Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition
Abstract
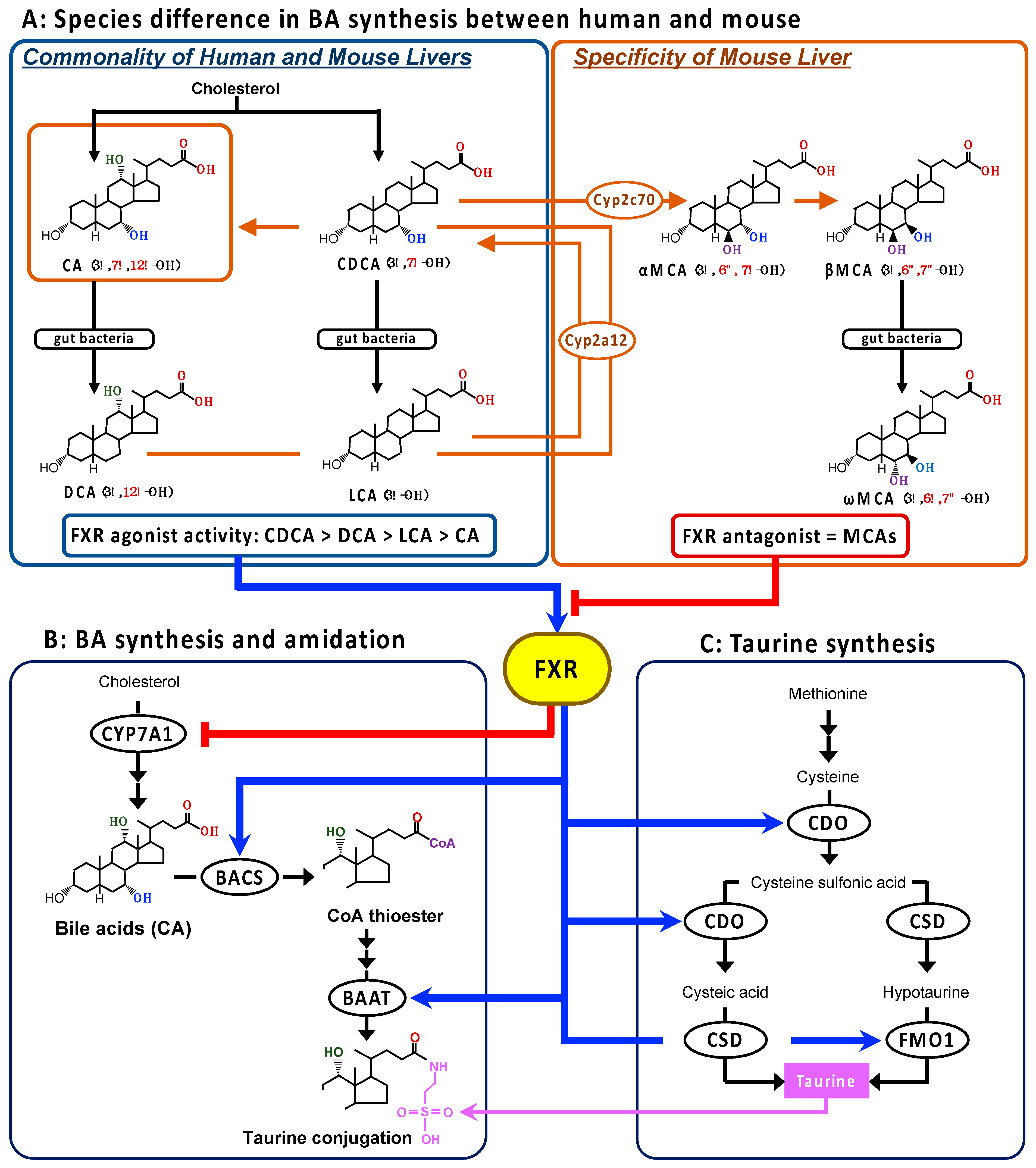
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. BA Analysis
2.4. Amino Acid Analysis
2.5. Total RNA Extraction and RT-PCR Analysis
2.6. Statistical Analysis
3. Results
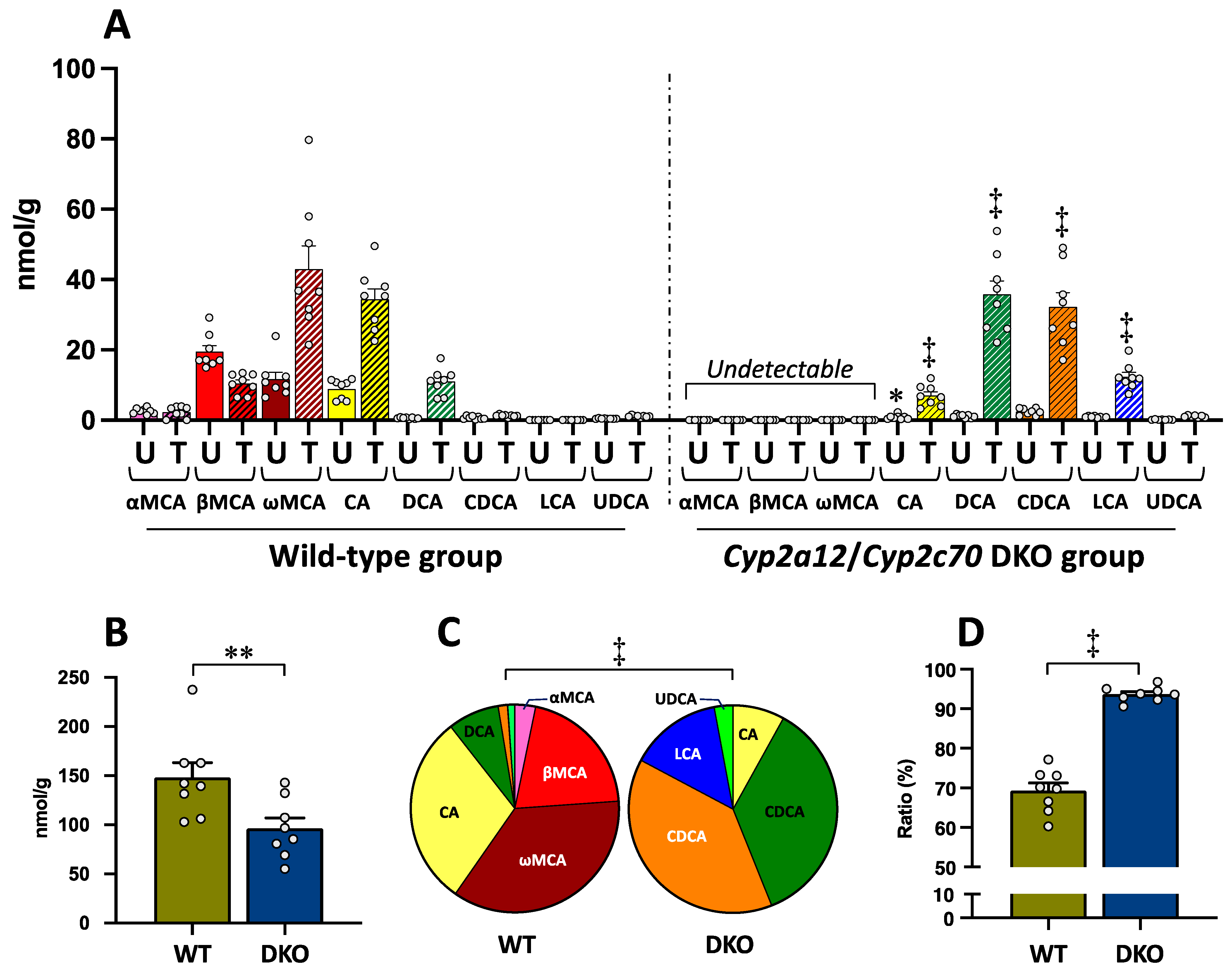
3.1. Bile Acid Concentrations in the Liver
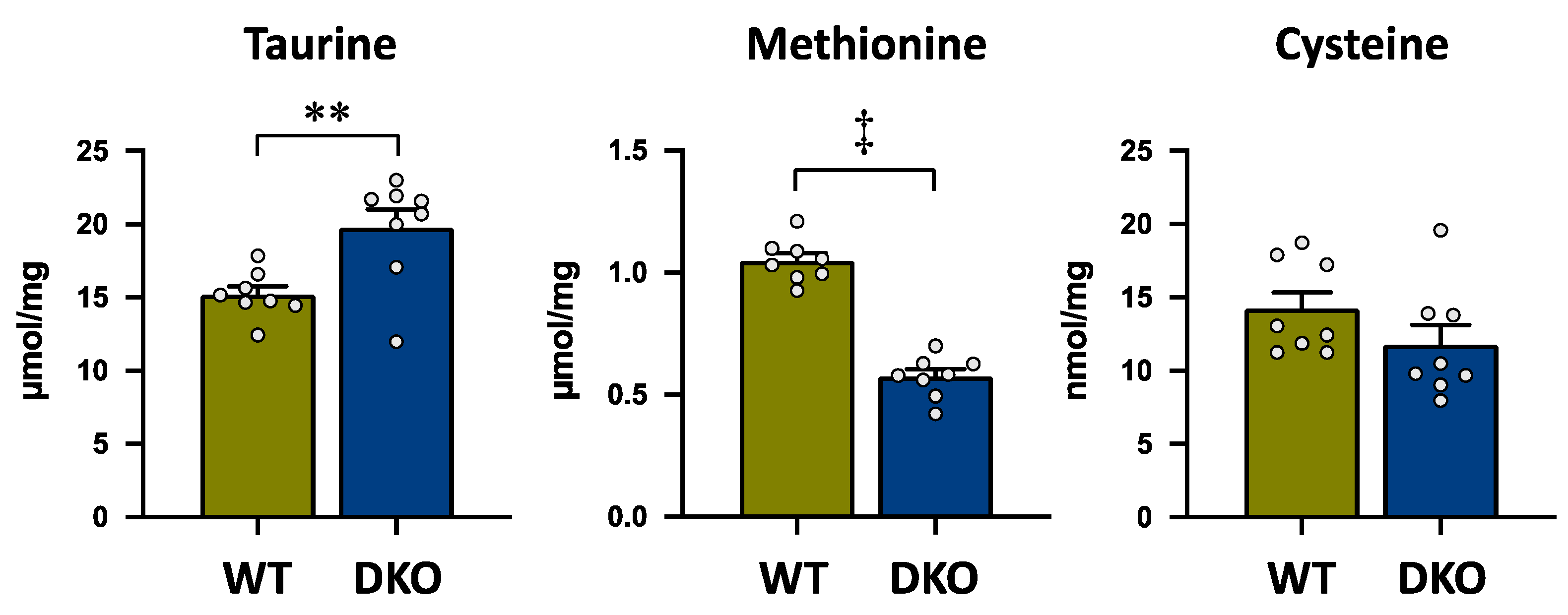
3.2. Amino Acid Concentrations in the Liver
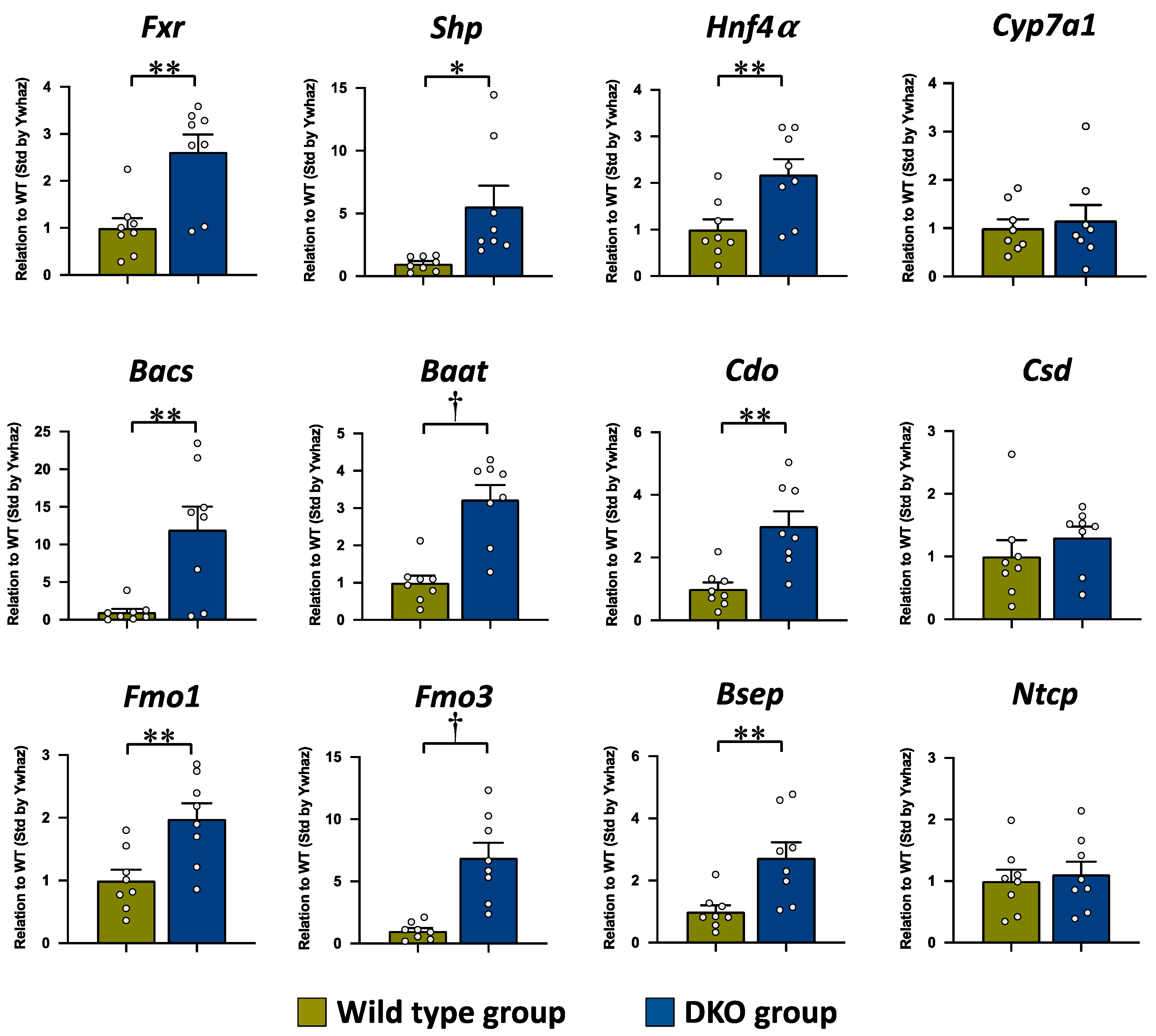
3.3. The mRNA Expression Levels in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APDS | 3-Aminopyridyl-N-hydroxysuccinimidyl carbamate |
| BA | bile acid |
| BAAT | bile acid-coenzyme A:amino acid N-acetyltransferase |
| BACS | ATP-dependent microsomal bile acid coenzyme A synthetase |
| BSEP | bile salt export pump |
| CA | cholic acid |
| CDCA | chenodeoxycholic acid |
| CDO | cysteine dioxygenase |
| CoA | coenzyme A |
| CSD | cysteine sulfinate decarboxylase |
| CYP | cytochrome P450 |
| DCA | deoxycholic acid |
| DKO | double knockout |
| FXR | farnesoid X receptor |
| FMO1/3 | flavin containing monooxygenase 1/3 |
| GCA | glycocholic acid |
| GCDCA | glycochenodeoxycholic acid |
| GDCA | glycodeoxycholic acid |
| GLCA | glycolithocholic acid |
| GUDCA | glycoursodeoxycholic acid |
| GW4064 | 3-[2-[2-chloro4-[[3-3(2,6-dichlorophenyl-5-(1-methylethyl-4-isoxazolyl]methoxy]phenyl]ethenyl]benzoic acid |
| HNF4α | hepatocyte nuclear factor 4α |
| HPLC-ESI-MS/MS | high-performance liquid chromatography–electrospray ionization tandem mass spectrometry |
| IS | internal standard |
| LCA | lithocholic acid |
| LXRα | liver X receptor α |
| MCA | muricholic acid |
| NTCP | sodium/taurocholate co-transporter peptide |
| PBS | phosphate-buffered saline |
| SE | standard error |
| SHP | small heterodimer partner |
| TBA | total bile acid |
| TCA | taurocholic acid |
| TCDCA | taurochenodeoxycholic acid |
| TDCA | taurodeoxycholic acid |
| TLCA | taurolithocholic acid |
| TUDCA | Tauroursodeoxycholic acid |
| TαMCA | tauro-α-muricholic acid |
