Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids
Abstract
:1. Introduction
2. Identification of Secondary Metabolites
2.1. Alkaloids
2.2. Phenanthrenes
2.3. Bibenzylates
2.4. Other Secondary Metabolites
| NO. | Compound | Plant Source | Reference |
|---|---|---|---|
| Alkaloids | |||
| 1 | Dendrobine | C, P | [31,32] |
| 2 | N-isopentenyl-dendrobinium | C, P | [31,32] |
| 3 | N-isopentenyl-dendroxinium | C, P | [31,32] |
| 4 | Nobilonine | C, P | [31,32] |
| 5 | Dendramine | P | [32] |
| 6 | Mubironine A | P | [32] |
| 7 | Findlayine D | P | [32] |
| 8 * | dendronboic acid | N | [8] |
| 9 | N-p-Cinnamoyl-tyramine | P | [32] |
| 10 * | N-methoxylcarbonyldendrobine | N | [8] |
| 11 | Crepidine | P | [32] |
| 12 | Dendrocrepidine B | P | [32] |
| 13 | Dendrocrepine | P | [32] |
| 14 | Dendrocrepine C | P | [32] |
| 15 | Credidamine | P | [32] |
| 16 | Dendrocrepidine D | P | [32] |
| 17 | Homocrepidine B | P | [32] |
| 18 * | Crepidtumines A | C | [10] |
| 19 * | Crepidtumines B | C | [10] |
| 20 * | Crepidatumines C | C | [9] |
| 21 * | Crepidatumines D | C | [9] |
| 22 | ginsenine | BU, R | [33,34] |
| 23 | anocetochine | BU | [33] |
| 24 | Neoechinulin A | L | [35] |
| 25 | Indole-3-aldehyd | ST | [36] |
| 26 # | acortatarin A | J | [37] |
| 27 | Huperzine A | BU | [33] |
| Phenanthrenes | |||
| 28 * | 2,2′,2″,7,7′,7″-hexahydroxy-4,4′,4″-trimethoxy-[9,9′,9″,10,10′,10″]-hexahydro-1,8,1′,6″-triphenanthrene | ST | [12] |
| 29 | 2,5-dihydroxy-4-methoxy-9,10-dihydrophenanthrene | PL, M | [20,38] |
| 30 | 2,5,7-trihydroxy-4-methoxy-9,10-dihydrophenanthrene | B | [39] |
| 31 | coelonin | H | [40] |
| 32 | 4,7-dimethoxy-9,10-dihydrophenanthren-2-ol | ST | [36] |
| 33 | 2-methoxy-9,10-dihydrophenanthrene-4,5-diol | ST | [36] |
| 34 | 4-methoxy-9,10-dihydrophenanthrene-1,2,7-triol | ST | [36] |
| 35 | 1,4,7-trihydroxy-2-methoxy-9,10-dihydrophenanthrene | PL | [20] |
| 36 | calanhydroquinone C | PL | [20] |
| 37 # | 2,7-Dihydroxy-3,5-dimethoxy-9,10-dihydrophenanthrene | ST | [12] |
| 38 # | 2,3,7-Trihydroxy-4-methoxy-9,10-dihydrophenanthrene | ST | [12] |
| 39 * | spiranthesphenanthrene A | SI | [13] |
| 40 * | spiranthesphenanthrene B | SI | [13] |
| 41 * | spiranthesphenanthrene C | SI | [13] |
| 42 * | spiranthesphenanthrene D | SI | [13] |
| 43 * | spiranthesphenanthrene E | SI | [13] |
| 44 * | spiranthesphenanthrene F | SI | [13] |
| 45 | Dendrocandin P1 | O | [41] |
| 46 | Dendrocandin P2 | O | [41] |
| 47 * | bletilore A | ST | [36] |
| 48 | chrysotoxol A | L | [35] |
| 49 | 3,7-dihydroxy-2,4-dimethoxy-phenanthrene | PL | [20] |
| 50 | 2,5-dihydroxy-4-methoxyphenanthrene | HA, M | [22,38] |
| 51 | 2,5-dihydroxy-4,9-dimethoxyphenanthrene | HA | [22] |
| 52 * | 2-hydroxy-3,4,7-trimethoxyphenanthrene | O | [42] |
| 53 | 2,4,8-trimethoxy phenanthrene-3,7-diol | N | [43] |
| 54 | 5,7-dimethoxyphenanthrene-2,6-diol | ST | [36] |
| 55 | 1,5-dimethoxyphenanthrene-2,7-diol | ST | [36] |
| 56 | 7-hydroxy-2-methoxy-1,4-phenanthrenequinone | HA | [22] |
| 57 * | Bulbocodioidins A (9R,9S) | BUL | [14] |
| 58 * | Bulbocodioidins B (9R,9S) | BUL | [14] |
| 59 * | Bulbocodioidins C (9R,9S) | BUL | [14] |
| 60 * | Bulbocodioidins D (10S,10R) | BUL | [14] |
| 61 * | 2,3-dimethoxyl-7-hydroxyl-1,4-phenanthrenedione | F | [15] |
| 62 * | 2-methoxyl-3-methyl-7-hydroxyl-9,10-dihydro-1,4-phenanthrenedione | F | [15] |
| 63 | densiflorol B | N | [43] |
| 64 | cypripedin | N | [43] |
| 65 | 3-hydroxymethyl-9-methoxy-2-(4′-hydroxy-3′, 5′-dimethoxyphenyl)-2,3,6,7-tetrahydrophenanthro [4, 3-b] furan-5, 11-diol | M | [38] |
| 66 # | 4,7,4′,7′-tetrahydroxy-2,2′-dimethoxy-1,1′-biphenanthrene | ST | [12] |
| Bibenzylates | |||
| 67 # | 3-Hydroxy-5-methoxybibenzyl | ST | [12] |
| 68 | 3,3′,5-trihydroxybibenzyl | L | [35] |
| 69 | batatasin III | L, M, B, H | [35,38,39,40] |
| 70 | 3,4′-dihydroxy-5-methoxybibenzyl | H | [40] |
| 71 | 3-hydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 72 | 3-O-methylgigantol | H | [40] |
| 73 | gigantol | H | [40] |
| 74 | 3,4-dihydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 75 # | Moscatilin | PL, H | [20,40] |
| 76 | 3,5,5′-trihydroxy-4′-methoxybibenzyl | ST | [36] |
| 77 | 3-O-methyldihydropinosylvin | ST, M | [36,38] |
| 78 | Dihydropinosylvin | ST | [36] |
| 79 | 3,5,5′-trihydroxybibenzyl | ST | [36] |
| 80 | 3,5,4′-trihydroxybibenzyl | ST | [36] |
| 81 | 4,3′,5′-trihydroxy-3-methoxybibenzyl | HA | [22] |
| 82 | 4,3′-dihydroxy-3,5′-dimethoxybibenzyl | HA | [22] |
| 83# | 3′-hydroxy-3,4,4′,5-tetramethoxybibenzyl | PL | [20] |
| 84 | 4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl | M, HA | [22,38] |
