Intracellular Acidification in a Rat C6 Glioma Model following Cariporide Injection Investigated by CEST-MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Culture and Cell Preparation for Implantation
2.2. Chemicals
2.3. Animal Model/Preparation
2.4. Study Overview
2.5. In Vivo Imaging
2.6. Image Analysis
2.7. Statistical Analysis
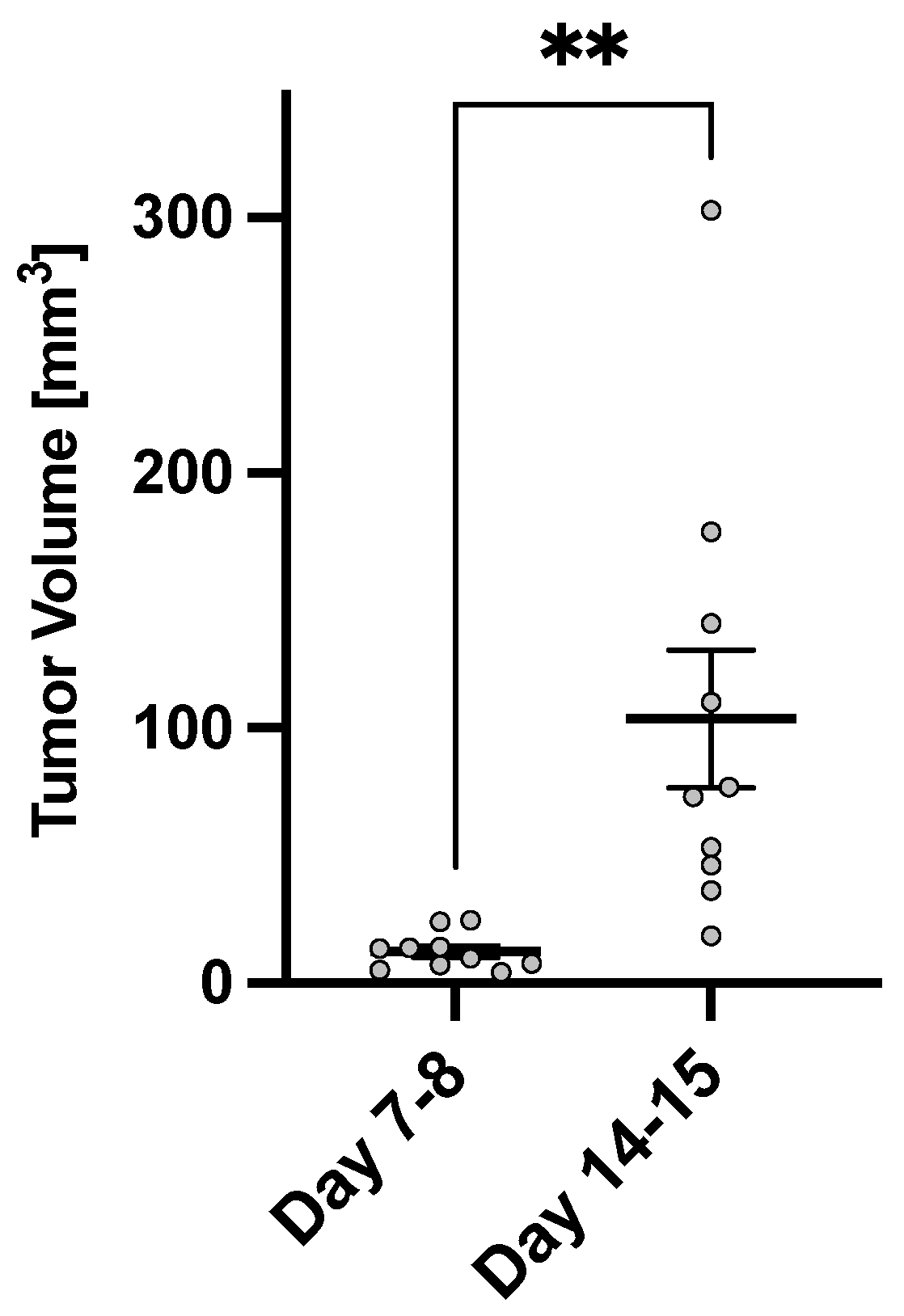
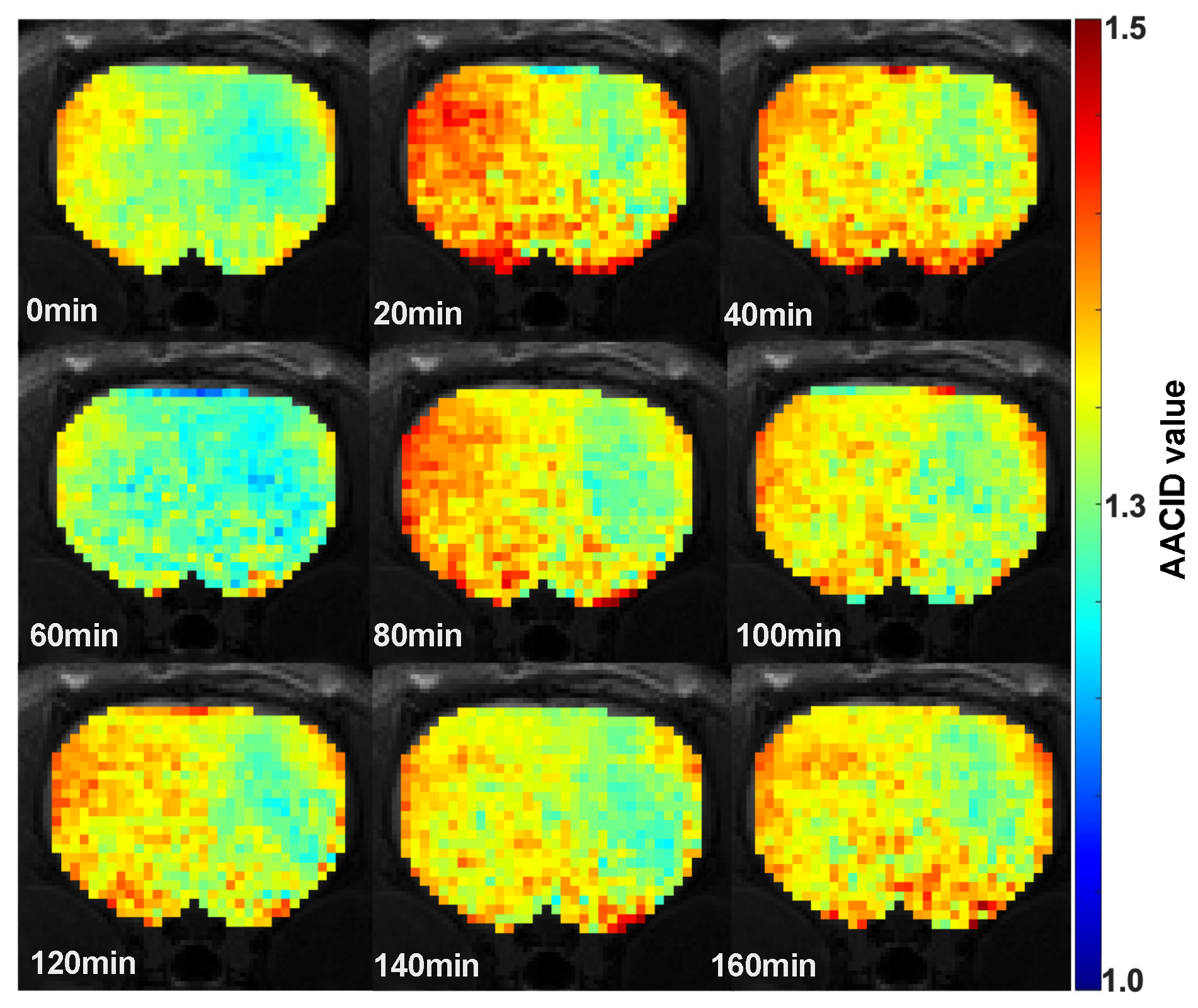
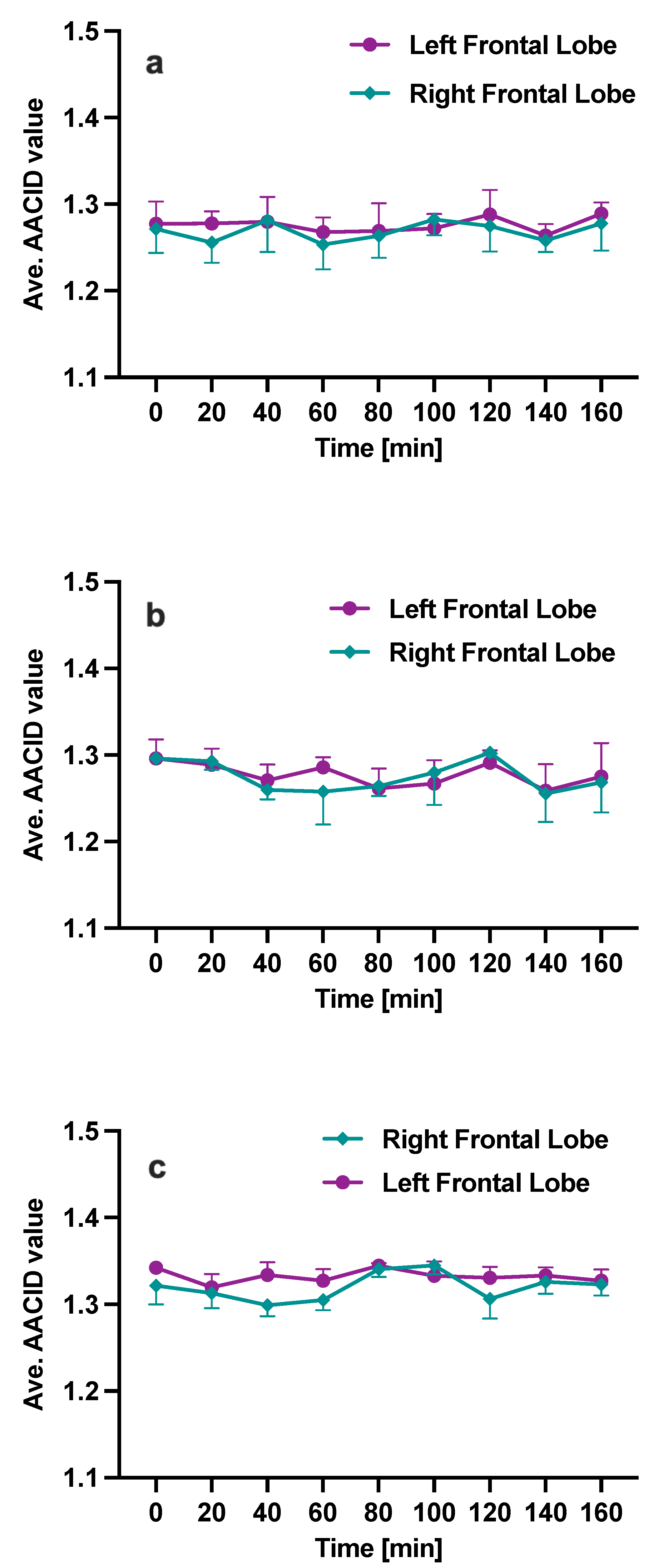
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| GBM | Glioblastoma Multiform |
| AA | Anaplastic Astrocytomas |
| IDH | Isocitrate Dehydrogenase |
| KPS | Karnofsky Performance Scale |
| pHi | Intracellular pH |
| pHe | Extracellular pH |
| NHE | Na+/H+ exchangers |
| NHE1 | Na+/H+ exchanger1 |
| IGF | Insulin-like Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| GFAP | Glial Fibrillary Acidic Protein |
| MRI | Magnetic Resonance Imaging |
| 31P-MRS | Phosphorus Magnetic Resonance Spectroscopy |
| CEST | Chemical Exchange Saturation Transfer |
| WASSR | WAter Saturation Shift Referencing |
| AACID | Amine and Amide Concentration-Independent Detection |
| RF | Radio Frequency |
| APT | Amide Proton Transfer |
| SNR | Signal-to-Noise Ratio |
| ROI | Region Of Interest |
| PBS | phosphate buffered saline |
| DMSO | Dimethyl Sulfoxide |
| SQ | Subcutaneous |
| IP | Intraperitoneal |
| SCID | Severe Combined Immunodeficiency |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Gieryng, A.; Pszczolkowska, D.; Bocian, K.; Dabrowski, M.; Rajan, W.D.; Kloss, M.; Mieczkowski, J.; Kaminska, B. Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas. Sci. Rep. 2017, 7, 17556. [Google Scholar] [CrossRef]
- Agnihotri, S.; Burrell, K.E.; Wolf, A.; Jalali, S.; Hawkins, C.; Rutka, J.T.; Zadeh, G. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch. Immunol. Ther. Exp. 2013, 61, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, A.B.; Suki, D.; Yang, D.; Shi, W.; Aldape, K. The natural history of EGFR and EGFRvIII in glioblastoma patients. J. Transl. Med. 2005, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Bellizzi, A.; Caldeira, S.; Albarani, V.; Malanchi, I.; Poignee, M.; Alunni-Fabbroni, M.; Casavola, V.; Tommasino, M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000, 14, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Fliegel, L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006, 84, 1081–1095. [Google Scholar] [CrossRef]