| TβMCA | tauro-β-muricholic acid |
| TωMCA | tauro-ω-muricholic acid |
| UDCA | ursodeoxycholic acid |
| WT | wild type |
References
- Hosokawa, Y.; Matsumoto, A.; Oka, J.; Itakura, H.; Yamaguchi, K. Isolation and characterization of a cDNA for rat liver cysteine dioxygenase. Biochem. Biophys. Res. Commun. 1990, 168, 473–478. [Google Scholar] [CrossRef]
- Do, K.Q.; Tappaz, M.L. Specificity of cysteine sulfinate decarboxylase (CSD) for sulfur-containing amino-acids. Neurochem. Int. 1996, 28, 363–371. [Google Scholar] [CrossRef]
- Kaisaki, P.J.; Jerkins, A.A.; Goodspeed, D.C.; Steele, R.D.; Kaisakia, P.J. Cloning and characterization of rat cysteine sulfinic acid decarboxylase. Biochim. Biophys. Acta 1995, 1262, 79–82. [Google Scholar] [CrossRef]
- Reymond, I.; Sergeant, A.; Tappaz, M. Molecular cloning and sequence analysis of the cDNA encoding rat liver cysteine sulfinate decarboxylase (CSD). Biochim. Biophys. Acta 1996, 1307, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Tappaz, M.; Bitoun, M.; Reymond, I.; Sergeant, A. Characterization of the cDNA coding for rat brain cysteine sulfinate decarboxylase: Brain and liver enzymes are identical proteins encoded by two distinct mRNAs. J. Neurochem. 1999, 73, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Matsuzaki, Y. Taurine and liver diseases: A focus on the heterogeneous protective properties of taurine. Amino Acids 2014, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura-Shinya, Y.; Ebina, K.; Komine, S.; Ra, S.G.; Ishikura, K.; Ohmori, H.; Honda, A. N-acetyltaurine and acetylcarnitine production for the mitochondrial acetyl-CoA regulation in skeletal muscles during endurance exercises. Metabolites 2021, 11, 522. [Google Scholar] [CrossRef]
- Huxtable, R.J. Does taurine have a function? Introduction. Fed. Proc. 1980, 39, 2678–2679. [Google Scholar]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–491. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ito, T.; Baseggio Conrado, A.; Murakami, S. Editorial for special issue on Regulation and effect of taurine on metabolism. Metabolites 2022, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, H. Present status of research on catabolism and excretion of cholesterol. Adv. Lipid Res. 1963, 1, 335–385. [Google Scholar] [CrossRef] [PubMed]