| 85 | dendrosinen B | B | [39] |
| 86 | 4,3′-dihydroxy-3,5-dimethoxybibenzyl | HE | [21] |
| 87 | 4′, 5-dihydroxy-3, 3′-dimethoxybiphezyl | B | [39] |
| 88 | 3, 3′-dihydroxy-4, 5-dimethoxybiphezyl | B | [39] |
| 89 # | 3-methylgigantol | PL | [20] |
| 90 * | 2-chloro-3,4′-dihydroxy-3′,5-dimethoxybibenzyl | PL | [20] |
| 91 | 4,5-dihydroxy-3,3′,α-trimethoxybibenzyl | HE | [21] |
| 92 | dendrocandin A | H | [40] |
| 93 | (S)-3,4,α-trihydroxy-4′,5-dimethoxybibenzyl | H | [40] |
| 94 | densiflorol A | H | [40] |
| 95 | 4,4′-dihydroxyl-3,5-dimethoxylbibenzyl | L | [35] |
| 96 | 4,α-dihydroxy-3,5,3′-trimethoxybibenzyl | HE | [21] |
| 97 | dendrosinen D | B | [39] |
| 98 * | 3,4,α-trihydroxy-5,3′-dimethoxybibenzyl | HE | [21] |
| 99 | trigonopol B | L | [35] |
| 100 | 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl | HE | [21] |
| 101 * | 3, α-dihydroxy-4,5,3′-trimethoxybibenzyl | HA | [22] |
| Other secondary metabolites | |||
| 102 # | p-hydroxybenzyl methyl ether | ST | [12] |
| 103 | p-Hydroxybenzyl ether | ST | [12] |
| 104 | p-hydroxybenzyl alcohol | ST | [12] |
| 105 | 4-methoxy-phenylethanol | S | [44] |
| 106 | dihydroconiferyl alcohol | HU | [45] |
| 107 | anoectosterol | BU, R | [33,34] |
| 108 # | (E) -4- (2-methoxyvinyl) benzene-1,2-diol | N | [43] |
| 109 | p-hydroxybenzyl alcohol | ST | [36] |
| 110 | 3-hydroxybenzaldehyde | S | [44] |
| 111 | 3,4-dihydroxy-5-methoxy benzaldehyde | HU | [45] |
| 112 # | 3,5-dihydroxy-4-hydroxy benzaldehyde | HU | [45] |
| 113 | 4-hydroxy-3-methoxy benzaldehyde | HU | [45] |
| 114 | 5-hydroxymethyl furfural | HU | [45] |
| 115 | coumarin | N | [43] |
| 116 # | moellendorffiline | N | [43] |
| 117 # | isopimpinellin | N | [43] |
| 118 | syringaresinol | L, HE | [21,35] |
| 119 | neoolivil | B | [39] |
| 120 | Matairesinol | S | [44] |
| 121 # | methyl melilotate | M | [38] |
| 122 # | ethyl melilotate | M | [38] |
| 123 * | methyl 2-(acetyloxy) benzenepropanoate | M | [38] |
| 124 | Dihydroconiferyl dihydrop-hydroxycinnamate | B | [39] |
| 125 | eis-p-hydroxyl ethyl cinnamate | S | [44] |
| 126 | p-hydroxyphenylpropionic ethyl ester | S | [44] |
| 127 | methyl 3-(4-hydroxyphenyl) propionate | HU | [45] |
| 128 | (9Z,12Z)-methyl octadeca-9,12-dienoate | S | [44] |
| 129 | hexadecanoic acid 2,3-dihydroxypropyl ester | HE | [21] |
| 130 | p-hydroxyphenyl-propionic acid | B | [39] |
| 131 | p-hydroxybenzoic acid | HU | [45] |
| 132 | p-hydroxycinnamic ac | B | [39] |
| 133 | ferulic acid | B | [39] |
| 134 | caffeic acid | B | [39] |
| 135 | 4-Hydroxy-2-methoxy-3,6-dimethylbenzoic acid | H | [40] |
| 136 # | 2-Hydroxy-4-methoxy-3,6-dimethylbenzoic acid | B | [39] |
| 137 | dihydroferulic acid | HU | [45] |
| 138 # | 4-O-β-D-glucopyranosyl coniferyl aldehyde | HU | [45] |
| 139 | 4-allyl-2,6-dimethoxyphenyl glucopyranoside | HU | [45] |
| 140 # | 3,4,5-trihydroxyallylbenzene-3-O-β-D-glucopyranosyl-4-O-β-D-glucopyranoside | HU | [45] |
| 141 | (7S,8R)-syringylglycerol-8-O-4′-sinapyl ether 4-O-β-D-glucopyranoside | HU | [45] |
| 142 # | 3,4,5-trimethoxyphenol-1-O-β-D-glucopyranoside | HU | [45] |
| 143 | gastrodin | HU | [45] |
| 144 | (+)-syringaresinol-4-O-β-D-glucopyranoside | HU | [45] |
| 145 | Liriodendrin | HU | [45] |
| 146 | quercetin | BU | [33] |
| 147 | 8-p-hydroxybenzyl quercetin | BU | [33] |
| 148 | 5,4′-dihydroxy-6,7,3′-trimethoxyflavone | BU | [33] |
| 149 | naringenin | L, N, HU | [35,43,45] |
| 150 | 5,4′-dihydroxy-7, 3′, 5′-trimethoxyflavanone | L | [35] |
| 151 | 5,7, 4′-trihydroxy-3′, 5′-dimethoxyflavanone | L | [35] |
| 152 # | carthamidin | H | [40] |
| 153 # | periloyrine | HU | [45] |
| 154 | N-trans-cinnamoyltyramine | HE | [21] |
| 155 # | (9Z,11E) -13-hydroxy-9,11-octadecadienoic acid | M | [38] |
3. Pharmacological Activity and Mechanism of Secondary Metabolites of Medicinal Orchids
3.1. Antibacterial Activity
3.2. Regulation of Free Radical Metabolism and Antioxidants
3.3. Biological Activity against Cancer Cells
3.4. Other Biological Functions and Potential Applications
3.5. Potential Activity in Organic Extracts
4. Conventional Methods for Increasing the Yield of Secondary Metabolites of Medicinal Orchids
4.1. Plant Medium System
4.2. Biotransformation Pathways
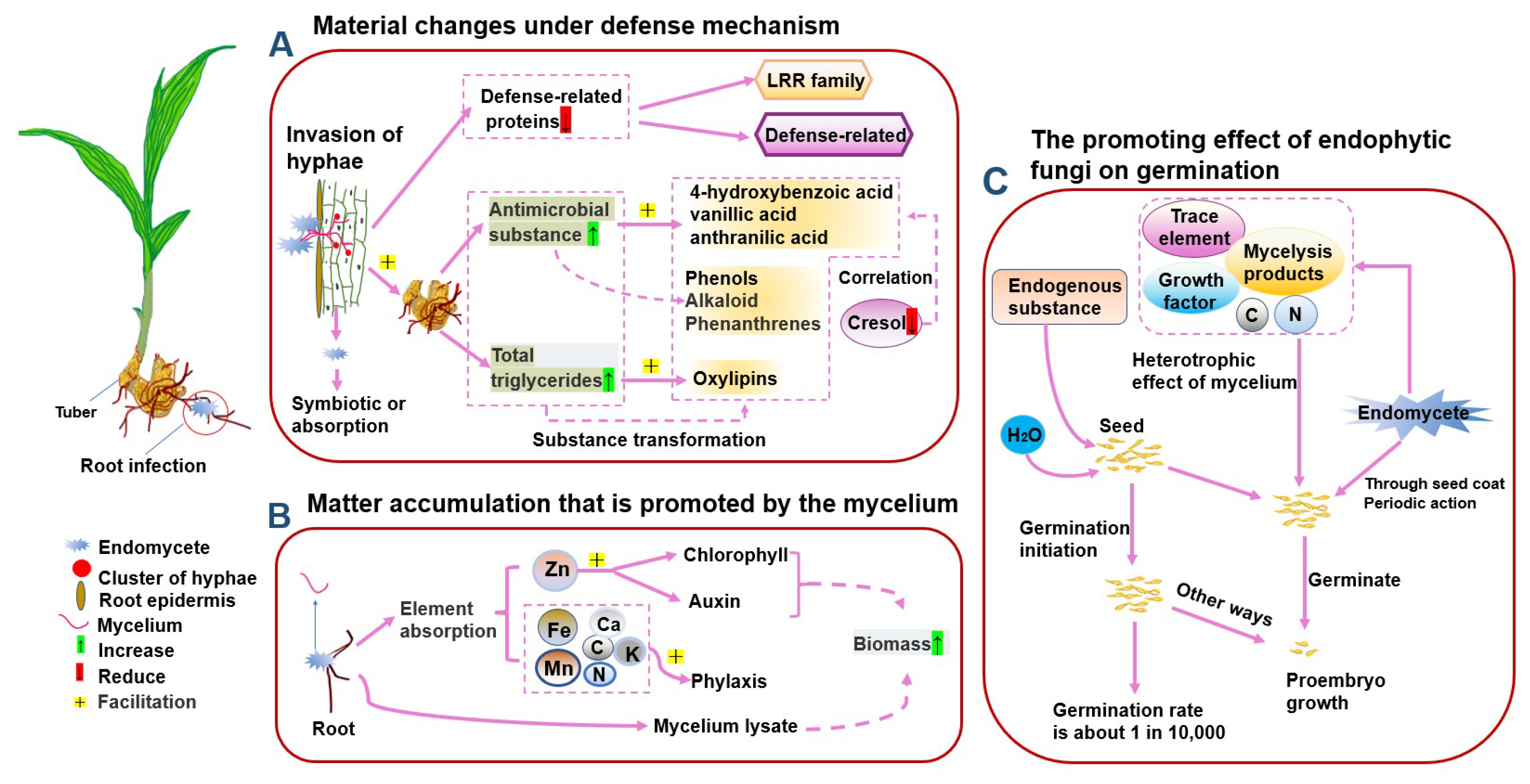
4.3. Accumulation-Promoting Effect of Endophytic Fungi
4.4. Synthesis of Secondary Metabolites under Environmental Stress
5. Prospectives
5.1. Combined Application of Active Secondary Metabolites
5.2. Application Optimization of Active Secondary Metabolites
5.3. Modern Reproduction and Breeding of Medicinal Orchids
Author Contributions
Funding