- Harguindey, S.; Arranz, J.L.; Wahl, M.L.; Orive, G.; Reshkin, S.J. Proton Transport Inhibitors as Potentially Selective Anticancer Drugs. Anticancer. Res. 2009, 29, 2127–2136. [Google Scholar] [PubMed]
- Harguindey, S.; Orive, G.; Pedraz, J.L.; Paradiso, A.; Reshkin, S.J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—One single nature. Biochim. Biophys. Acta BBA Rev. Cancer 2005, 1756, 1–24. [Google Scholar] [CrossRef]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef]
- Loo, S.Y.; Chang, M.K.X.; Chua, C.S.H.; Kumar, A.P.; Pervaiz, S.; Clement, M.V. NHE-1: A Promising Target for Novel Anti-cancer Therapeutics. Curr. Pharm. Des. 2012, 18, 1372–1382. [Google Scholar] [CrossRef]
- Pouyssegur, J.; Chambard, J.C.; Franchi, A.; Paris, S.; Obberghen-Schilling, E.V. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: Coupling to ribosomal protein S6 phosphorylation. Proc. Natl. Acad. Sci. USA 1982, 79, 3935–3939. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Cardone, R.A.; Harguindey, S. Na+/H+ Exchanger, pH Regulation and Cancer. Recent Patents Anti-Cancer Drug Discov. 2012, 8, 85–99. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2009, 11, 50–61. [Google Scholar] [CrossRef]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am. J. Cancer Res. 2022, 12, 138. [Google Scholar] [PubMed]
- Lagana, A.; Vadnais, J.; Le, P.U.; Nguyen, T.N.; Laprade, R.; Nabi, I.R.; Noel, J. Regulation of the formation of tumor cell pseudopodia by the Na+/H+ exchanger NHE1. J. Cell Sci. 2000, 113, 3649–3662. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Bellizzi, A.; Albarani, V.; Guerra, L.; Tommasino, M.; Paradiso, A.; Casavola, V. Phosphoinositide 3-Kinase Is Involved in the Tumor-specific Activation of Human Breast Cancer Cell Na+/H+ Exchange, Motility, and Invasion Induced by Serum Deprivation. J. Biol. Chem. 2000, 275, 5361–5369. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Dong, W.; Song, Z.; Dou, K. Over-expression of Na+/H+ exchanger 1 and its clinicopathologic significance in hepatocellular carcinoma. Med Oncol. 2009, 27, 1109–1113. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.; Kunkler, I.; Langdon, S. The Impact of Tumour pH on Cancer Progression; Strategies for Clinical Intervention. Explor. Target. Anti-Tumour Ther. 2020, 1, 71. [Google Scholar] [CrossRef]
- Stock, C.; Pedersen, S.F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective. Semin. Cancer Biol. 2017, 43, 5–16. [Google Scholar] [CrossRef]