- Sjovall, J. Dietary glycine and taurine on bile acid conjugation in man; bile acids and steroids 75. Proc. Soc. Exp. Biol. Med. 1959, 100, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Falany, C.N.; Johnson, M.R.; Barnes, S.; Diasio, R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:Amino acid n-acyltransferase. J. Biol. Chem. 1994, 269, 19375–19379. [Google Scholar] [CrossRef]
- Danielsson, H.; Sjovall, J. Bile acid metabolism. Annu. Rev. Biochem. 1975, 44, 233–253. [Google Scholar] [CrossRef]
- Katafuchi, T.; Makishima, M. Molecular basis of bile acid-FXR-FGF15/19 signaling axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef]
- Solaas, K.; Ulvestad, A.; Soreide, O.; Kase, B.F. Subcellular organization of bile acid amidation in human liver: A key issue in regulating the biosynthesis of bile salts. J. Lipid Res. 2000, 41, 1154–1162. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Houten, S.M. Peroxisomes and bile acid biosynthesis. Biochim. Biophys. Acta 2006, 1763, 1427–1440. [Google Scholar] [CrossRef] [Green Version]
- Bremer, J. Species differences in the conjugation of free bile acids with taurine and glycine. Biochem. J. 1956, 63, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Bruusgaard, A.; Thaysen, E.H. Increased ratio of glycine-taurine conjugated bile acids in the early diagnosis of terminal ileopathy. Preliminary report. Acta Med. Scand. 1970, 188, 547–548. [Google Scholar] [CrossRef]
- Hardison, W.G. Hepatic taurine concentration and dietary taurine as regulators of bile acid conjugation with taurine. Gastroenterology 1978, 75, 71–75. [Google Scholar] [CrossRef]
- Modica, S.; Gadaleta, R.M.; Moschetta, A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal. 2010, 8, e005. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef]