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Su, W.H.; Zhang, G.F.; Li, X.H.; Ou, X.K. Relationship between accumulation of secondary metabolism in medicinal plant and environmental condition. Chin. Tradit. Herb. Drugs 2005, 36, 139–142. [Google Scholar] [CrossRef]
- Liu, S.J. Distribution and functions of plants’ secondary metabolites. J. Taishan Univ. 2003, 25, 91–94. [Google Scholar] [CrossRef]
- Dong, Y.L.; Pan, X.W. Introduction to plant secondary metabolites. Biotechnol. Bull. 2002, 37, 17–19. [Google Scholar] [CrossRef]
- Liu, H.D.; Pan, L.L.; Zhou, X.; Wan, N.; Wu, Y.F.; Li, B. Research progress on chemical constituents and pharmacological activities of alkaloids in Orchidaceae plants. Chin. Tradit. Herb. Drugs 2019, 50, 731–744. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, Y.C.; Han, B.; Yang, Y.B.; Wang, Z.; Sun, Z.Y. Research progress on constituents of alkaloids in plants from Dendrobium Sw. Chin. Tradit. Herb. Drugs 2019, 50, 3246–3254. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, J.; Zhang, J.H.; Zhu, G.F.; Liang, C.L.; Ye, Q.S. Comparison of Polysaccharide and Alkaloid Contents in Dendrobium. Chin. Agric. Sci. Bull. 2015, 31, 242–246. [Google Scholar] [CrossRef]
- Zhang, M.S.; Linghu, L.; Wang, G.H.; He, Y.Q.; Sun, C.X.; Xiao, S.J. Dendrobine-type alkaloids from Dendrobium nobile. Nat. Prod. Res. 2022, 36, 5393–5399. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Yang, R.; Zhou, H.; Bai, Y.; Yu, M.; Ding, G.; Li, B. Crepidatumines C and D, Two New Indolizidine Alkaloids from Dendrobium crepidatum Lindl. ex Paxt. Molecules 2019, 24, 3071. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Yang, R.; Li, Z.; Zhou, H.; Bai, Y.; Yu, M.; Li, B.; Ding, G. Crepidtumines A and B, Two Novel Indolizidine Alkaloids from Dendrobium crepidatum. Sci. Rep. 2020, 10, 9564. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, H.; Ding, X.; Liu, J.; Wang, X.; Hu, L.; Liu, M.; Zhang, C. Anti-inflammatory octahydroindolizine alkaloid enantiomers from Dendrobium crepidatum. Bioorg. Chem. 2020, 100, 103809. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C. Establishment of Bletilla Striata Liquid Suspension Culture System and Determination of Secondary Metabolites. Master’s Degree, Zunyi Medical University, Zunyi, China, 2019. [Google Scholar]
- Liu, L.; Yin, Q.M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.K.; Luo, X.; Zhang, X.W. Bioactivity-Guided Isolation of Cytotoxic Phenanthrenes from Spiranthes sinensis. J. Agric. Food Chem. 2019, 67, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.Y.; Wang, C.; Han, S.W.; Sun, M.H.; Li, S. Phenanthrenequinone enantiomers with cytotoxic activities from the tubers of Pleione bulbocodioides. Org. Biomol. Chem. 2019, 17, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.N.; Wu, Y.P.; Chen, Y.J.; Liu, W.J.; Wang, J.X.; He, F.; Jiang, L. Two new stilbenoids from aerial parts of Flickingeria fimbriata. J. Asian Nat. Prod. Res. 2019, 21, 117–122. [Google Scholar] [CrossRef]
- Ren, G.; Chen, Y.T.; Ye, J.B.; Zhong, G.Y.; Xiao, C.Y.; Deng, W.Z.; Chen, Y.L. Phytochemical investigation of leaves of Dendrobium officinale. Chin. Tradit. Herb. Drugs 2020, 51, 3637–3644. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, J.H.; Xu, H.; Chou, G.X.; Wang, Z.T. Bibenzyls from Dendrobium officinale. China J. Chin. Mater. Medica 2021, 46, 3853–3858. [Google Scholar] [CrossRef]
- Meng, W.T.; Meng, X.; Niu, L.T.; Zhang, S.S.; Ouyang, C.J.; Ding, C.H.; Zhu, L.J.; Zhang, X. A new bibenzyl derivative from stems of Dendrobium officinale. China J. Chin. Mater. Medica 2023, 48, 700–706. [Google Scholar] [CrossRef]
- Wang, Y.C.; Han, B.; Li, Z.J.; Nie, X.T.; Yang, M.Z.; Wang, W.; Sun, Z.Y. Determination of the Compounds of Dendrobium pendulum Roxb.by UPLC-Q-TOF-MS. Chin. Pharm. J. 2021, 56, 708–714. [Google Scholar]
- Chen, D.N.; Wang, Y.Y.; Liu, W.J.; Chen, Y.J.; Wu, Y.P.; Wang, J.X.; He, F.; Jiang, L. Stilbenoids from aerial parts of Dendrobium plicatile. Nat. Prod. Res. 2020, 34, 323–328. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Z.Y.; Shang, Z.M.; Zhang, M.S.; Li, X.F.; Zhang, J.Y.; Xiao, S.J. Chemical constituents of Dendrobium hercoglossum. Chin. Tradit. Herb. Drugs 2020, 51, 3126–3130. [Google Scholar] [CrossRef]
- Shang, Z.M.; Xia, D.; Cheng, L.; Liu, G.Y.; Zhang, M.S.; Zhang, J.Y.; Li, X.F.; Xiao, S.J. Chemical constituents from Dendrobium hancockii. Chin. Tradit. Herb. Drugs 2019, 50, 3760–3763. [Google Scholar] [CrossRef]