- Harguindey, S.; Arranz, J.; Orozco, J.P.; Rauch, C.; Fais, S.; Cardone, R.; Reshkin, S.J. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs—An integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J. Transl. Med. 2013, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Orozco, J.P.; Cuenca, M.; Fernández, M.C.; Arranz, J.L. New and powerful NHE1 inhibitors as potential anticancer drugs in bedside oncology: A prospective program of preclinical studies in cats and dogs with spontaneous malignant tumors. In Proceedings of the 4th Annual Meeting of the International Society of Proton Dynamics in Cancer, Garching, Germany, 10–12 October 2013; Conference Abstract. Frontiers: Lausanne, Switzerland, 2014. [Google Scholar] [CrossRef]
- Lee, Y.J.; Bae, J.H.; Kim, S.A.; Kim, S.H.; Woo, K.M.; Nam, H.S.; Cho, M.K.; Lee, S.H. Cariporide enhances the DNA damage and apoptosis in acid-tolerable malignant mesothelioma H-2452 cells. Mol. Cells 2017, 40, 567. [Google Scholar] [CrossRef] [PubMed]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. In vivo detection of acute intracellular acidification in glioblastoma multiforme following a single dose of cariporide. Int. J. Clin. Oncol. 2018, 23, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Benda, P.; Lightbody, J.; Sato, G.; Levine, L.; Sweet, W. Differentiated rat glial cell strain in tissue culture. Science 1968, 161, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Curtin, J.F.; Nichols, W.S.; Muhammad, A.G.; King, G.D.; Pluhar, G.E.; McNiel, E.A.; Ohlfest, J.R.; Freese, A.B.; Moore, P.F.; et al. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J. Neuro-Oncol. 2007, 85, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105. [Google Scholar] [CrossRef]
- Guo, P.; Hu, B.; Gu, W.; Xu, L.; Wang, D.; Huang, H.J.S.; Cavenee, W.K.; Cheng, S.Y. Platelet-Derived Growth Factor-B Enhances Glioma Angiogenesis by Stimulating Vascular Endothelial Growth Factor Expression in Tumor Endothelia and by Promoting Pericyte Recruitment. Am. J. Pathol. 2003, 162, 1083–1093. [Google Scholar] [CrossRef]
- Nakada, M.; Niska, J.A.; Tran, N.L.; McDonough, W.S.; Berens, M.E. EphB2/R-Ras Signaling Regulates Glioma Cell Adhesion, Growth, and Invasion. Am. J. Pathol. 2005, 167, 565–576. [Google Scholar] [CrossRef]
- Sibenaller, Z.A.; Etame, A.B.; Ali, M.M.; Barua, M.; Braun, T.A.; Casavant, T.L.; Ryken, T.C. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg. Focus 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Doblas, S.; He, T.; Saunders, D.; Pearson, J.; Hoyle, J.; Smith, N.; Lerner, M.; Towner, R.A. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J. Magn. Reson. Imaging 2010, 32, 267–275. [Google Scholar] [CrossRef]