- Miyazaki, T.; Honda, A.; Matsuzaki, Y. Regulation of taurine conjugation and biosynthesis by bile acids through farnesoid x receptor activation. Hepatol. Res. 2014, 44, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Pircher, P.C.; Kitto, J.L.; Petrowski, M.L.; Tangirala, R.K.; Bischoff, E.D.; Schulman, I.G.; Westin, S.K. Farnesoid x receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 2003, 278, 27703–27711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, T.A.; Matsumoto, Y.; Matsumoto, H.; Xie, Y.; Hirschberger, L.L.; Stipanuk, M.H.; Anakk, S.; Moore, D.D.; Watanabe, M.; Kennedy, S.; et al. Cysteine sulfinic acid decarboxylase regulation: A role for farnesoid x receptor and small heterodimer partner in murine hepatic taurine metabolism. Hepatol. Res. 2014, 44, E218–E228. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 2020, 61, 54–69. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Carotti, A.; Marinozzi, M.; Custodi, C.; Cerra, B.; Pellicciari, R.; Gioiello, A.; Macchiarulo, A. Beyond bile acids: Targeting farnesoid x receptor (FXR) with natural and synthetic ligands. Curr. Top. Med. Chem. 2014, 14, 2129–2142. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell. Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Honda, A.; Miyazaki, T.; Morishita, Y.; Hirayama, T.; Iwamoto, J.; Nakamoto, N.; Ikegami, T. Sex-, age-, and organ-dependent improvement of bile acid hydrophobicity by ursodeoxycholic acid treatment: A study using a mouse model with human-like bile acid composition. PLoS ONE 2022, 17, e0271308. [Google Scholar] [CrossRef]
- Iwamoto, J.; Honda, A.; Miyazaki, T.; Monma, T.; Ueda, H.; Morishita, Y.; Yara, S.I.; Hirayama, T.; Ikegami, T. Western diet changes gut microbiota and ameliorates liver injury in a mouse model with human-like bile acid composition. Hepatol. Commun. 2021, 5, 2052–2067. [Google Scholar] [CrossRef]