- Liu, H.; Wei, X.Y.; Ma, H.; Qu, H.; Sun, Y. Analysis on pigment composition of petals of several native Cymbidium Sw.species. Jiangsu J. Agric. Sci. 2022, 38, 1657–1677. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Nie, X.T.; Li, Z.J.; Zhang, M.; Yang, Y.B.; Wang, W.; Sun, Z.Y. Research Progress on Active Ingredients of Flavonoids in Traditional Chinese Medicine Dendrobium. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 197–206. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, Y.; Lei, Z.; Wei, G. UHPLC-ESI-MS Analysis of Purified Flavonoids Fraction from Stem of Dendrobium denneaum Paxt. and Its Preliminary Study in Inducing Apoptosis of HepG2 Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 8936307. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, W.Y.; Zou, H.; Dai, Y.M. Transcriptome Analysis on Pathway of and Genes Related to Flavonoid Synthesis in Dendrobium officinale. Fujian J. Agric. Sci. 2019, 34, 1019–1025. [Google Scholar] [CrossRef]
- Lyu, C.G.; Yang, J.; Kang, C.Z.; Li, Z.H.; Ma, Z.H.; Guo, L.P.; Wang, Y.P. Determination of 10 Flavonoids by UPLC-MS/MS and Analysis of Polysaccharide Contents and Compositions in Dendrobii Officinalis Caulis from Different Habitats. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 47–52. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhou, C.H.; Zhang, L.K.; Jiang, M.; Xie, Z.S.; Yuan, Y.; Huang, Y.C.; Luo, Y.Y.; Wei, G. Isolation and Identification of Main Flavonoid Glycosides of Dendrobium officinale from Danxia Species and Yunnan Guangnan Species. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 29–34. [Google Scholar] [CrossRef]
- Li, Z.B.; Meng, Y.J.; Hu, L.; Zhang, L.; Huang, Y.H.; Yang, L.E.; Liang, Z.Y.; Huang, Y.C.; Wei, G. Analysis of the Chemical Composition of Flavonoids in Dendrobium fimbriatum Hook. Based on HPLCESI-MSn. Tradit. Chin. Drug Res. Clin. Pharmacol. 2022, 33, 1254–1260. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Zhang, J.Y.; Huang, Y.C.; Zhou, C.J.; Wang, Y.W.; Zhou, C.H.; Xing, S.P.; Shun, Q.S.; Xu, Y.X.; Wei, G. Identification of flavonoids in Dendrobium huoshanense and comparison with those in allied species of Dendrobium by TLC, HPLC and HPLC coupled with electrospray ionization multi-stage tandem MS analyses. J. Sep. Sci. 2019, 42, 1088–1104. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhou, W.Y.; Han, B.; Wang, Y.C.; Zeng, Y.Y.; Lu, S.C.; Sun, Z.Y. Study on alkaloids from stems of Dendrobium crepidatum based on UPLC-Q-TOF-MS. Nat. Prod. Res. Dev. 2020, 32, 482–488+426. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhang, M.; Han, B.; Zhai, F.F.; Liu, L.; Sun, Z.Y.; Li, Z.J. Study on alkaloids in the stems of Dendrobium pendulum based on UPLC-Q-TOF-MS. Nat. Prod. Res. Dev. 2021, 33, 2019–2028. [Google Scholar] [CrossRef]
- Qian, L.P.; Que, H.Q.; Peng, H.Y.; Li, W.; Chen, A.H.; Guo, S.M.; Li, Y.C.; Lin, S. A Study on the Chemical Constituents of Anoectochilus burmannicus Rolfe. Chin. J. Ethnomed. Ethnopharm. 2020, 29, 43–48. [Google Scholar]
- Qian, L.P.; Li, W.; Peng, H.Y.; Que, H.Q.; Shun-min, G.; Sui, L. Research on chemical constituents of Anoectochilus roxburghii. China Med. Pharm. 2021, 11, 73–76. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, H.P.; He, W.B.; Yang, W.L.; Ni, F.Y.; Huang, Z.W.; Hu, H.Y.; Wang, J. Polyphenols from Dendrobium loddigesii and their biological activities. Acta Sci. Nat. Univ. Sunyatseni 2019, 58, 96–102. [Google Scholar] [CrossRef]
- Gang, Y.Q. Studies on Chemical Constituents from Bletilla Striata and Their Biological Activities. Master’s Thesis, South-Central University for Nationalities, Wuhan, China, 2020. [Google Scholar]
- Song, S.P.; Jiang, F.; Li, C.H.; Wei, T.; Yu, Y.F.; Tian, X.H. Chemical Constituents from Liparis japonica. Chin. Pharm. J. 2018, 53, 104–108. [Google Scholar]
- Yang, Z.Y.; Zhang, Y.; Yang, J.; Luo, W.L.; Zhang, M.S.; Wang, G.; Sun, C.X.; Dong, M.J.; Xiao, S.J. Chemical constituents from Dendrobium moschatum. Chin. Tradit. Pat. Med. 2022, 44, 3517–3521. [Google Scholar] [CrossRef]
- Shang, Z.M.; Cheng, L.; Liu, G.Y.; Zhang, M.S.; Li, X.F.; Xiao, S.J. Chemical constituents of Dendrobium bellatulum. Chin. Tradit. Herb. Drugs 2019, 50, 2036–2040. [Google Scholar] [CrossRef]
- Yang, X.B.; Yan, S.; Hu, J.M.; Zhou, J. Chemical constituents from Dendrobium heterocarpum Lindl. Nat. Prod. Res. Dev. 2019, 31, 1745–1752. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New phenanthrene and 9,10-dihydrophenanthrene derivatives from the stems of Dendrobium officinale with their cytotoxic activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef]
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2020, 34, 1694–1701. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, Q.F.; Xia, J.; Wang, L.; Tao, L.; Shen, X.C. Antitumor Phenanthrene Constituents of Dendrobium nobile. Chin. Pharm. J. 2018, 53, 1722–1725. [Google Scholar]