- Moroz, M.A.; Huang, R.; Kochetkov, T.; Shi, W.; Thaler, H.; de Stanchina, E.; Gamez, I.; Ryan, R.P.; Blasberg, R.G. Comparison of Corticotropin-Releasing Factor, Dexamethasone, and Temozolomide: Treatment Efficacy and Toxicity in U87 and C6 Intracranial GliomasComparison of hCRF and Dexamethasone Efficacy in Glioma Models. Clin. Cancer Res. 2011, 17, 3282–3292. [Google Scholar] [CrossRef]
- Korzowski, A.; Weinfurtner, N.; Mueller, S.; Breitling, J.; Goerke, S.; Schlemmer, H.P.; Ladd, M.E.; Paech, D.; Bachert, P. Volumetric mapping of intra-and extracellular pH in the human brain using 31P MRSI at 7T. Magn. Reson. Med. 2020, 84, 1707–1723. [Google Scholar] [CrossRef]
- Wenger, K.J.; Hattingen, E.; Franz, K.; Steinbach, J.P.; Bähr, O.; Pilatus, U. Intracellular pH measured by 31P-MR-spectroscopy might predict site of progression in recurrent glioblastoma under antiangiogenic therapy. J. Magn. Reson. Imaging 2017, 46, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, P.C.M.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Schüre, J.R.; Shrestha, M.; Breuer, S.; Deichmann, R.; Hattingen, E.; Wagner, M.; Pilatus, U. The pH sensitivity of APT-CEST using phosphorus spectroscopy as a reference method. NMR Biomed. 2019, 32, e4125. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, X.; Chan, K.W.Y.; McMahon, M.T. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013, 26, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Payen, J.F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Terreno, E.; Castelli, D.D.; Aime, S. Encoding the frequency dependence in MRI contrast media: The emerging class of CEST agents. Contrast Media Mol. Imaging 2010, 5, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Malloy, C.R.; Sherry, A.D. MRI Thermometry Based on PARACEST Agents. J. Am. Chem. Soc. 2005, 127, 17572–17573. [Google Scholar] [CrossRef]
- McVicar, N.; Li, A.X.; Suchý, M.; Hudson, R.H.E.; Menon, R.S.; Bartha, R. Simultaneous in vivo pH and temperature mapping using a PARACEST-MRI contrast agent. Magn. Reson. Med. 2012, 70, 1016–1025. [Google Scholar] [CrossRef]