- Yamashita, M.; Honda, A.; Shimoyama, S.; Umemura, M.; Ohta, K.; Chida, T.; Noritake, H.; Kurono, N.; Ichimura-Shimizu, M.; Tsuneyama, K.; et al. Breach of tolerance versus burden of bile acids: Resolving the conundrum in the immunopathogenesis and natural history of primary biliary cholangitis. J. Autoimmun. 2023, 136, 103027. [Google Scholar] [CrossRef]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Murakami, M.; Iwamoto, J.; Honda, A.; Tsuji, T.; Tamamushi, M.; Ueda, H.; Monma, T.; Konishi, N.; Yara, S.; Hirayama, T.; et al. Detection of gut dysbiosis due to reduced clostridium subcluster XIVa using the fecal or serum bile acid profile. Inflamm. Bowel Dis. 2018, 24, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Shoda, J.; Mahara, R.; Osuga, T.; Tohma, M.; Ohnishi, S.; Miyazaki, H.; Tanaka, N.; Matsuzaki, Y. Similarity of unusual bile acids in human umbilical cord blood and amniotic fluid from newborns and in sera and urine from adult patients with cholestatic liver diseases. J. Lipid Res. 1988, 29, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Sasaki, S.I.; Toyoda, A.; Wei, F.Y.; Shirai, M.; Morishita, Y.; Ikegami, T.; Tomizawa, K.; Honda, A. Impaired bile acid metabolism with defectives of mitochondrial-tRNA taurine modification and bile acid taurine conjugation in the taurine depleted cats. Sci. Rep. 2020, 10, 4915. [Google Scholar] [CrossRef] [Green Version]
- Namikawa-Kanai, H.; Miyazaki, T.; Matsubara, T.; Shigefuku, S.; Ono, S.; Nakajima, E.; Morishita, Y.; Honda, A.; Furukawa, K.; Ikeda, N. Comparison of the amino acid profile between the nontumor and tumor regions in patients with lung cancer. Am. J. Cancer Res. 2020, 10, 2145–2159. [Google Scholar] [PubMed]
- Shimbo, K.; Oonuki, T.; Yahashi, A.; Hirayama, K.; Miyano, H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2009, 23, 1483–1492. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time pcr experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Veeravalli, S.; Phillips, I.R.; Freire, R.T.; Varshavi, D.; Everett, J.R.; Shephard, E.A. Flavin-containing monooxygenase 1 catalyzes the production of taurine from hypotaurine. Drug. Metab. Dispos. 2020, 48, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell. Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.; Nomura, Y.; Kadowaki, M.; Takase, H.; Takano, K.; Takeuchi, N. Age-related changes in cholesterol and bile acid metabolism in rats. J. Lipid Res. 1978, 19, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Vignozzi, L.; Maggi, M.; Adorini, L. Farnesoid x receptor activation improves erectile dysfunction in models of metabolic syndrome and diabetes. Biochim. Biophys. Acta 2011, 1812, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H.; Blake, W.R.; Conley, J. Unusual organic osmolytes in deep-sea animals: Adaptations to hydrostatic pressure and other perturbants. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.; Hayes, K. The biology of taurine in nutrition and development. In Advances in Nutritional Research; H. H. Draper. 9; Springer: Boston, MA, USA, 1980; Chapter 9; pp. 231–299. [Google Scholar]
- Styles, N.A.; Shonsey, E.M.; Falany, J.L.; Guidry, A.L.; Barnes, S.; Falany, C.N. Carboxy-terminal mutations of bile acid CoA:N-acyltransferase alter activity and substrate specificity. J. Lipid Res. 2016, 57, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Carey, M.C. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J. Lipid Res. 1982, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, Y.; Qi, Y.; Yang, W.; Li, Y. Farnesoid x receptor agonists as therapeutic target for cardiometabolic diseases. Front. Pharmacol. 2020, 11, 1247. [Google Scholar] [CrossRef]
- Inoue, Y.; Yu, A.M.; Yim, S.H.; Ma, X.; Krausz, K.W.; Inoue, J.; Xiang, C.C.; Brownstein, M.J.; Eggertsen, G.; Bjorkhem, I.; et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J. Lipid Res. 2006, 47, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Crestani, M.; Sadeghpour, A.; Stroup, D.; Galli, G.; Chiang, J.Y. Transcriptional activation of the cholesterol 7alpha-hydroxylase gene (CYP7A) by nuclear hormone receptors. J. Lipid Res. 1998, 39, 2192–2200. [Google Scholar] [CrossRef]