- Cai, C.H.; Tan, C.Y.; Chen, H.Q.; Wang, H.; Mei, W.L.; Song, X.Q.; Dai, H.F. Chemical constituents from Dendrobium sinense (Ⅱ). Guihaia 2020, 40, 1368–1374. [Google Scholar] [CrossRef]
- Zhao, H.S.; Xu, F.G.; Chen, X.X.; Hu, J.M.; Zeng, F.J.; Peng, D.Y.; Wu, D.L. Chemical constituents of Dendrobium huoshanense C.Z.Tang et S.J.Cheng. Nat. Prod. Res. Dev. 2021, 33, 1491–1498. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Chen, F.; Zhang, Q.; Chu, S.R. Effect of dihydrophenanthropyrans of bletilla striata on common clinical pathogenic bacteria. Anhui Med. Pharm. J. 2020, 24, 800–804+852. [Google Scholar] [CrossRef]
- Sun, M.H.; Ma, X.J.; Shao, S.Y.; Han, S.W.; Jiang, J.W.; Zhang, J.J.; Li, S. Phenanthrene, 9,10-dihydrophenanthrene and bibenzyl enantiomers from Bletilla striata with their antineuroinflammatory and cytotoxic activities. Phytochemistry 2021, 182, 112609. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.W.; Ti, H.H.; Wang, S.M. Study on the chemical constituents, antimicrobial and anti-inflammatory activities of Pholidotachinensis Lindl. J. Guangdong Pharm. Univ. 2021, 37, 1–7. [Google Scholar] [CrossRef]
- Yan, X.; Tang, B.; Liu, M. Phenanthrenes from Arundina graminifolia and in vitro evaluation of their antibacterial and anti-haemolytic properties. Nat. Prod. Res. 2018, 32, 707–710. [Google Scholar] [CrossRef]
- Dong, Y.F.; Li, W.Y.; Ye, R.C.; Wang, L. Antimicrobial and antioxidant activities of total alkaloids of Liparis nervosa(Thunb.)Lindl. J. Sichuan Univ. 2010, 47, 669–673. [Google Scholar] [CrossRef]
- Liu, S.; Huang, X.Y.; Su, Y.H.; Bai, X.; Zhu, P.C.; Li, D.L.; Tang, J.N. Antibacterial mechanism of crude extracts of lactic acid bacteria isolated from traditional fermented food against methicillin-resistant Staphylococcus aureus. J. Food Saf. Qual. 2023, 14, 66–73. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Zhu, X.M.; Xie, Y.L. Research progress of natural products’ inhibition on the growth and aflatoxin synthesis of Aspergillusflavus. J. Henan Univ. Technol. 2021, 42, 132–140. [Google Scholar] [CrossRef]
- Luo, C.; Zhuo, D.; Lu, X.X. Research progress in antioxidant mechanism of natural products. Sci. Technol. Food Ind. 2009, 30, 335–339. [Google Scholar] [CrossRef]
- Fang, Z.; Xue, B.; Liu, L.Z.; Yang, Y. Free Radical Types in Cells and Generation Mechanism. J. Anhui Agric. Sci. 2015, 43, 20–22+40. [Google Scholar] [CrossRef]
- Yan, S.; Ma, R.j.; Yang, L.; Li, J.y.; Yang, X.B.; Hu, J.M. Chemical constituents and skin caring activities of Dendrobium loddigesii. Nat. Prod. Res. Dev. 2019, 31, 615–620. [Google Scholar] [CrossRef]
- Lv, W.W.; Zhao, M.; Qin, H.C.; Duan, S.M.; Li, K.; Liao, C.H.; Zhou, P. Study on Determination of Anthocyanin from Bletilla striata Flower and Antioxidant Activity in vitro. Genom. Appl. Biol. 2017, 36, 5269–5276. [Google Scholar] [CrossRef]
- Cao, M.M.; Chen, W.Q. Interpretation on the global cancer statistics of GLOBOCAN 2020. Chin. J. Front. Med. Sci. 2021, 13, 63–69. [Google Scholar]
- Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin alphav and integrin beta3. BMC Complement. Altern. Med. 2017, 17, 553. [Google Scholar] [CrossRef]
- Cardile, V.; Avola, R.; Graziano, A.C.E.; Russo, A. Moscatilin, a bibenzyl derivative from the orchid Dendrobium loddigesii, induces apoptosis in melanoma cells. Chem. Biol. Interact. 2020, 323, 109075. [Google Scholar] [CrossRef]
- Chen, W.K.; Chen, C.A.; Chi, C.W.; Li, L.H.; Lin, C.P.; Shieh, H.R.; Hsu, M.L.; Ko, C.C.; Hwang, J.J.; Chen, Y.J. Moscatilin Inhibits Growth of Human Esophageal Cancer Xenograft and Sensitizes Cancer Cells to Radiotherapy. J. Clin. Med. 2019, 8, 187. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Luo, W.J.; Wang, G.H.; Zhang, X.; Chen, Z.N.; Wang, N.L.; Yao, X.S. Inhibitory effect of bibenzyls from Dendrobium Nobile on the proliferation of high invasive hepatoma cell line FHCC-98. Chin. J. Tissue Eng. Res. 2006, 10, 150–152. [Google Scholar] [CrossRef]
- Wang, G.H.; Guo, X.Y.; Wang, N.L.; Zhang, J.C.; Yang, M.S.; Yao, X.S. Study on Cytotoxicity of Four 9,10-Dihydrophenanthrenes. Chin. Pharm. J. 2007, 42, 181–183. [Google Scholar] [CrossRef]
- Li, J.J.; Ren, F.C.; Hu, J.M.; Zhou, J. Chemical constituents and cytotoxic activities of Dendrobium wardianum. Chin. Tradit. Herb. Drugs 2020, 51, 1819–1824. [Google Scholar] [CrossRef]
- Suzuki, R.; Tanaka, T.; Yamamoto, M.; Sakagami, H.; Tomomura, M.; Tomomura, A.; Satoh, K.; Shirataki, Y. In search of new biological activities of isolates from Odontoglossum Harvengtense ‘Tutu’. Vivo 2012, 26, 993–999. [Google Scholar]