- Jones, K.M.; Pollard, A.C.; Pagel, M.D. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J. Magn. Reson. Imaging 2017, 47, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.E.; Korzowski, A.; Regnery, S.; Goerke, S.; Breitling, J.; Floca, R.O.; Debus, J.; Schlemmer, H.P.; Ladd, M.E.; Bachert, P.; et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J. Magn. Reson. Imaging 2019, 50, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M.; Randtke, E.A.; Yoshimaru, E.S.; Howison, C.M.; Chalasani, P.; Klein, R.R.; Chambers, S.K.; Kuo, P.H.; Pagel, M.D. Clinical translation of tumor acidosis measurements with AcidoCEST MRI. Mol. Imaging Biol. 2017, 19, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro-Oncology 2018, 20, 1661–1671. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef]
- Irrera, P.; Roberto, M.; Consolino, L.; Anemone, A.; Villano, D.; Navarro-Tableros, V.; Carella, A.; Dastrù, W.; Aime, S.; Longo, D.L. Effect of Esomeprazole Treatment on Extracellular Tumor pH in a Preclinical Model of Prostate Cancer by MRI-CEST Tumor pH Imaging. Metabolites 2022, 13, 48. [Google Scholar] [CrossRef]
- McVicar, N.; Li, A.X.; Meakin, S.O.; Bartha, R. Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR Biomed. 2015, 28, 566–575. [Google Scholar] [CrossRef]
- Marathe, K.; McVicar, N.; Li, A.; Bellyou, M.; Meakin, S.; Bartha, R. Topiramate induces acute intracellular acidification in glioblastoma. J. Neuro-Oncol. 2016, 130, 465–472. [Google Scholar] [CrossRef]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. Dichloroacetate induced intracellular acidification in glioblastoma: In vivo detection using AACID-CEST MRI at 9.4 Tesla. J. Neuro-Oncol. 2017, 136, 255–262. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, G.; Shen, Z.; Zhuang, C.; Xie, Y.; Zhang, X.; Yang, Z.; Guan, J.; Shen, Y.; Chen, Y.; et al. Noninvasive detection of extracellular pH in human benign and malignant liver tumors using CEST MRI. Front. Oncol. 2020, 10, 578985. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef]
- Gilbert, K.M.; Schaeffer, D.J.; Gati, J.S.; Klassen, L.M.; Everling, S.; Menon, R.S. Open-source hardware designs for MRI of mice, rats, and marmosets: Integrated animal holders and radiofrequency coils. J. Neurosci. Methods 2019, 312, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- McVicar, N.; Li, A.X.; Goncalves, D.F.; Bellyou, M.; Meakin, S.O.; Prado, M.A.; Bartha, R. Quantitative Tissue Ph Measurement during Cerebral Ischemia Using Amine and Amide Concentration-Independent Detection (AACID) with MRI. J. Cereb. Blood Flow Metab. 2014, 34, 690–698. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Cody Hazlett, H.; Gimpel Smith, R.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Greenhouse, S.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Wong, P.; Kleemann, H.; Tannock, I. Cytostatic potential of novel agents that inhibit the regulation of intracellular pH. Br. J. Cancer 2002, 87, 238–245. [Google Scholar] [CrossRef]