- Stroup, D.; Chiang, J.Y. Hnf4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1). J. Lipid Res. 2000, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, G.P.; Lee, Y.H.; Lambert, G.; Ward, J.M.; Gonzalez, F.J. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 2001, 21, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chiang, J.Y. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): Roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J. Biol. Chem. 2001, 276, 41690–41699. [Google Scholar] [CrossRef] [Green Version]
- Honda, A.; Salen, G.; Matsuzaki, Y.; Batta, A.K.; Xu, G.; Hirayama, T.; Tint, G.S.; Doy, M.; Shefer, S. Disrupted coordinate regulation of farnesoid X receptor target genes in a patient with cerebrotendinous xanthomatosis. J. Lipid Res. 2005, 46, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Yokogoshi, H.; Mochizuki, H.; Nanami, K.; Hida, Y.; Miyachi, F.; Oda, H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J. Nutr. 1999, 129, 1705–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, N.; Umeda, C.; Oda, H.; Yokogoshi, H. The effect of taurine on the cholesterol metabolism in rats fed diets supplemented with cholestyramine or high amounts of bile acid. J. Nutr. Sci. Vitaminol. (Tokyo) 2003, 49, 21–26. [Google Scholar] [CrossRef]
- Murakami, S.; Fujita, M.; Nakamura, M.; Sakono, M.; Nishizono, S.; Sato, M.; Imaizumi, K.; Mori, M.; Fukuda, N. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin. Exp. Pharmacol. Physiol. 2016, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.H.; Jia, Y.; Jun, H.J.; Lee, J.H.; Hwang, K.Y.; Choi, D.W.; Um, S.J.; Lee, B.Y.; You, S.G.; Lee, S.J. Taurine is a liver X receptor-alpha ligand and activates transcription of key genes in the reverse cholesterol transport without inducing hepatic lipogenesis. Mol. Nutr. Food Res. 2012, 56, 900–911. [Google Scholar] [CrossRef]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, B.; Watson, M.A.; Kim, H.; Miao, J.; Kemper, J.K.; Kliewer, S.A. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol. Endocrinol. 2003, 17, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Accession Number | Sequence (5′-3′) | Product Size (bp) | |