- Wang, T.S.; Lu, Y.M.; Ma, G.X.; Pan, Y.; Xu, G.J.; Xu, L.S.; Wang, Z.T. In vitro inhibition activities of leukemial K562 cells growty by constituents from D. Chrysotoxum. Nat. Prod. Res. Dev. 1997, 1–3. [Google Scholar] [CrossRef]
- Jao, C.W.; Lin, W.C.; Wu, Y.T.; Wu, P.L. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef]
- Fu, J.N.; Zhao, H.J.; Wang, Y.X. Effect of gastrodin on proliferation and apoptosis of glioma cells. Chin. J. Clin. Pharmacol. 2022, 38, 518–522. [Google Scholar] [CrossRef]
- Sun, A.; Liu, J.; Pang, S.; Lin, J.; Xu, R. Two novel phenanthraquinones with anti-cancer activity isolated from Bletilla striata. Bioorg Med. Chem. Lett. 2016, 26, 2375–2379. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.; Zhang, X.; Wang, W.; Dong, X.; Yan, X.; Hu, R. Bioactivity-guided isolation of biphenanthrenes from Liparis nervosa. Fitoterapia 2016, 115, 15–18. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wu, Q.; Shi, J.S.; Lu, Y.L. Component Analysis of Dendrobium nobile Lindl. Alkaloids and its Inhibitory Effect on the Apoptosis of PC12 Cell Induced by Aβ1-42. Chin. Pharm. J. 2021, 56, 1059–1067. [Google Scholar]
- Xie, H.; Feng, S.; Farag, M.A.; Sun, P.; Shao, P. Synergistic cytotoxicity of erianin, a bisbenzyl in the dietetic Chinese herb Dendrobium against breast cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 149, 111960. [Google Scholar] [CrossRef] [PubMed]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Assessment of Antioxidant and Cytotoxic Activities of Extracts of Dendrobium crepidatum. Biomolecules 2019, 9, 478. [Google Scholar] [CrossRef]
- Schuster, R.; Zeindl, L.; Holzer, W.; Khumpirapang, N.; Okonogi, S.; Viernstein, H.; Mueller, M. Eulophia macrobulbon—An orchid with significant anti-inflammatory and antioxidant effect and anticancerogenic potential exerted by its root extract. Phytomedicine 2017, 24, 157–165. [Google Scholar] [CrossRef]
- Warinhomhoun, S.; Khine, H.E.E.; Sritularak, B.; Likhitwitayawuid, K.; Miyamoto, T.; Tanaka, C.; Punsawad, C.; Punpreuk, Y.; Sungthong, R.; Chaotham, C. Secondary Metabolites in the Dendrobium heterocarpum Methanolic Extract and Their Impacts on Viability and Lipid Storage of 3T3-L1 Pre-Adipocytes. Nutrients 2022, 14, 2886. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. Establishment of Bletilla striata (Thunb.) Rchb.f Cell Suspension Culture System and Determination of Secondary Metabolites. Master’s Degree, Yunnan Agricultural University, Kunming, China, 2012. [Google Scholar]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; ShangGuan, Y.; Liu, H.; Xu, D. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb.f. using a callus suspension culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef] [PubMed]
- Pujari, I.; Thomas, A.; Rai, P.S.; Satyamoorthy, K.; Babu, V.S. In vitro bioproduction and enhancement of moscatilin from a threatened tropical epiphytic orchid, Dendrobium ovatum (Willd.) Kraenzl. 3 Biotech 2021, 11, 507. [Google Scholar] [CrossRef]
- Deng, H.; Chen, N.F.; Chen, C.W.; Han, B.X.; He, X.M. A Method for the Production of Dendrobium Huoshanensis Alkaloids by Suspension Culture Cells. CN105906641A, 20 May 2016. [Google Scholar]
- Deng, H.; Chen, N.F.; Chen, C.W.; Han, B.X.; Sun, C.B. A Method for the Production of Dendrobium Huoshanensis Flavone by Suspension Culture Cells. CN105838749A, 20 May 2016. [Google Scholar]
- Wang, C.Q. Temperature Difference on Syringin and Coniferin Contain in Dendrobium Catenatum and Related Gene Expression. Master’s Degree, Fujian Agriculture and Forestry University, Fuzhou, China, 2019. [Google Scholar]
- Dai, J.G.; Lu, D.D.; Cui, Y.J.; Guo, H.Z.; Zheng, J.H.; Guo, D.A. Biotransformation of gastrodin by cell suspension culture of platycodongrandiflorum. Acta Pharm. Sin. 2001, 36, 942–943. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Zeng, X.Y.; Xu, D.H.; Luo, Y.F.; Liu, T.S.; Jiang, L.M.; Wang, Z.; Meng, L. Method and Application of Biotransformation Synthesis of Gastrodin. CN109609434A, 23 November 2018. [Google Scholar]
- Cai, J.; Ding, J.Y.; Hua, Y.A.; Li, N. Establishment of biotransformation system of the gastrodin biosynthesis by hairy root of Panaxginseng. J. Plant Resour. Environ. 2005, 14, 29–31. [Google Scholar] [CrossRef]
- Gong, J.S.; Ma, W.P.; Pu, J.X.; Xu, S.G.; Zheng, S.Q.; Xiao, C.J. Production of gastrodin through biotransformation of p-hydroxybenzaldehyde by cell suspension cultures of Datura tatula L. Sheng Wu Gong. Cheng Xue Bao = Chin. J. Biotechnol. 2006, 22, 800–804. [Google Scholar] [CrossRef]