- Braganhol, E.; Huppes, D.; Bernardi, A.; Wink, M.R.; Lenz, G.; Battastini, A.M.O. A comparative study of ectonucleotidase and P2 receptor mRNA profiles in C6 cell line cultures and C6 ex vivo glioma model. Cell Tissue Res. 2009, 335, 331–340. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Fu, W.M.; Chen, M.; Qiu, Z. Establishment of C6 brain glioma models through stereotactic technique for laser interstitial thermotherapy research. Surg. Neurol. Int. 2015, 6, 51. [Google Scholar] [CrossRef]
- Silva, A.C.D.; Cabral, F.R.; Mamani, J.B.; Malheiros, J.M.; Polli, R.S.; Tannus, A.; Vidoto, E.; Martins, M.J.; Sibov, T.T.; Pavon, L.F.; et al. Tumor growth analysis by magnetic resonance imaging of the C6 glioblastoma model with prospects for the assessment of magnetohyperthermia therapy. Einstein (São Paulo) 2012, 10, 11–15. [Google Scholar] [CrossRef]
- Albatany, M.; Ostapchenko, V.G.; Meakin, S.; Bartha, R. Brain tumor acidification using drugs simultaneously targeting multiple pH regulatory mechanisms. J. Neuro-Oncol. 2019, 144, 453–462. [Google Scholar] [CrossRef]
- Lim, H.; Albatany, M.; Martínez-Santiesteban, F.; Bartha, R.; Scholl, T.J. Longitudinal measurements of intra-and extracellular pH gradient in a rat model of glioma. Tomography 2018, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J.; Cloughesy, T.F.; Liau, L.M.; Prins, R.M.; Antonios, J.P.; Li, D.; Yong, W.H.; Pope, W.B.; Lai, A.; Nghiemphu, P.L.; et al. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro-Oncology 2015, 17, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Thews, O.; Riemann, A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metastasis Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef]
- Riemann, A.; Ihling, A.; Reime, S.; Gekle, M.; Thews, O. Impact of the tumor microenvironment on the expression of inflammatory mediators in cancer cells. In Oxygen Transport to Tissue XXXVIII; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–111. [Google Scholar] [CrossRef]
- Qi, Q.; Fox, M.S.; Lim, H.; Bartha, R.; Scholl, T.J.; Hoffman, L.; Lee, T.Y.; Thiessen, J.D. Multimodality in vivo imaging of perfusion and glycolysis in a rat model of C6 glioma. Mol. Imaging Biol. 2021, 23, 516–526. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local InvasionAcid-Mediated Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Honasoge, A.; Sontheimer, H. Involvement of tumor acidification in brain cancer pathophysiology. Front. Physiol. 2013, 4, 316. [Google Scholar] [CrossRef]
