10 ± 0.41 g | 11.65 ± 0.3 gh | 5.42 ± 0.30 f | |
| Siwi | EtOAc | 0.640 ± 0.11 k | 16.22 ± 0.30 d | 13.06 ± 0.30 b |
| EtOH/H2O 70% | 42.36 ± 0.23 e | 12.35 ± 0.02 fg | 9.84 ± 0.021 c | |
| MeOH/H2O 80% | 45.70 ± 0.42 c | 12.70 ± 0.26 f | 6.42 ± 0.26 e | |
| H2O | 30.22 ± 0.36 h | 11.54 ± 0.3 gh | 3.97 ± 0.30 g | |
| Sukkari | EtOAc | 1.101 ± 0.01 i | 29.61 ± 0.26 a | 17.40 ± 0.26 a |
| EtOH/H2O 70% | 49.86 ± 0.28 b | 11.42 ± 0.28 h | 8.70 ± 0.28 d | |
| MeOH/H2O 80% | 51.20 ± 0.34 a | 10.80 ± 0.26 h | 5.77 ± 0.26 f | |
| H2O | 34.60 ± 0.29 f | 9.22 ± 0.26 i | 1.17 ± 0.26 h |
| Variety | Extract | IC50 (μg·mL−1) |
|---|---|---|
| Ajwa | EtOAc | 316.97 ± 0.29 c |
| EtOH/H2O 70% | 470.06 ± 0.29 g | |
| MeOH/H2O 80% | 352.27 ± 0.29 d | |
| H2O | 867.07 ± 0.30 l | |
| Siwi | EtOAc | 161.55 ± 0.30 b |
| EtOH/H2O 70% | 631.69 ± 0.64 j | |
| MeOH/H2O 80% | 504.53 ± 0.26 h | |
| H2O | 396.37 ± 0.30 e | |
| Sukkari | EtOAc | 132.40 ± 0.26 a |
| EtOH/H2O 70% | 626.89 ± 0.28 i | |
| MeOH/H2O 80% | 425.62 ± 0.26 f | |
| H2O | 807.89 ± 0.26 k |
| No. | Rt (min) | Compound | Quantification (µg·g−1) | ||
|---|---|---|---|---|---|
| Ajwa | Siwi | Sukkari | |||
| 1 | 4.0 | Gallic acid | 5.30 ± 0.00 | n.d. | 8.30 ± 0.01 |
| 2 | 6.7 | Protocatechuic acid | n.d. | n.d. | 16.68 ± 0.00 |
| 3 | 10.0 | p-hydroxybenzoic acid | 59.13 ± 0.02 | n.d. | 81.34 ± 0.01 |
| 4 | 12.0 | Catechin | n.d. | n.d. | 63.31 ± 0.00 |
| 5 | 12.3 | Chlorogenic acid | 5.26 ± 0.00 | n.d. | 50.35 ± 0.01 |
| 6 | 13.5 | Caffeic acid | 3.04 ± 0.03 | n.d. | 13.01 ± 0.02 |
| 7 | 14.4 | Syringic acid | 27.82 ± 0.01 | n.d. | 17.11 ± 0.00 |
| 8 | 16.2 | Vanillic acid | n.d. | n.d. | 7.07 ± 0.00 |
| 9 | 20.5 | Ferulic acid | 5.41 ± 0.01 | n.d. | 122.83 ± 0.01 |
| 10 | 21.2 | Sinapic acid | 22.86 ± 0.00 | 2.97 ± 0.01 | 51.74 ± 0.00 |
| 11 | 26.2 | p-coumaric acid | n.d. | 14.81 ± 0.00 | n.d. |
| 12 | 35.8 | Cinnamic acid | 2.96 ± 0.02 | n.d. | 7.82 ± 0.01 |
| 13 | 36.3 | Quercetin | 5.88 ± 0.00 | n.d. | 7.35 ± 0.00 |
| 14 | 40.5 | Kaempferol | n.d. | n.d. | 20.48 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbaky, A.S.; Tammam, M.A.; Ali, M.Y.; Sharaky, M.; Selim, K.; Semida, W.M.; Abd El-Mageed, T.A.; Ramadan, M.F.; Oraby, H.F.; Diab, Y.M. Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits. Metabolites 2023, 13, 816. https://doi.org/10.3390/metabo13070816
Abdelbaky AS, Tammam MA, Ali MY, Sharaky M, Selim K, Semida WM, Abd El-Mageed TA, Ramadan MF, Oraby HF, Diab YM. Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits. Metabolites. 2023; 13(7):816. https://doi.org/10.3390/metabo13070816
Chicago/Turabian StyleAbdelbaky, Ahmed S., Mohamed A. Tammam, Mohamed Yassin Ali, Marwa Sharaky, Khaled Selim, Wael M. Semida, Taia A. Abd El-Mageed, Mohamed Fawzy Ramadan, Hesham F. Oraby, and Yasser M. Diab. 2023. "Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits" Metabolites 13, no. 7: 816. https://doi.org/10.3390/metabo13070816
APA StyleAbdelbaky, A. S., Tammam, M. A., Ali, M. Y., Sharaky, M., Selim, K., Semida, W. M., Abd El-Mageed, T. A., Ramadan, M. F., Oraby, H. F., & Diab, Y. M. (2023). Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits. Metabolites, 13(7), 816. https://doi.org/10.3390/metabo13070816