- Pujari, I.; Babu, V.S. Rhizobium rhizogenes infection in threatened Indian orchid Dendrobium ovatum mobilises “Moscatilin” to enhance plant defensins. 3 Biotech 2022, 12, 119. [Google Scholar] [CrossRef]
- Li, Z.; Wen, W.; Qin, M.; He, Y.; Xu, D.; Li, L. Biosynthetic Mechanisms of Secondary Metabolites Promoted by the Interaction Between Endophytes and Plant Hosts. Front. Microbiol. 2022, 13, 928967. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Jia, M.; Chen, L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbiol. 2017, 43, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; ShangGuaan, Y.N.; Huang, C.Y.; Wu, M.K.; Xu, L.L.; Xu, D.L. Co-culture System of Bletilla striata Callus With Endophytic Fungi and Changing Contents of Secondary Metabolites. North. Hortic. 2021, 485, 128–134. [Google Scholar]
- Wang, J.; Li, J.F.; Zhang, T.T.; Sun, T.F.; Liu, W.H. Effect of endophytic fungi on expression of key enzyme genes in polysaccharide and alkaloid synthesis from Dendrobium officinale. Chin. Tradit. Herb. Drugs 2019, 50, 5838–5846. [Google Scholar] [CrossRef]
- Gao, W.W.; Guo, S.W. Effects of Endophytic Fungal Hyphae and Their Metabolites on the Growth of Dendrobium candidum and Anoectochilus roxburghii. Acta Acad. Med. Sin. 2001, 23, 556–559. [Google Scholar]
- Hampejsová, R.; Berka, M.; Berková, V.; Jersáková, J.; Domkářová, J.; Von Rundstedt, F.; Frary, A.; Saiz-Fernández, I.; Brzobohatý, B.; Černý, M. Interaction With Fungi Promotes the Accumulation of Specific Defense Molecules in Orchid Tubers and May Increase the Value of Tubers for Biotechnological and Medicinal Applications: The Case Study of Interaction Between Dactylorhiza sp. and Tulasnella calospora. Front. Plant Sci. 2022, 13, 757852. [Google Scholar] [CrossRef]
- Yang, J.W.; Ling, H.; Zhang, Y.; Zeng, X.; Guo, S.X. Effects of endophytic fungi on seed germination of medicinal plants of Orchidaceae: A review. Mycosystema 2018, 37, 22–34. [Google Scholar] [CrossRef]
- Yeow, L.C.; Chew, B.L.; Sreeramanan, S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol. Rep. 2020, 27, e00497. [Google Scholar] [CrossRef]
- Ko, S.S.; Jhong, C.M.; Shih, M.C. Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef]
- Li, D.; Ye, G.; Li, J.; Lai, Z.; Ruan, S.; Qi, Q.; Wang, Z.; Duan, S.; Jin, H.L.; Wang, H.B. High light triggers flavonoid and polysaccharide synthesis through DoHY5-dependent signaling in Dendrobium officinale. Plant J. Cell Mol. Biol. 2023. [Google Scholar] [CrossRef]
- Ting, L.; Shangwen, Z.; Bin, Y.; Ping, M.; Weihua, Y.; Qian, S.; Xiangjun, Z. Effects of Se Application on Growth, SOD Activity and Contents of Polysaccharide and Chlorophyll of Dendrobium officinale Tissue Culture Seedlings. Guangxi For. Sci. 2022, 51, 798–802. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, H.; Shen, H.; Dong, Y.; Sun, X.; Cai, Y.; Fan, H. Effects of low phosphorus stress on the main active ingredients and antioxidant activities of Dendrobium officinale. Ind. Crops Prod. 2021, 173, 114095. [Google Scholar] [CrossRef]
- Guo, Y.M.; Zeng, L.J.; Liang, S.Y.; Deng, R.Q.; Ye, H.N.; Liao, S.X. Effect of UV-B Radiation on Growth and Main Secondary Metabolites of Dendrobium officinale Kimuraet Migo. North. Hortic. 2016, 368, 154–156. [Google Scholar]
- Mo, Y.; Zeng, L.; Huang, H.; Feng, H.; Ye, J.; Li, J.; Zhou, C. Effects of UV—B Radiation on PhOtOsynthetic Pigments, Flavonoids and PAL Activity in Dendrobium officinole. Guizhou Agric. Sci. 2015, 43, 34–37. [Google Scholar] [CrossRef]





| Plant Source | Active Substance | Activities and Indicators | Cell Model | Reference |
|---|---|---|---|---|
| CH | Erianin | Inhibits the proliferation of cancer cells, promotes G2/M phase arrest and induces apoptosis and has a potential role in ferroptosis and the inhibition of the migration of lung cancer cells. | Lung cancer cell lines H1299 and H460 | [61] |
| N | Crepidatin(1), Chrysotobibenzyl (2), 4,4′-Dihydroxy-3,3′,5-trimethoxydibenzyl (3) | Inhibit cancer cell proliferation. The IC50 values of (1)–(3) were 74.30 ± 0.98 μmol/L, 56.60 ± 0.92 μmol/L and 8.68 ± 0.95 μmol/L, respectively. | Hepatocellular carcinoma cell line FHCC-98 | [62] |
| HA | Phoyunnanin E | Destroys the original morphology of H460 nucleus, promotes apoptosis and necrosis, and inhibits the migration of H460, H292 and A549 human lung cancer cells. | Human lung cancer H460, H292, A549 cells | [58] |
| Y | 4,5-Dihydroxy-2-methoxy-9,10-dihydrophenanthrene (1),4,7-Dihydroxy-2-methoxy-9,10-dihydrophenanthrene (2) | Inhibition of the value added. The IC50 values of (1–2) were 25.5 and 29.1 μmol/L, respectively. (1) It can block HepG2 cells in the G2/M phase and induce apoptosis. | Human hepatoma cell line HepG2 | [63] |