- Ma, D.; Fang, Q.; Wang, P.; Gao, R.; Wu, W.; Lu, T.; Cao, L.; Hu, X.; Wang, J. Induction of heme oxygenase-1 by Na+/H+ exchanger1 protein plays a crucial role in imatinib-resistant chronic myeloid leukemia cells. J. Biol. Chem. 2015, 290, 12558–12571. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–6707. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, J.; Wei, W.; Lv, Y.; Zhang, X.; Yao, Y.; Wang, L.; Ling, T.; Zou, X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. Oncotargets Ther. 2018, 11, 6705–6722. [Google Scholar] [CrossRef] [PubMed]
- Rolver, M.G.; Elingaard-Larsen, L.O.; Andersen, A.P.; Counillon, L.; Pedersen, S.F. Pyrazine ring-based Na+/H+ exchanger (NHE) inhibitors potently inhibit cancer cell growth in 3D culture, independent of NHE1. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Windschuh, J.; Zaiss, M.; Meissner, J.E.; Paech, D.; Radbruch, A.; Ladd, M.E.; Bachert, P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Cai, K.; Haris, M.; Hariharan, H.; Reddy, R. On B1 inhomogeneity correction of in vivo human brain glutamate chemical exchange saturation transfer contrast at 7T. Magn. Reson. Med. 2013, 69, 818–824. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaffari, M.; Nyström, N.N.; Li, A.; Bellyou, M.; Scholl, T.J.; Bartha, R. Intracellular Acidification in a Rat C6 Glioma Model following Cariporide Injection Investigated by CEST-MRI. Metabolites 2023, 13, 823. https://doi.org/10.3390/metabo13070823
Mozaffari M, Nyström NN, Li A, Bellyou M, Scholl TJ, Bartha R. Intracellular Acidification in a Rat C6 Glioma Model following Cariporide Injection Investigated by CEST-MRI. Metabolites. 2023; 13(7):823. https://doi.org/10.3390/metabo13070823
Chicago/Turabian StyleMozaffari, Maryam, Nivin N. Nyström, Alex Li, Miranda Bellyou, Timothy J. Scholl, and Robert Bartha. 2023. "Intracellular Acidification in a Rat C6 Glioma Model following Cariporide Injection Investigated by CEST-MRI" Metabolites 13, no. 7: 823. https://doi.org/10.3390/metabo13070823
APA StyleMozaffari, M., Nyström, N. N., Li, A., Bellyou, M., Scholl, T. J., & Bartha, R. (2023). Intracellular Acidification in a Rat C6 Glioma Model following Cariporide Injection Investigated by CEST-MRI. Metabolites, 13(7), 823. https://doi.org/10.3390/metabo13070823