|---|---|---|---|---|
| Bacs | NM_009512.2 | F | TCT ATG GCC TAA AGT TCA GGC G | 75 |
| R | CTT GCC GCT CTA AAG CAT CC | |||
| Baat | NM_007519 | F | GTG TAG AGT TTC TCC TGA GAC AT | 199 |
| R | CTG GGT ACA GGT GGG TAG AC | |||
| Bsep | NM_021022 | F | AGC AGG CTC AGC TGC ATG AC | 122 |
| R | AAT GGC CCG AGC AAT AGC AA | |||
| Cdo | NM_033037.4 | F | GGG GAC GAA GTC AAC GTG G | 162 |
| R | ACC CCA GCA CAG AAT CAT CAG | |||
| Csd | NM_001359126 | F | CCA GGA CGT GTT TGG GAT TGT | 193 |
| R | ACC AGT CTT GAC ACT GTA GTG A | |||
| Cyp7a1 | NM_007824 | F | AAG AGC AAC TAA ACA ACC TG | 244 |
| R | TTC CCA CTT TCA TCA AGG TA | |||
| Fmo1 | NM_010231.3 | F | CCA TCA AGT GCT GCC TGG AA | 143 |
| R | CCT GCT GCT GTT AGA AAC CAC AGA | |||
| Fmo3 | NM_008030.1 | F | CCA CAG CAG GGA CTA TAA GGA A | 129 |
| R | GAG CTG ATG GTG ACC TTC TGA | |||
| Fxr | NM_001163700 | F | GGT CAT GCA GAC CTG TTG GAA | 142 |
| R | TGA CGA TCG CTG TGA GCA GA | |||
| Hnf4α | NM_008261.3 | F | ATG CCT GCC TCA AAG CCA TC | 67 |
| R | ATC TTG CCC GGG TCA CTC A | |||
| Ntcp | NM_001177561 | F | AAG GCC ACA CTA TGT ACC CTA CGT C | 106 |
| R | GAT GCT GTT GCC CAC ATT GA | |||
| Shp | NM_011850 | F | CAA GGA GTA TGC GTA CCT GA | 232 |
| R | GAT AGG GCG GAA GAA GAG AT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, T.; Ueda, H.; Ikegami, T.; Honda, A. Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition. Metabolites 2023, 13, 824. https://doi.org/10.3390/metabo13070824
Miyazaki T, Ueda H, Ikegami T, Honda A. Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition. Metabolites. 2023; 13(7):824. https://doi.org/10.3390/metabo13070824
Chicago/Turabian StyleMiyazaki, Teruo, Hajime Ueda, Tadashi Ikegami, and Akira Honda. 2023. "Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition" Metabolites 13, no. 7: 824. https://doi.org/10.3390/metabo13070824
APA StyleMiyazaki, T., Ueda, H., Ikegami, T., & Honda, A. (2023). Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition. Metabolites, 13(7), 824. https://doi.org/10.3390/metabo13070824