| W | 4-hydroxy-3-methoxy cinnamaldehyde, 3,7-Dimethoxy-5-hydroxy-1,4-phenanthraquinone | Cancer cells are widely toxic. The IC50 values of the two compounds were 0.908–8.84 mol/L, and the inhibitory effect on tumor cells was stronger than that of the positive drug cisplatin. | Human promyelocytic leukemia cell line HL-60, human non-small cell lung cancer cell line A-549, human colon cancer cell line SW480, human SMMC-7721, human breast cancer cell MCF-7 | [64] |
| SU | 2,5-dihydroxy-4,9-dimethoxyphenanthrene (1), 4-methoxyphenanthrene-2,7-diol (2), 2,3-dimethoxy-1,4-phenanthrenequinone (3), 3,5,7-trihydroxyflavone (4) | They inhibited the growth of cancer cells (TS = 1.1–2.7) and had a certain tumor-specific cytotoxicity. Activity (1) > (4) > (2) > (3) | Human oral squamous cell carcinoma cells (HSC-2, HSC-3, HSC-4, Ca9-22), human promyelocytic leukemia cells HL-60 | [65] |
| CH | Erianin, Chrysotoxine, Chysotoxene, confusarin | Proliferation inhibition (at an IC50 concentration of 50%, the inhibition rate was 0.0065,5.43,0.32,46.15 g/mL) at 72 h, with the increase in the compound concentration, the inhibitory effect was enhanced. | Chronic myeloid leukemia cell line K562 | [66] |
| MI | phaitanthrin A(1),tryptanthrine(2) | Strong cytotoxicity. (1) The IC50 values for the two cancer cells were 33.8 and 27.0 μmol/L, respectively. (2) The IC50 values were 11.1 and 9.0 μmol/L, respectively. | Breast cancer cell line MCF-7, lung cancer cell line NCI-H460 | [67] |
| E | Gastrodin | Led to the dose-dependent inhibition of cell proliferation and concentration-dependent induction of glioma cell apoptosis. The expression of p62 protein was significantly upregulated, the expression of LC3-II (or LC3-I) was decreased and Beclin1 protein was downregulated. | Glioma cell T98G | [68] |
| ST | 7-hydroxy-2-methoxy-phenanthrene-3,4-dion,3′,7′,7-trihydroxy-2,2′,4′-trimethoxy-[1,8′-biphenanthrene]-3,4-dione | The two compounds have strong cytotoxicity. The IC50 values were close to that of the positive drug cisplatin. It can effectively induce the arrest of A549 cells in the G0/G1 phase, increase the production of reactive oxygen species (ROS) and promote the apoptosis of cancer cells. | Human lung cancer alveolar basal epithelial cells A549, human breast cancer cells MCF-7, human colon cancer cells HT-29 | [69] |
| NE | 2,2’,7’-trihydroxy-4,7,5’,6’-tetramethoxy-1,1’-biphenanthrene (1),2,7,2′-trihy- droxy-4,4′,7′-trimethoxy-1,1′-biphenanthrene (2),2,2′-dihydroxy-4,4′,7,7′-tetramethoxy-1,1′-biphenanthrene (3) | Strong cytotoxicity. (1–3) The IC50 values of HGC-27 were 8.21–9.95 μmol/L; (1) (3) The IC50 of HT-29 was 8.53–9.27 μmol/L. | Human colon cancer cell line HT-29, human gastric cancer cell line HGC-27 | [70] |
| PL | 2-chloro-3,4′-dihydroxy-3′,5-dimethoxybibenzyl | Strong cytotoxicity. The IC50 values for cancer cells were 3.41, 3.02 and 2.80 M, respectively. | MDA-MB231, HepG2 and A549 cells | [20] |
| N | densiflorol B (1), cypripedin (2), moscatin (3) | Significantly inhibit the proliferation of cancer cells. The IC50 of (1–3) were 2.99, 5.01 and 72.68 μmol/L, respectively. | MCF-7 breast cancer cells | [43] |
| O | orchinol | Significant killing effect on model cancer cells. The IC50 values were 11.96 and 8.92 μM, respectively. | Human promyelocytic leukemia cell lines HI-60 and THP-1 cells | [41] |
| SI | spiranthesphenanthrene A | The cytotoxicity was higher than that of cisplatin (IC50 = 19.0 ± 7.3 μM). By significantly increasing the level of E-cadherin and reducing the levels of vimentin, N-cadherin and Snail, the migration of cancer cells was significantly inhibited. | B16–F10 cancer cells | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Wu, F.; Chen, M.; Xiao, Z.; Xu, Y.; Xu, M.; Liu, J.; Xu, D. Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids. Metabolites 2023, 13, 829. https://doi.org/10.3390/metabo13070829
Li K, Wu F, Chen M, Xiao Z, Xu Y, Xu M, Liu J, Xu D. Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids. Metabolites. 2023; 13(7):829. https://doi.org/10.3390/metabo13070829
Chicago/Turabian StyleLi, Kunqian, Fengju Wu, Mengzhu Chen, Zhihao Xiao, Ya Xu, Mengwei Xu, Jingyi Liu, and Delin Xu. 2023. "Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids" Metabolites 13, no. 7: 829. https://doi.org/10.3390/metabo13070829
APA StyleLi, K., Wu, F., Chen, M., Xiao, Z., Xu, Y., Xu, M., Liu, J., & Xu, D. (2023). Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids. Metabolites, 13(7), 829. https://doi.org/10.3390/metabo13070829








